Abstract
The present study evaluated the hypoglycemic activity of Aloe extract on streptozotocin-induced diabetic mice and focuses its effect on GLUT-4 gene expression under in vitro cell-culture system. Administration of extract at the dosage of 130 mg/kg body weight per day for 4 weeks resulted in significant decrease in blood glucose and total cholesterol in streptozotocin (60 mg/kg body weight) induced diabetic mice. The hypoglycemic effect was compared with metformin. The activities of carbohydrate metabolizing enzymes were brought back to near normal level after the treatment and glucose homeostasis was maintained. Lyophilized aqueous Aloe extract (1 mg/ml) upregulated the GLUT-4 mRNA synthesis in mouse embryonic NIH/3T3 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a chronic metabolic disease of human beings having several clinical complications including retinopathy, nephropathy, neuropathy, cardiomyopathy, and delayed wound healing [1, 2]. The incidence of diabetes is increasing rapidly and this will lead to a worldwide increase in the cost of management of the disease and its complications. The world health organization suggests that by 2030 there will be more than 366 million affected individual worldwide [3]. In recent years, several synthetic drugs have been developed to combat against diabetes but the situation has only marginally improved. Furthermore, these synthetic drugs are not able to combat with all the pathological complications and mostly palliative in its effect. Herbal medications have been used successfully by mankind since ancient time to counteract diabetes and its associated complications [4]. These herbal plants could be an alternative therapy for diabetes and its complications. Since the last two decades, several reports have shown that Aloe preparations have beneficial therapeutic effects on diabetes in human patients [5–7] as well as in diabetic animal models [8–14]. Aloe spp. is documented in ethno-botanical survey as one of potential anti-diabetic plants [15]. The dried sap of the Aloe plant had been used for diabetes in the Arabian Peninsula. Its ability to lower the blood glucose was studied in five patients with noninsulin-dependent diabetes and in Swiss albino mice made diabetic using alloxan [16]. The study concluded that Aloe contains hypoglycemic agents which lower the blood glucose by unknown mechanisms. Effects of Aloe preparations on expression profiles of major genes responsible for glucose uptake are largely unknown. The present investigations was undertaken to evaluate the glucose lowering effects of Aloe extract in streptozotocin-induced diabetic mice and attempts have been made to study its effect on GLUT-4 gene expression in mouse NIH/3T3 cells.
Materials and Methods
Plant Collection
Aloe germplasm named Aloe vera (green variety) was collected from Defense Research Laboratory, Pithoragarh, Uttarakhand, India.
Preparation of Plant Material
Harvested Aloe leaves were collected in clean stainless steel trays. Bulk amount of leaves was washed under running tap water after collection of alloin and was wiped out with dry muslin cloth. Further, leaves were chopped with clean sharp knife into small pieces of size 1–2 mm thick and 4 cm long. Afterward, drying of chopped material was done in a dryer under hot circulating air at 40 °C for 72–96 h. Finally, grinding was done in a grinding mill having stainless steel blades. Ground powder was filled in air tight containers and stored at room temperature. Aqueous extract from Aloe vera was prepared by using standard protocol. Briefly, dry leaf powder of Aloe was mixed in water at 60 °C covered with parafilm and kept in shaker incubator overnight. Supernatant was filtered with Whatman filter paper no. 1 and centrifuged at 5,000 RPM for 10 min. The supernatant collected was dried to form gel in fan-equipped incubator at 50 °C. Water extract was lyophilized and stored at 4 °C.
Spectral Analysis of Lyophilized Aloe Extract
Aqueous solution of lyophilized powder (1 mg/ml) was made and its absorption spectrum was scanned between the wavelength region of 190–600 nm using Shimadzu 1,700 UV–Vis spectrophotometer.
In Vivo Study
Experimental Animals
Ten to 12 weeks old Swiss albino mice were divided into four groups (I, II, III, and IV), with eight mice in each group. Mice were maintained in clean and ambient environment (NIH guidelines) and permission has been taken from institute’s ethics committee. Mice were fed with normal diet or, whenever indicated, specialized diet.
Inductions of Diabetes Mellitus Followed by Aloe Extract Treatment
Overnight starved experimental mice from groups II, III, and IV (as discussed above) were injected with streptozotocin (Sigma, USA) at a dose of 60 mg/kg BW. The chemical was injected through intra-peritoneal (i.p.) mode within 10 min after dissolving in 0.025 M sodium citrate at pH 4.0. The mice in group I was injected with sodium citrate buffer as vehicle control. Fasting blood glucose (FBG) was estimated at the time of induction of diabetes and post-prandial glucose was checked regularly until stable hyperglycemia was achieved. The mice exhibiting blood glucose levels ~250 mg/dl were included in the study as stable hyperglycemic animals. Once the stable hyperglycemia was achieved, the mice belonging to group III was treated with oral dose of Aloe extract (130 mg/kg BW) and the mice belonging to group IV was treated with oral dose of metformin (50 mg/kg body weight), once every day for 4 weeks while groups I and II mice received normal diet. Doses were selected based on 2 weeks trial of glucose tolerance test (GTT) with different set of various doses of Aloe extract and metformin.
Effect of Fasting Blood Glucose Level
Fasting blood glucose was measured after 21 days of treatment with Aloe extract, during which the animals were fed with normal diet. For the determination of FBG, on completion of the 21 days of treatment, the mice were fasted overnight, the blood was collected from the tip of the tail vein and the blood glucose was measured using GOD–POD glucose estimation kit (Excel Diagnostics Pvt. Ltd. India). The results were expressed in terms of milligrams per deciliter of blood.
Glucose Tolerance Test
In order to determine the effect of Aloe extract on insulin activity, the GTT was carried out on all four groups of mice, i.e., normal (nondiabetic) control, diabetic control, diabetic + Aloe treated, and diabetic + metformin treated. GTT was performed by oral administration of glucose load of 1 g/kg bw in 0.1 ml water to overnight fasted animals. Blood samples were collected from the tail vein at 30, 60, 90, and 120 min after the oral glucose load and treated as before for plasma glucose analysis.
Estimation of Lipid Profile in Blood Samples
On completion of the treatment, blood samples were collected and lipid profiles for all four groups of animals were performed using commercially available kits. Total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, and triglyceride (TG) levels in serum were determined according to the instructions of the manufacturer (Trans-Asia Bio Medical limited, Mumbai, India). For the determination of very-low-density lipoprotein (VLDL) and low-density lipoprotein (LDL) cholesterol, Friedwald’s formula was used which states: \( {\text{VLDL cholesterol}} = {\text{Triglyceride}}/{\text{5 and LDL cholesterol}} = {\text{Total cholesterol}} - ({\text{VLDL}} + {\text{HDL cholesterol}}) \).
Biochemical Estimation of Enzyme Activities and Tissue Glycogen Content
Glucose-6-Phosphatase in Liver
Glucose-6-phosphatase catalyzes the conversion of glucose-6-phosphate to glucose [17]. The mice from all the four groups were sacrificed and the collected liver was homogenized in ice-cold sucrose solution (250 mM). To 0.1 ml of sucrose/EDTA buffer, 0.1 ml of 100 mM glucose-6-phosphate, 0.1 ml of imidazole buffer (100 mM, pH 6.5), and 0.1 ml of liver homogenate were added with thorough mixing. The tubes were then incubated at 37 °C for 15 min. The reaction was terminated by the addition of 2 ml of TCA/ascorbate (10%:3%, w/v), and the solution was centrifuged at 3,000 rpm for 10 min. To 1 ml of clear supernatant, 0.5 ml of ammonium molybdate (1%, w/v) and 1 ml sodium citrate (2%, w/v) were added and the absorbance was measured at 700 nm. The enzyme activity was expressed as unit per gram wet tissue.
Hexokinase Activity in Liver
The hexokinase activity was tested based on the reduction of NAD+ through a coupled reaction with glucose-6-phosphate dehydrogenase as per the method described earlier [18]. Briefly, the excised liver tissue homogenate was prepared in normal saline. To 0.1 ml of homogenate, 2.28 ml of Tris–magnesium chloride buffer (200 mM Tris and 20 M MgCl2, pH 8.0) was added along with 0.5 ml of 0.67 M glucose, 0.1 ml of 16 mM ATP, 0.1 ml of 6.8 mM NAD, and 0.01 ml of 300 U/ml glucose-6-phosphate dehydrogenase. The solution was mixed thoroughly, and the absorbance was measured at 340 nm after 5 min of incubation. The activity was expressed as units per gram wet tissue.
Tissue Levels of Glycogen Content
Glycogen content of liver and skeletal muscles was measured according to earlier established methods [19]. Briefly, the samples were homogenized separately in warm 80% ethanol at a concentration of 100 mg/ml and then centrifuged at 10,000 rpm for 20 min. The residue was collected and allowed to dry in a boiling water bath. To each residue, 5 ml of distilled water and 6 ml of perchloric acid were added. The extraction was further carried out at low temperature for 20 min. The collected extract was centrifuged at 10,000 rpm for 15 min and 0.2 ml of the supernatant was transferred to a test tube and the volume was made up to 1 ml by addition of distilled water. To each tube 4 ml of anthrone reagent was added and incubated at 95 °C in a boiling water bath for 10 min. The absorbance of the samples was measured at 630 nm after cooling the tubes to room temperature. The amount of glycogen in tissue samples was expressed in microgram of glucose per milligram tissue.
In Vitro Study
Mouse Cell Line
NIH/3T3 cell line was procured from the National Centre for Cell Sciences, Pune, and grown at 37 °C under 5% CO2 in Dulbecco’s modified minimum essential medium (DMEM), supplemented with 10% NBCS, penicillin (100,000 IU/L), streptomycin (0.1 g/L), and with other additions, i.e., 0.1% DMSO, insulin (0.1 IU/ml), metformin (1 mg/ml), or Aloe extract (1 mg/ml) as indicated.
Determination of Acute Toxicity of Aloe Extract on Mouse Cell Line
Trypan blue exclusion assay was used to assess the potential cytotoxicity of Aloe extract on NIH/3T3 cell line. Lyophilized powder of Aloe extract was dissolved in sterile ultra pure water supplemented with DMSO (0.1% final concentration) and sterilized by filtration using Millipore syringe filter (0.22 μm pore size). Different concentrations of Aloe extract solutions were prepared in DMEM (0.1, 0.5, 1.0, 2.5, 5.0, and 10 mg/ml) and were used to inoculate healthy cells in duplicates along with control. Trypan blue, 0.4%, was used for determination of cell viability at different intervals by using an improved Neubauer pattern hemocytometer.
RT-PCR Amplification of GLUT-4 mRNA
In the present study, Aloe extract (hot water) treated NIH/3T3 cells were analyzed for GLUT-4 gene modulation at transcript level by semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR). NIH/3T3 cells were serum starved for 2 h and inoculated with growth media containing Aloe extract (1.0 mg/ml), insulin (0.1 IU/ml), metformin (1 mg/ml), and vehicle control. After inoculation, these were incubated at 37 °C under 5% CO2. RNA templates were isolated in triplicate from vehicle control cells, Aloe-extract-treated, metformin-treated, and insulin-treated cells using TRI reagent. Briefly, culture flasks were washed with ice-cold PBS before processing for RNA isolation. To amplify GLUT-4 gene, oligonucleotide primers were designed with the use of mouse GLUT-4 gene sequence available from GenBank and synthesized from Genei Pvt. Ltd., Bangalore, India. The primer pairs used for analysis of GLUT-4 transcripts were f 5′ agg cac cct cac tac gct ct 3′ and r 5′ gcc aca atg aac cag gga atg 3′ (product size ~1 kb). PCR products of 18S rRNA, using primer pairs f 5′ cca gta agt gcg ggt cat a 3′ and r 5′ cct tgt tac gac ttt tac ttc c 3′ were used as internal control (193 bp). Reverse transcription was performed directly on total cellular RNA (1.5 ng) by using Qiagen one-step RT-PCR kit.
Densitometry Analysis
After the completion of RT-PCR cycles, signals of amplicons in agarose gel were quantified arbitrarily. Densitometry analysis was done with the help of gene profiler software, Alpha Innotech Corporation USA. Three independent experiments were conducted. Briefly, individual gels were scored by placing the curser over individual band and recording the relative densitometry values of three independent gels used for expression analysis.
Statistical Analysis
Values are presented as means±SEM. The statistical significance was evaluated by one-way ANOVA using the statistical software Origin 6.1 (Origin Lab Corporation, USA).
Results and Discussion
Spectral Analysis
In wavelength range of 190–600 nm five peaks were observed at 190, 200, 270, 290, 300, and 350 nm wavelength. Multiple peaks observed in this experiment indicated the diverse composition of Aloe extract. Some of which might possesses the anti-diabetic property. Several previous studies indicated the presence of anthraquinone aloin, polysachharides, reducing sugars, organic acids, glycoproteins, sterols, terpenes, etc. in Aloe preparations [20, 21].
Effect of Aloe Extract on Fasting Blood Glucose Level and on Glucose Tolerance
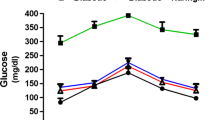
Fasting blood glucose level was significantly higher for the diabetic group as compared to treated and control group of mice (P < 0.05). Diabetic mice supplemented with Aloe extract for 4 weeks showed a remarkable improvement in overall glucose response as compared to untreated diabetic mice. FBG level in Aloe extract and metformin-treated groups were significantly lower as compared to streptozotocin-induced diabetic mice. The blood glucose lowering activity of Aloe extract is almost comparable to that of metformin-treated mice (Fig. 1). Although metformin is not the right drug for the cure of type I diabetes, yet it was used in the present study based on the fact that it can also potentiate the hypoglycemic condition and may reduce the insulin dose requirement in type 1 diabetes [22]. However, its effect on type I diabetic condition is yet to be illustrated. Hence further studies are needed with other drugs which are better known for the control of type 1 diabetes than metformin. The levels of blood glucose in control and Aloe-treated and metformin-treated groups demonstrated a significant change in blood glucose level after administration of glucose (1 g/kg BW). The mice of diabetic group had a significant elevation in blood glucose level throughout the total measurement period (150 min) as compared to control (Fig. 1) and also it did not return to the initial value (zero min level) even at the end of the experiment conducted. Several previous studies indicated the glucose-lowering effect of Aloe products in streptozotocin-induced diabetic mice [10, 14]. Oral GTT data over 150 min suggest that Aloe extract reduced plasma glucose concentration in diabetic mice. Since streptozotocin selectively destroys beta cells of the pancreas, findings suggest that glucose-lowering activity of Aloe extract is possibly mediated by mechanism (s) that does not involve insulin.
Oral glucose tolerance test: effect of Aloe extract on fasting blood glucose (FBG) and oral glucose tolerance in diabetic mice. After 21 days of treatment with Aloe extract, four groups of animals viz group 1—normal (nondiabetic) control, groups 2, 3, and 4—streptozotocin-induced diabetic mice; group 2 diabetic control, group 3 diabetic + Aloe (130 mg/ml) treated, and group 4 diabetic + metformin (50 mg/ml) treated. Results are presented as mean±SEM, (n = 8); a and b indicate the significant level of difference in values (P < 0.05) for diabetic control and Aloe-treated group, respectively, as compared to normal control
Effect of Aloe Extract on Plasma Lipid Profile
Different parameters of blood lipid profiles were tested in streptozotocin-induced diabetic mice after the treatment with Aloe extract. After 4 weeks of Aloe and metformin treatment, the levels of TC, LDL, TG, and VLDL in the plasma of diabetic mice were significantly lowered (P < 0.05). TG levels in the plasma in group treated with Aloe extract and metformin was 85.5% and 82%, respectively, of that of untreated diabetic group. TC levels in the plasma in the group treated with Aloe extract and metformin was 73.95% and 80%, respectively, of that of untreated diabetic group. TC and LDL were remarkably better controlled in Aloe-treated group as compared to metformin (Table 1). Hyperlipidemia is a common finding in patients with diabetes mellitus and is responsible for vascular complications [23, 24]. The administration of Aloe extract significantly decreased serum TG, LDL, and TC in treated diabetic mice. These results are also comparable with those of previous researchers [14].
Effect of Aloe Extract on Enzyme Activities and Tissue Glycogen Content
The result (Fig. 2) shows the activity of liver glucose-6-phosphatase and hexokinase in control and experimental group of mice. After 4 weeks of treatment with Aloe extract, there was a significant reduction in glucose-6-phosphatase activity in diabetic treated groups (groups III and IV) as compared to untreated streptozotocin-induced diabetic mice (group II). By contrast, treatment of the diabetic mice with Aloe extract (130 mg/kg BW) and metformin (50 mg/kg BW) led to 34% and 30%, respectively (P < 0.05), rise in liver hexokinase activity when compared to diabetic control (group II) where this activity is reduced substantially as compared to that of normal (nondiabetic) control (group I).
Enzyme activity: effect of Aloe extract on hepatic glucose 6 phosphatase (G-6-Pase) and hexokinase activity in streptozotocin-induced diabetic mice. Data are expressed as mean±SEM, (n = 8); b and d indicates the significant level of differences in hepatic G-6-Pase activity as compared to normal (nondiabetic) control (P < 0.05); a and c indicates the significant level of differences in hepatic hexokinase activity as compared to normal (nondiabetic) control (P < 0.05)
Glycogen content of liver and skeletal muscle were estimated after 4 weeks of the treatment with Aloe extract along with normal control (group I), diabetic control (group II), and metformin-treated mice (group IV). Figure 3 shows the level of glycogen in the liver and muscle tissue of control and experimental groups of mice. In diabetic mice both liver and muscle glycogen contents decreased significantly, by 69% and 50%, respectively, as compared to normal control (P < 0.05). Treatment with Aloe extract and metformin led to 27% and 50% increase, respectively, in hepatic glycogen contents in diabetic treated groups as compared to diabetic control (P < 0.05). Glycogen is the primary intracellular storable form of sugar and its level in various tissues, especially livers and skeletal muscles are a direct reflection of normal carbohydrate metabolism. In streptozotocin-treated mice there is relative deficiency of insulin [25] which leads to inhibition of influx of glucose in liver and muscle. Our study showed that although the levels of glycogen in Aloe-treated diabetic mice could not achieve the level same as that of normal control, the Aloe extract could significantly improve the hepatic glycogen contents. This indicates one of the possible ways the Aloe extract might act by improving the process of glycogenesis.
Glycogen content: effect of Aloe extract on liver and muscle tissue glycogen contents in streptozotocin-induced diabetic mice. Data are presented as mean±SEM, (n = 8); b and d indicates the significant level of differences in hepatic glycogen contents as compared to normal (nondiabetic) control (P < 0.05); a and c indicates the significant level of differences in muscle glycogen contents as compared to normal (nondiabetic) control (P < 0.05)
Effect of Aloe Extract on GLUT-4 mRNA in NIH/3T3 Cells
Supported by in vivo glucose lowering activity of Aloe extract, we evaluated the extract for GLUT-4 gene expression in embryonic mouse fibroblast cells. Since no significant difference in NIH/3T3 cells growth pattern were observed at 1.0 mg/ml concentration of Aloe extract, this concentration was chosen as the optimal concentration to evaluate the anti-diabetic effect. Densitometry scanning of agarose gel (Figure 4a) indicates an increase in GLUT-4 transcripts by ~2.1764-fold by Aloe extract (1.0 mg/ml) as compared to control, which is also comparable with metformin (~4.4117-fold) and insulin (~3.4779-fold) used as standard references. 18S rRNA was used as internal control (Fig. 4b). Cell-culture assay has been used because of its simplicity and satisfactory reproducibility. Additionally, this model does not raise any ethical issue as well its ability to screen a large number of plants with different combination at initial stage. RT-PCR analysis showed elevated GLUT-4 expression in Aloe-extract-treated cells. Glucose transporter proteins play important role in glucose transport between cells and interstitial fluids. These proteins facilitate glucose entry into the cells via diffusion along a glucose concentration gradient [26]. Metformin was reported to have glucose lowering effect by increased skeletal myocyte glucose uptake [27, 28]. Potential sites of action of metformin are insulin receptors and glucose transporters [29]. The most plausible explanations for the over-expression of GLUT-4 gene under the influence of Aloe extract are: the upregulation of GLUT-4 caused by Aloe extract might be mediated by Aloe’s stimulatory effects on cytoskeletal proteins which play an essential role in vesicle trafficking needed during GLUT-4 transport from cytoplasm to the plasma membrane. This effect might be further transduced in the cytoplasm by one or other known/unknown signal transduction pathway which led to over-expression of GLUT-4 gene. The microtubule network and actin cytoskeleton play a role in GLUT-4 trafficking, either by linking signaling components or by directing movements of vesicles in the cell. GLUT-4 gene expression may be targeted by insulin-receptor-mediated signals. Further work is under progress to establish the role of GLUT-4 genes in Aloe content-mediated glucose-lowering effects. Finding from present investigation indicate the hypoglycemic and hypolipidemic effects of aqueous Aloe extract on streptozotocin-induced diabetic mice. Furthermore, in vitro results suggest that Aloe extract modulate GLUT-4 transcripts. The experimental results provided here could make the basis of understanding the exact molecular pathway (s) by which Aloe active principle(s) carry anti-diabetic action. Plant possesses different toxic residues and hence needs toxicological analysis before being used as drug formulation. Acute toxicity studies have been performed in normal healthy mice using Aloe extract (data not shown). There was no mortality observed in Aloe-treated mice. The tremendous use of Aloe spp. as therapeutics accompanied by an upsurge of both clinical and chemical research which is reaching more closely towards the active ingredients and their biological activity. The emphasis is changing towards definition of active constituent or constituents so that they can be used accurately in the formulations [30]. This kind of study would be useful for designing an appropriate formulation for treatment of diabetes, in which different active principles act synergistically and targets different signaling pathways of carbohydrate metabolism.
a and b Semi-quantitative RT-PCR and densitometry analysis of GLUT-4 gene. a Total RNA isolated from the cells after 36 h of treatment. Cells were incubated with Aloe extract (1 mg/ml), metformin (1 mg/ml), and insulin (0.1 IU/ml) showed elevated levels of GLUT-4 transcript as compared with control cells. Lane 1 control, lane 2 Aloe extract (1 mg/ml), lane 3 metformin (1 mg/ml), lane 4 insulin (0.1 IU/ml), and M indicates 100 bp DNA ladder (three independent experiments were conducted and a representative agarose gel is shown here) and analysis of 18S rRNA transcripts in NIH/3T3 cells. Cells treated as indicated above were analyzed for 18S rRNA transcript used as internal control. Lane 1 control, lane 2 Aloe extract (1 mg/ml), lane 3 metformin (1 mg/ml), lane 4 insulin (0.1 IU/ml), lane 5 template control, M indicates 100 bp DNA ladder. b Densitometry analysis was done with the help of gene profiler software, Alpha Innotech Corporation USA. The average values of three independent experiments are shown here and standard deviations (error bars) are shown for one experiment out of a total of three
In conclusion, the results of in vivo and in vitro studies strongly demonstrate that the water soluble fraction of Aloe spp. possess glucose-lowering activities and some of its component (s) modulates GLUT-4 mRNA expression. Further, investigation will be required to investigate GLUT-4 gene status in mice and fraction responsible for glucose-lowering effect.
References
Arky, R. A. (1982). Clinical correlates of metabolic derangements of diabetes mellitus. In G. P. Kozak (Ed.), Complications of diabetes mellitus (pp. 16–20). Philadelphia: W.B. Saunders.
Marcovecchio, M., Mohn, A., & Chiarelli, F. (2005). Type 2 diabetes mellitus in children and adolescents. Journal of Endocrinology Investigation, 28, 853–863.
Wild, S., Roglie, G., Green, A., et al. (2004). Global prevalence of diabetes estimates for the year 2000 and projections for 2030. Diabetes Care, 27(5), 1047–1059.
Bailey, L. J., & Day, C. (1989). Traditional plant medicine as treatment for diabetes. Diabetes Care, 12, 553–564.
Bunyapraphatsara, N., Chasrakaew, W., Pornchirasilp, S., et al. (1995). Antidiabetic effect of fresh and preserved Aloe gel. Thai Journal of Phytopharmacy, 2, 1–7.
Yongchaiyudha, S., Rungpitarangsi, V., Bunyapraphatsara, N., et al. (1996). Antidiabetic activity of Aloe vera juice l. Clinical trial in new cases of diabetes mellitus. Phytomedicine, 3, 241–243.
Yagi, A., Hegazy, S., Kabbash, A., et al. (2009). Possible hypoglycemic effect of Aloe vera L. high molecular weight fractions on type 2 diabetic patients. Saudi Pharmaceutical Journal, 17(3), 210–217.
Ajbanoor, M. A. (1990). Effects of Aloes on blood glucose levels in normal and Alloxan-diabetic mice. Journal of Ethnopharmacology, 28, 215–220.
Beppu, H., Nagamura, Y., & Fujita, K. (1993). Hypoglycaemic and anti-diabetic effects in mice of Aloe arborescens Miller var. natalensis Berger. Phytotherapy Research, 7, S37–S42.
Beppu, H., Shimpo, K., Chihara, T., et al. (2006). Antidiabetic effects of dietary administration of Aloe arborescens Miller components on multiple low-dose streptozotocin-induced diabetes in mice, investigation on hypoglycemic action and systemic absorption dynamics of Aloe components. Journal of Ethnopharmacology, 103, 468–477.
Rajasekaran, S., Sivagnanam, K., Ravi, K., et al. (2004). Hypoglycemic effect of Aloe vera gel on streptozotocin-induced diabetes in experimental rats. Journal of Medicinal Food, 7, 61–66.
Rajasekaran, S., Sivagnanam, K., & Subramanian, S. (2005). Modulatory effects of Aloe vera leaf gel extract on oxidative stress in rats treated with streptozotocin. The Journal of Pharmacy and Pharmacology, 57, 241–246.
Rajasekaran, S., Ravi, K., Sivagnanam, K., et al. (2006). Beneficial effects of Aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clinical and Experimental Pharmacology & Physiology, 33, 232–237.
Kim, K., Kim, H., Kwon, J., et al. (2009). Hypoglycemic and hypolipidemic effects of processed Aloe vera gel in a mouse model of non-insulin-dependent diabetes mellitus. Phytomedicine, 16, 856–863.
Gbolade, A. A. (2009). Inventry of antidiabetic plants in selected districts of lagos state, Nigeria. Journal of Ethnopharmacology, 121, 135–139.
Ghannam, N., & Geissman, E. S. (1986). The anti-diabetic activity of Aloes, preliminary clinical and experimental observations. Hormone Research, 24, 288–294.
Schaftingen, E. V., & Gerin, I. (2002). The glucose-6-phosphatase system. Journal of Biochemistry, 362, 513–532.
Brandstrup, N., Kirk, J. E., & Bruni, C. (1957). Determination of hexokinase in tissues. Journal of Gerontology, 12, 166–171.
Sadasivam, S., & Manickam, A. (1996). Carbohydrates. In S. Sadasivam & A. Manickam (Eds.), Methods in biochemistry (pp. 11–12). New Delhi: New Age International Private Limited.
Obata, M., Ito, S., & Beppu, H. (1993). Mechanism of antiinflammatory and antithermal burn action of Cpase from Aloe arborescence Miller var. natalensis berger in rats and mice. Phytotherapy Reasearch, 7(7), S30–S33.
Hamman, J. H. (2008). Composition and applications of Aloe vera gel. Molecules, 13, 1599–1616.
Vella, S., Buetlow, L., Royle, P., et al. (2010). The use of metformin in type 1 diabetes: a systemic review of efficacy. Diabetologia, 53(5), 809–820.
Perkins, J. M., & Davis, S. N. (2007). The rationale for prandial glycemic control in diabetes mellitus. Insulin, 2(2), 52–60.
Valdivielso, P., Puerta, S., Rioja, J., et al. (2010). Postprandial apolipopreotein B48 is associated with asymptomatic peripheral arterial diseases, a study in patients with type 2 diabetes and controls. Clinica Chimica Acta, 411, 433–437.
Liu, K., Paterson, A. J., Konard, R. J., et al. (2002). Streptozotocin, an O-Glc NAcase inhibitor, blunts insulin and growth hormone secretion. Molecular and Cellular Endocrinology, 194, 135–146.
Mueckler, M. (1994). Facilitative glucose transporters. European Journal of Biochemistry, 219, 713–725.
Hundal, H. S., Ramlal, T., Reyes, R., et al. (1992). Cellular mechanism of Metformin action involves glucose transporter translocation from an intracellular pool to the plasma membrane in L6 muscles cells. Endocrinology, 131, 1165–1173.
Galuska, D., Nolte, L. A., Zierath, J. R., et al. (1994). Effects of Metformin on insulin-stimulated glucose transport in isolated skeletal muscles obtained from patients with NIDDM. Diabetologia, 37, 826–832.
Giannarelli, R., Aragona, M., Coppelli, A., et al. (2003). Reducing insulin resistance with metformin, the evidence today. Diabetes & Metabolism, 29, S628–S635.
Reynolds, T. (1998). Aloe vera, what are the active ingredients? Proceedings of the Active Ingredients Conference, Paris, 1998, 85–93.
Acknowledgment
The authors would like to thank Dean, College of postgraduate studies, G. B. Pant University of Agriculture and Technology, Pantnagar, for providing necessary facilities. First author also acknowledge Department of Biotechnology, Government of India, for providing merit scholarship during his Master’s degree program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R., Sharma, B., Tomar, N.R. et al. In Vivo Evalution of Hypoglycemic Activity of Aloe spp. and Identification of Its Mode of Action on GLUT-4 Gene Expression In Vitro. Appl Biochem Biotechnol 164, 1246–1256 (2011). https://doi.org/10.1007/s12010-011-9210-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9210-6