Abstract
In this study, Streptomyces roseosporus was subjected to helium–neon (He–Ne) laser (632.8 nm) irradiation to improve the production ability of extracellular antibiotic daptomycin. Under the optimum irradiation dosage of 18 mW for 22 min, a stable positive mutant strain S. roseosporus LC-54 was obtained. The maximum A21978C (daptomycin is a semisynthetic antimicrobial substance derived from the A21978C complex) yield of this mutant strain was 296 mg/l, which was 146% higher than that of the wild strain. The mutant strain grew more quickly and utilized carbohydrate sources more efficiently than the wild strain. The batch culture kinetics was investigated in a 7 l bioreactor. The logistic equation for growth, the Luedeking–Piret equation for daptomycin production, and Luedeking–Piret-like equations for carbon substrate consumption were established. This model appeared to provide a reasonable description for each parameter during the growth phase and fitted fairly well with the experiment data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a growing need to develop new antibiotics to combat the worsening problem relating to antibiotic resistance among potentially life-threatening pathogens [1, 2]. The high prevalence of the problem among staphylococci has driven the increased use of certain agents of last resort such as vancomycin. However, cases of methicillin-resistant Staphylococcus aureus, penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant Enterococcus spp. have been well reported in the USA and around the world. Infections caused by these pathogens may become more common with time. Due to the increasing spread of resistant organisms, the discovery of novel antibiotic agents that can combat these resistant pathogens is very important.
Daptomycin is a cyclic lipopeptide antibiotic with activity limited to gram-positive organisms [3]. Daptomycin’s mechanism of action possibly involves disrupting amino acid transport by the cell membrane and altering cytoplasmic membrane potential, which may account for its efficacy against strains resistant to other antibiotics in clinical use [4–6]. Daptomycin is a semisynthetic antimicrobial derived from the bioprecursor A21978C [7], which was first isolated from the culture filtrates of Streptomyces roseosporus by several resin procedures [8]. In order to obtain a higher production of daptomycin, it is important to improve A21978C yield. However, few study about the efficient improvement of this bioprecursor has been reported.
Low-power He–Ne laser irradiation is an attractive and preferable method to mutate microorganisms [9, 10]. Kohli and Gupta investigated the mechanism of He–Ne laser preirradiation-induced protection against UVC damage in wild-type Escherichia coli K12 strain AB1157 and its isogenic DNA-repair mutant strains [11]. However, few literatures about the application of low-power He–Ne laser irradiation in the antibiotic biotechnology such as daptomycin production have been mentioned.
Industrial fermentation is gradually moving away from the traditional and largely empirical approach towards simpler and better controlled process [12]. Knowledge and understanding of the kinetics of antibiotics production are of great economic importance. The rational design and optimization of the reactor also require a quantitative understanding of production kinetics. Mathematical models are valuable for fermentation process operation, which are proposed for a limited number of antibiotic production systems. For example, a simple model was utilized to investigate the effect of specific growth rate on the biosynthesis of erythromycin by S. erythraeus in phosphate-limited chemostat cultures [13]. For improving the production of A21978C, kinetic study is also necessary.
The aim of this study was to improve the production of A21978C with S. roseosporus. He–Ne laser irradiation method was applied to mutate the A21978C-producing strain S. roseosporus. The mathematical models for simulating the A21978C production behaviors including cell growth, substrate consumption, and A21978C production were then established.
Materials and Methods
Strain
The wild-type A21978C-producing stain S. roseosporus NRRL11375 was conserved in our lab.
Culture Medium
The slant medium was G1 medium containing (per liter): 20 g of starch, 1 g of KNO3, 0.5 g of MgSO4·7H2O, 0.5 g of NaCL, 0.5 g of K2HPO4·3H2O, and 0.1 g of Fe2(SO4)3 (pH 7.5). The seed medium contains 5 g glucose, 5 g peptone, 5 g yeast extract, 15 g dextrin, 5 g peanut powder, 0.5 g K2HPO4·3H2O, 0.5 g MgSO4·7H2O, 0.2 g and CaCO3, dissolved in 1 l water, with pH adjusted to 7.5. The fermentation medium was as follows: 38 g dextrin, 1 g casein, 5 g peanut powder, 0.5 g K2HPO4·3H2O, 0.5 g MgSO4·7H2O, and 0.2 g CaCO3, dissolved in 1 l water, with pH adjusted to 7.5.
Cultivation and A21978C Production
Spore was scraped out of slant and maintained as a frozen suspension in 20% glycerol with 106/ml at −20 °C. Firstly, seed culture was prepared by inoculating 1 ml of spore suspension into 250 ml serum bottle with 30 ml primary culture medium and incubated under aerobic condition at 30 °C, with a shaking speed of 200 rpm for 48 h, where cells were at their midlogarithmic phase. Then 2% (v/v) of seed was inoculated into 7.5 l BIOFLO 110 bioreactor with 4-l working volume (New Brunswick Scientific Company, USA) and incubated for 144 h at 30 °C with agitation of 200–400 rpm, aeration of 0.8 v/v/m (pH 7.0).
Irradiation Procedure
Laser irradiation was performed at a wavelength of 632.8 nm using HN-1000 He–Ne laser (Guangzhou Institute of Laser Application Technology, PR China). Laser irradiation was transmitted from the laser to the spore suspension by an 800-μm optical fiber, which was fixed with a delivery arm. The delivery arm permitted precise positioning of fiber tip 23 mm above the cell suspension and allowed the laser beam width of 10 mm, which indicated that the focal spot was fixed at 0.785 cm2. Laser irradiation experiments were performed at a wide range of energy doses by varying output power and exposure time. All the experiments of He–Ne laser irradiation were operated under dark conditions at 30 °C. The laser irradiation fluence was calculated with the following formula:
where Q is laser irradiation fluence (J/cm2); W is output power of laser irradiation (mW); T is laser irradiation time (min); and S is focal spot of laser irradiation (cm2). A 0.1-ml spore suspension of the wild strain (106/ml) was put in a quartz cuvette (cross section, 10 × 10 mm, capacity 3.5 ml) and exposed to He–Ne laser under the given irradiation condition. After each irradiation, spore suspension was immediately diluted, and 0.1 ml of each gradient was plated on Gause’s synthetic agar medium. The spores were cultivated aerobically in the dark at 30 °C for 7 days. The single colony was selected and conserved in 4 °C for further screening.
The genetic characteristics of the select positive mutant strain was carried out by the following method: at the end of the production process, 1-ml cell suspension was transferred into another 250-ml serum bottle with 30-ml seed medium and cultivated under the same conditions as 2.3, which was as the seed culture for the next cultivation.
Analysis
The concentration of the components of A21978C was measured by Agilent 1100 HPLC (Agilent Company, USA). The analytical column was from Agilent Industries C18 (25 cm × 4.6 mm). The mobile phase consisted 0.1% trifluoroacetic acid in water and acetonitrile (55:45, v/v). Total sugars in the supernatants of all extracts were measured by the hot anthrone method [14]. A 10-ml aliquot of the fermentation broth was sampled to determine the cell dry weight. The sample was harvested by vacuum and washed with 0.9% NaCl solution. Cell dry weight was determined by filtering the fluid through a preweighed filter paper (Whatman GF/C) and dried at 80 °C in a vacuum oven to constant weight.
Statistics
All experiments were repeated three times. The data shown in the tables and figures in the “Results and Discussion” section were the mean values of the experiments.
Results and Discussion
He–Ne Laser Irradiation on A21978C-Producing Strain
A wide dose ranging from 1.91 to 47.77 J/cm2 were used in laser irradiation experiments, and the controlled output power from 5 to 25 mW and exposure time from 5 to 25 min were performed.
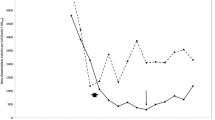
Figure 1 shows the results of mutagenesis and mortality under different irradiation parameters. It was observed that the minimum cell mortality and positive mutation rate occurred at 5-mW output powers, indicating that irradiation with lower output power had less effect on this strain. Spore mortality rate increased with increasing power doses and irradiation time, while mutagenesis rate decreased after the optimum energy density. Moreover, if one of these two parameters was fixed, spore mortality rate would increase with the increase of the other parameter. In addition, no high positive mutation frequency was acquired under the condition of low mortality rate. At 20 mW for 20 min, 3.5% positive mutation rate was obtained, while mortality rate reached 48%. The corresponding fluence of 30.57 J/cm2 was the optimum energy density.
The results of mutagenesis under different irradiation parameters. It was observed that the minimum cell mortality and positive mutation rate occurred at 5-mW output powers, indicating that irradiation with lower output power had less effect on this strain. Spore mortality rate increased with increasing power doses and irradiation time, while mutagenesis rate decreased after the optimum energy density. At 20 mW for 20 min, 3.5% positive mutation rate was obtained, while mortality rate reached 48%. The corresponding fluence of 30.57 J/cm2 was the optimum energy density
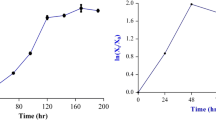
In order to further investigate the effects of the output power on the mortality rate and positive mutation frequency at the fluence of 30.57 J/cm2, irradiation experiments were conducted as follows: (I) 14 mW for 28.6 min, (II) 16 mW for 25 min, (III) 18 mW for 22.2 min, (IV) 20 mW for 20 min, and (V) 22 mW for 18.2 min. As shown in Fig. 2, the maximum positive mutation frequency occurred for experiment III, in which 4.8% positive mutation frequency was obtained. The results show that the ideal positive mutation frequency was determined by both the fluence and the output power, and the effort was increased with the increase of output power. Positive mutation rate increased with the output power and then decreased because of the higher mortality rate in experiment V. The optimum output power was near 18 mW, where the survival rate was 60% and the positive mutation frequency was 4.8%.
Efforts of the different output power. The maximum positive mutation frequency occurred for experiment III, in which 4.8% positive mutation frequency was obtained. Positive mutation rate increased with the output power and then decreased because of the higher mortality rate in experiment V. The optimum output power was near 18 mW, where the survival rate was 60% and the positive mutation frequency was 4.8%
Based on the above optimum He–Ne laser irradiation parameters (the output power of 18 mW, the exposure time of 22.2 min, and the corresponding fluence of 30.57 J/cm2), a total of 101 positive mutant strains was acquired with higher A21978C production ability. The mutant strain with the highest A21978C yield ability was selected and named as LC-54. The A21978C production capability, colony characteristics, and cell conformation of the mutant strain LC-54 was proven to be stable for ten generations (data not shown), which exhibits the heredity stability of the mutant strain LC-54. And LC-54 was used in following studies.
A21978C Production
Figure 3 indicates the cell growth, A21978C production and carbon consumption of LC-54 and the wild strain at the initial total carbohydrate of 38 g/l. After a short lag phase, both strains entered the exponential growth phase. The production of antibiotic started at 20 h, where the cells entered the midexponential phase and reached the max production at 136 h. Therefore, cell growth and A21978C production took place asynchronous, which follow a trophophase–idiophase fermentation, the typical pattern of antibiotic production [15]. LC-54 could accumulate 296 mg/l of A21978C within 144 h, which was 176 mg more than that of the wild strain. Moreover, carbon sources were consumed very quickly, and the cell dry weight increases sharply in both the mutant strain and the wild strain in the first 60 h. But in the last 84 h the wild strain utilizes less carbon sources, and 13.8 g/l of total carbohydrate concentration was left in the broth at the end of cultivation. On the contrary, the mutant consumed substrate efficiently, grew faster, and produced more antibiotic compared to the wild strain. It may be attributed to the ability of enzymes that catalyze the synthesis of A21978C which had been enhanced when laser beam imposed on cells, leading to the increase of the A21978C production of S. roseosporus.
Comparison of cell growth, A21978C production, and substrate consumption between LC-54 and wild strain. The production of antibiotics started at 20 h, where the cells entered the midexponential phase and reached the max production at 136 h. The mutant strain consumed substrate efficiently, grew faster, and produced more antibiotic compared to the wild strain
Kinetic Model
The model employs rate equations for biomass (x), antibiotic (P), and glucose (S) to describe the fermentation process.
Microbial Growth
From the examination of the large number of experimental data, it has been found that the Monod kinetic model was not applicable to this particular fermentation system. Therefore, the logistic equation, which is a reasonable method, was used as an alternative empirical function. In many fermentation systems such as antibiotic fermentations by many types of microorganisms, cell growth has been characterized by the logistic equation [16–18]. The logistic equation can be described as :
where u m is the maximum specific growth rate (h−1) and x m is the maximum attainable biomass concentration (g dry weight l−1).The integrated form of Eq. 2 using x = x 0 (t = 0) gives a sigmoidal variation of x as a function of t which may represent both an exponential and a stationary phase (Eq. 3).
Rearrangement of Eq. 2 yields Eq. 4:
Product Formation
The kinetics of product formation were based on the Luedeking–Piret equations. This model was originally developed for the formation of lactic acid by Lactobacillus delbrueckii [18]. According to this model, the product formation rate depends upon both the instantaneous biomass concentration, x, and growth rate, dx/dt, in a linear manner.
where α and β are the product formation constants which may vary with fermentation conditions. The benefit of this model is that b can be found from stationary-phase data. At stationary phase (dx/dt = 0 and x = x m), β can be obtained using the following equation:
Carbon Substrate Uptake
A carbon substrate such as dextrin is used to form cell material and metabolic products as well as the maintenance of cells. The glucose consumption equation given below is a Luedeking–Piret-like equation in which the amount of total carbohydrate used for product formation is assumed to be negligible
Where γ = 1/Y x/s and δ = m s . These two parameters can be evaluated by the same method as stated in the product formation kinetics. At stationary phase, dx/dt = 0 and x = x m. Therefore, δd can be obtained using the following equation:
The kinetic models (Eqs. 3 to 8) were fitted to the experimental data by nonlinear regression using MATLAB software. The model parameter values were first evaluated by solving Eqs. 3 to 8 and then the computer program was used as a search method to minimize the sum of squares of the differences between the predicted and measured values. The predicted values were then used to simulate the profiles of cell, substrate, and product concentrations during the fermentation. In order to determine the significance of the deviations between the experimental and calculated data, statistical analysis (t test) was also carried out by using SAS program.
As shown in Fig. 4, the simulation curves calculated according to the proposed kinetic models (Eqs. 3 to 8) fitted well to the experimental data of both the wild strain and the mutant strain in batch fermentation. Only a slight deviation of the calculated data from the experimental data of A21978C production was observed during the initial stages of the fermentation. t test results indicated that the deviations between the calculated and experimental data are not significant at a significance probability of 5%. This result suggests that the proposed models based on logistic and Luedeking–Piret equations are sufficient to describe growth of S. roseosporus, carbohydrate consumption, and A21978C production.
The kinetic parameters of the mutant strain and the wild strain were given in Table 1, which can be used to verify the experimental data of A21978C production by S. roseosporus. Although the maximum cell concentration and the maximum specific growth rate (u max) of the mutant strain and the wild strain were similar, a growth-associated rate constant and a non-growth-associated rate constant for A21978C formation (α and β) of the mutant strain are about two times and four times higher than that of the wild strain, respectively. The values of α are not zero for both strains, suggesting that the production of A21978C is half-growth-associated. The value of the no-growth-associated rate constant for total carbohydrate consumption (δ) of the mutant was higher than that of the wild strain. The amount of total carbohydrate consumed during the stationary phase of the wild strain and the mutant was 9 and 15.8 g/l, respectively, which could be calculated from the values of δ and X max. The values indicated that LC-54 consumed more total carbohydrate for metabolite and A21978C production than the wild strain [19]. It should be also noted that the low cell activity remaining in the end of culture is another possible cause for low A21978C production of the wild strain.
The cultivation kinetic parameters of batch cultivations indicated clearly that A21978C was mainly produced in the stationary phase. During active growth, most of the carbon sources and nitrogen were utilized for cell growth and for enhancement of the synthesis of enzymes involved in A21978C biosynthesis [20]. During the stationary phase, the mutant strain had a higher γ and less total carbohydrate concentration, it meant that the cells had a higher activity in producing A21978C. According to this, if the cell activity can be stabilized, the amount of A21978C production will increase proportionally with the time of fermentation. High production of secondary metabolites in the mutant strain was considered due to overexpression of the differentiation phase proteins, which in turn can be determined by an increase in either transcription or translation [20].
Conclusions
In this study, a S. roseosporus strain with high A21978C production was obtained by mutagenesis with He–Ne laser irradiation, and the batch fermentation process of both the mutant and the wild strain were analyzed and modeled. The model provided a good description of cell growth, antibiotic production, and substrate consumption versus fermentation time. The predicted A21978C concentration showed slight deviation from experiment result, which might be due to the fact that the apparently complex antibiotic biosynthesis was described by simple Luedeking–Piret equations. However, it throws light on the microbial growth and uptake in bath culture and should therefore contribute to development of a precise description of antibiotic fermentation.
References
Fung, H. B., Chang, J. Y., & Kuczynski, S. (2003). Drugs, 63, 1459–1480.
Nichols, R. L. (1999). The Journal of Antimicrobial Chemotherapy, 44, 19–23.
Tally, F. P., & DeBruin, M. F. (2000). The Journal of Antimicrobial Chemotherapy, 46, 523–526.
Silverman, J. A. (2001). Antimicrobial Agents and Chemotherapy, 45, 1799–1802.
Sader, H. S., & Streit, J. M. (2006). Clinical Microbiology and Infection, 9, 844–852.
Straus, S. K., & Hancock, R. E. W. (2006). Biochimica et Biophysica Acta—Biomembranes, 9, 1215–1223.
Debono, M., Barnhart, M., Carrell, C. B., Hoffman, J. A., Occolowitz, J. L., Abbott, B. J., et al. (1987). The Journal of Antibiotics, 40, 761–777.
Debono, M., Abbott, B. J., & Molloy, R. M. (1988). The Journal of Antibiotics, 41, 1093–1105.
Karu, T., Tiphlova, O., Esenaliev, R., & Letokhov, V. (1994). Journal of Photochemistry and Photobiology. B: Biology, 24, 155–161.
Sorin, A., Constantin, S., Dana, G., Ionica, N., Geta, S., & Ion, I. S. (2000) In: Proceedings of SPIE-The International Society for Optical Engineering, pp 758–763.
Kohli, R., & Gupta, P. K. (2003). Journal of Photochemistry and Photobiology. B: Biology, 69, 161–167.
Trilli, A. (1990). Microbial growth dynamics. Oxford: IRL.
Trilli, A., Crossly, M. V., & Kontakou, M. (1987). Biotechnological Letters, 9, 765–770.
Van Handel, E. (1985). Journal of the American Mosquito Control Association, 1, 299–300.
Bu’Lock, J. D. (1961). Advances in Applied Microbiology, 3, 293–342.
Heijinen, J. J., Roels, J. A., & Stouthamer, A. H. (1979). Biotechnology and Bioengineering, 21, 2175–2201.
Goudar, C. T., & Joeris, K. (2005). Biotechnology Progress, 4, 1109–1118.
Martin, J. F., & McDaniel, L. E. (1975). Biotechnology and Bioengineering, 17, 925–938.
Huber, F. M., & Piper, R. L. (1988). Journal of Biotechnology, 7, 283–292.
McGowan, K. M., Long, S. D., & Pekala, P. H. (1995). Pharmacology and Therapeutics, 3, 465–505.
Acknowledgments
The authors wish to acknowledge the financial support provided by the National Natural Science Foundation of China (no. 20706042), National 973 Project of China (2007CB714302), National 863 Project of China (2007AA02Z200), and Program of Introducing Talents of Discipline to Universities (no. B06006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, W., Fan, J., Wen, J. et al. Kinetic Analysis and Modeling of Daptomycin Batch Fermentation by Streptomyces roseosporus . Appl Biochem Biotechnol 163, 453–462 (2011). https://doi.org/10.1007/s12010-010-9053-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-010-9053-6