Abstract
This study aimed to evaluate the influence of the culture medium supplementation with mineral ions, focusing on the growth of Streptococcus zooepidemicus as well as on the production and average molecular weight (MW) of hyaluronic acid (HA). The ions were investigated in terms of individual absence from the totally supplemented medium (C+) or individual presence in the non-supplemented medium (C−), where C+ and C− were used as controls. Differences between the effects were analyzed using the Tukey's test at p < 0.05. The adopted criteria considered required the ions, whose individual absence attained at 80% or less of the C+ and their individual presence was 20% or more than the C−. The supplementation was either inhibitory or acted in synergy with other ions, when the individual absence or presence was 20% higher than C+ or 20% lower than C−, respectively. Results showed that the effects of C+ or C− were equal for both the production of HA and its yield from glucose. However, C+ showed to be beneficial to cell growth while the individual absence of Na+ was beneficial to the production of HA. The highest MW of HA (7.4 × 107 Da) was observed in the individual presence of Na+ in spite of the lowest HA concentration (0.65 g.L−1). These results suggest that the quality of HA can be modulated through the mineral ion supplementation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hyaluronic acid (HA) is a linear polysaccharide with high molecular weight, composed of disaccharide units of d-glucuronic acid and N-acetylglucosamine linked alternately by β-1-3 and β-1-4 glycoside bonds [1]. Due to its unique physicochemical, rheological and biological properties, HA is a biopolymer with high aggregated value, which has been used in products of great importance for the medical, pharmaceutical and cosmetic industries [2].
Recently, the microbial production of HA has proved to be highly attractive since it does not offer any risks of viral contamination neither does it demand large sums of money for the purification of HA, which are characteristics of the HA produced from animal sources [3]. Moreover, microbial production provides opportunities for the optimization and quality control of the HA produced.
It is widely recognized that environmental conditions such as oxygen, pH, temperature, and medium supplementation with nutrients, vitamins and ions play an important role on the generation of products by fermentation [4]. Minerals such as magnesium (Mg++), potassium (K+), sodium (Na+), iron (Fe++ e Fe+++), zinc (Zn++), manganese (Mn++), molibdenium (Mo++), cobalt (Co++), copper (Cu++), and calcium (Ca++), which take part in various intracellular reactions and biological functions, supply essential elements for the cell growth [5].
Although the supplementation with ions of the various culture media reported in the literature for the production of HA using different Streptococci, there are no systematic studies focusing on the influence of ions on cell growth and production of HA.
As described in general information from the literature, Mg++ and Mn++ were identified as cofactors for glycosyltransferases involved in the synthesis of the disaccharides in polymer chains [6]. According to Petrová et al. [7], the activity of most glycosyltransferases depends on a divalent cation which contributes for the binding of sugar-nucleotides, through the stabilization of the conformation from the pyrophosphate fraction.
In the specific case of HA, divalent cations such as Ca++ play an important role on its helical conformation and biological function [8]. Stern [9] considered the concentration of Ca++ a determinant factor for the synthesis of HA in cultures of human cells. Similarly, the availability of Zn++ strongly contributes to the metabolic synthesis of HA in animals [10]. Monovalent cations (K+ and Na+) inhibited the hyaluronan synthase, which is the enzyme responsible for the binding of sugar nucleotides precursors of the HA synthesis [11]. Moreover, Na+ can contribute to the microbial production of HA, due to its role on the lactate excretion by the Streptococcus genus bacteria [12].
Recognizing the importance of ions in the metabolic processes and considering the lack of information in the literature focusing upon the real necessity for the ions which are used in the culture media for the microbial production of HA, a systematic study was conducted aiming to evaluate the influence of the mineral ions potassium, magnesium, manganese, iron, calcium, sodium zinc, and copper on the growth of Streptococcus zooepidemicus, yield coefficient of HA from glucose, as well as the production and molecular weight of HA.
Materials and Methods
Microorganism
Streptococcus equi subsp. zooepidemicus ATCC 39920 was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) as a lyophilized culture kept in ampoules.
Control Media
Two control media containing glucose and yield extract were used in the assays: one of them was supplemented with salts (Table 1), based on the composition reported by Swann et al. [13], which corresponds to the supplemented C+ control. The other medium, C− control, was composed of glucose and yeast extract only at the same concentration. Table 2 shows the content of ions in the yeast extract used in both media. Thus, C+ and C− control media were not positive and negative controls, respectively, though they represent the total supplemented (C+) and non-supplemented (C−) media. Stock solutions of glucose and salts were autoclaved separately and the FeSO4 solution was sterilized by filtration in 0.22 µm membranes (Millipore, Bedford, MA, USA). The pH was adjusted to 7.5 before sterilization.
Culture Maintenance and Pre-inoculum Preparation
The stock culture was maintained frozen in brain heart infusion (BHI) broth containing 10% glycerol and glass beads at −20 °C. The pre-inoculum was prepared by streaking the glass beads onto BHI agar plates supplemented with 5% sheep blood (Biotério Boa Vista, São Paulo, Brazil) and incubating at 37 °C during 24 h.
Inoculum Propagation
Aiming to exhaust the mineral ions accumulated inside the cell, the inoculum propagation was performed in four steps. In all the steps, the culture medium was the C− control. In the first step, a loop of slant from the reactivated colonies (pre-inoculum) was transferred to 5 mL of the culture medium and incubated at 37 °C along 12 h. The subsequent steps involved in the transfer of crescent inoculum volumes (1, 5, and 10 mL) propagated in the previous steps in 9, 45, and 90 mL of sterile medium, respectively, at the same temperature and time. The volumes from the third and fourth steps were stirred at 150 rpm.
Cultivations
The cultivations were carried out in 125 mL Erlenmeyer flasks with a working volume of 50 mL medium, inoculated with 10% v/v of the culture from the fourth step of propagation, stirred at 150 rpm and incubated at 37 °C during 24 h. Cultivations were conducted in duplicate and pH, cell mass, glucose, and HA concentrations, were determined for each flask at the initial and final times. The average molecular weight of HA was also determined. Analyses were performed in triplicate. The influence of the mineral ions was evaluated in terms of the individual absence or presence in the culture medium of the selected ion. The individual absence of the ions was studied in the totally supplemented culture media (C+), as described in Table 1, except for the salt containing the ion being evaluated. Effects of the ions individual presence were studied by adding the salt, which contained the ion under evaluation, to the medium containing glucose and yeast extract only (C−). Cultivations were carried out in sets of ten flasks containing the appropriate media for the ions evaluation and the control media, supplemented (C+) and non-supplemented (C−).
Analytical Methods
Cell Growth
Cell growth in the cultivations was determined by the cell dry weight according to gravimetric method and in the inoculum propagation steps by viable cells counting using a microdrop plating technique [14].
Glucose Concentration
Glucose concentration was determined through a glucose oxidase commercial kit (LABORLAB, São Paulo, Brazil).
HA Concentration
The culture broth was centrifuged at 3,200 rpm during 20 min. The cell-free broth was treated with ethanol in a proportion of 1.5:1 v/v ethanol/supernatant. The solution was cooled down, remaining at 4 °C during 1 h for the precipitation of HA. The HA precipitated was redissolved in a 0.15 mol.L−1 NaCl solution. Three precipitation and redissolution steps were performed to increase the yield of HA precipitated and its concentration was measured by the carbazole method [15].
HA Average Molecular Weight
The average molecular weight of HA was determined by size exclusion chromatography, using a Shimadzu chromatography system (Shimadzu Corporation, Kyoto, Japan), containing a 7.8 × 35 mm Polysep-GFC-P column guard (Phenomenex, Torrance, CA, USA) mounted in series with a 7.8 × 300 mm Polysep-GFC-P6000 column of the gel filtration (Phenomenex, Torrance, CA, USA) and a refraction index detector (Shimadzu RID-6A). The analysis conditions were: injected sample of 20 μL, 0.1 mol.L−1 sodium nitrate as the mobile phase, flow rate of 1.0 mL.min−1 and 25 °C temperature, as suggested by the column manufacturer. Dextran (American Polymer Standards, Mentor, OH, USA) with molecular weight ranging from 103 to 106 Da was used as a standard for the calibration curve as described by Balke et al. [16].
Ions Concentration
The concentration of the ions was determined in the medium containing glucose and yeast extract only (C− control). The ions Ca++, Cu++, Fe++, Mg++, Mn++, K+, and Zn++ were analyzed by optical emission spectrometry with plasma coupled individually (ICP-OES; Perkin Elmer-3000 DV, Norwalk, Connecticut, USA), while Na+ was quantified by atomic absorption spectrometry (AAS; NovAA 300-Analytik Jena AG, Jena, Turingia, Germany).
Statistical Analysis
The variance was analyzed by ANOVA method and the media compared by the Tukey's test at 5% probability level (p < 0.05).
Analysis Criteria
The following analysis criteria were adopted, aiming at a better evaluation of the results. The ions whose individual absence attained at 80% or less of C+ and their individual presence was 20% or more than C−, were considered required ions. The supplementation was considered either inhibitory or acted in synergy with other ions, when the individual absence or presence was 20% higher than C+ or 20% lower than C−, respectively.
Results and Discussion
Inoculum Propagation
Inoculum consisted of a previous starvation of the ions from the cells. Growth and viability of microorganisms in the incubation steps in the non-supplemented medium (C− control) are shown in Table 3. Results demonstrate the inoculum preparation's reproducibility for both treatment types in order to evaluate the influence of the ions on the cultivations. The same magnitude order of cell counting, along the four steps of incubation, assures similar starting conditions in the cultivations. The observed cell mass decline, following the incubations, demonstrates the starvation of the ions inside the cells.
The Influence of Ions on the Cultivations
The results below show the influence of the mineral ions K+, Mg++, Mn++, Fe++, Ca++, Na+, Zn++, and Cu++ on the growth of S. zooepidemicus, yield coefficient of HA from glucose and also the production and average molecular weight of HA, considering the criteria described in item 2.8.
Cell Growth
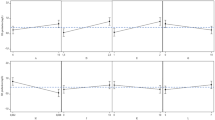
The influence of the supplementation with ions on the cell growth of S. zooepidemicus is presented in Fig. 1. According to the adopted criteria, the individual absence or presence of ions was classified as non-required. However, the total presence of ions (C+) was considered required for a better cell growth which evidences the ions synergic behavior. Surprisingly, the C− control medium was not strongly deleterious to cells since the cell growth attained at 69% in the C+ control.
Effects of the individual a absence or b presence of the mineral ions on the growth of Streptococcus zooepidemicus. a The reference value of the average cell growth (0.91 g.L−1) in the control medium supplemented with all of the evaluated ions (C+ control). b The reference value of the average cell growth (0.62 g.L−1) in the non-supplemented control medium (C− control). The letters above the bars mean the statistical comparisons performed by the Tukey's test at 5% probability (p < 0.05). Bars with the same letters are not significantly different
Production of Hyaluronic Acid
Results in Fig. 2 show that the total supplementation of the culture medium with ions did not have any influence on the production of HA (C+ = C−). The individual absence or presence revealed inhibitory effects especially due to Na+ whose individual absence resulted in an increase of 22% on the production of HA and its individual presence in a reduction of 37% related to the controls. Effects of Na+ on the production of HA can be associated with the metabolic energy deviation to maintain the protons potential inside the cells [12]. Additionally, according to Tlapak-Simmons [11] monovalent cations such as K+ and Na+ inhibited the hyaluronan synthase. The ion Fe++ showed an inhibitory effect on the individual presence only, suggesting a synergic behavior with the other ions.
Effects of the individual a absence or b presence of the mineral ions on the production of HA. a The reference value of the HA production (1.03 g.L−1) in the control medium supplemented with all of the evaluated ions (C+ control). b The reference value of the HA production (1.03 g.L−1) in the non-supplemented control medium (C− control). The letters above the bars mean the statistical comparisons performed by the Tukey's test at 5% probability (p < 0.05). Bars with the same letters are not significantly different
Yield Coefficient of HA from Glucose
Figure 3 shows the effects of the ions on the yield coefficient of HA from glucose (Y HA/S). Similar to the production of HA, no difference was identified between the total supplementation and non-supplementation of the culture medium with ions (C+ = C−). This result agrees with the individual behavior, in which none of the ions could be classified as required for the Y HA/S, according to the adopted criteria. However, the analysis of individual absence or presence spectra points out the Na+ as the main inhibitor of the Y HA/S. Minor effects can be attributed to Mn++ Zn++, and Cu++, which were non-required in the individual absence or individual presence only. Considering the lower concentration of these ions in the culture medium (below 0.25 mg.L−1) compared with the other ions, these effects suggest a synergic action with other ions.
Effects of the individual a absence or b presence of the mineral ions on the global yield coefficient of HA from glucose. a The reference value of the yield (0.17 g.g−1) in the control medium supplemented with all of the evaluated ions (C+ control). b The reference yield value (0.16 g.g−1) in the non-supplemented control medium (C− control). The letters above the bars mean the statistical comparisons performed by the Tukey's test at 5% probability (p < 0.05). Bars with the same letters are not significantly different
The HA Molecular Weight
The total supplementation of the culture medium with ions was not favorable to the molecular weight (MW) of HA, as showed in Fig. 4 (C− > C+). The spectra show that the individual absence of the ions had similar influence to that of the C+ control, except for Mg++, whose individual absence or presence led the MW of HA to reach the same level of the C− control. Thus, the main inhibition observed in the C+ control can be related to Mg++.
Effects of the individual a absence or b presence of the mineral ions on the molecular weight (MW) of HA. a The reference value of the MW of HA (2.0 × 107 Da) in the control medium supplemented with all of the evaluated ions (C+ control). b The reference value of the MW (3.3 × 107 Da) in the non-supplemented control medium (C− control). The letters above the bars mean the statistical comparisons performed by the Tukey's test at 5% probability (p < 0.05). Bars with the same letters are not significantly different
According to the criteria, none of the individual ions could be classified as required based on the spectrum of the individual presence, except for Na+ which caused the MW of HA to increase in more than 20% of the C− control. Hence, the individual supplementation of the culture medium with Na+ represents the best condition for a higher MW of HA (7.4 × 107 Da) in the cultivation studied in this work, in spite of the lowest concentration of HA (Fig. 2).
Conclusions
The experimental results indicate that the non-supplemented culture medium used in this work (C− control), containing 25 g.L−1 glucose and 60 g.L−1 yeast extract, does not require ion supplementation in order to favor the production of HA. The total supplementation of the culture medium (C+ control) was the best situation for the cell growth only. However, the best condition for the production of HA as well as for the yield coefficient of HA from glucose was the individual absence of Na+. Opposite conditions benefit the production and MW of HA. The highest MW of the polymer was observed in the individual presence of Na+ despite the lowest concentration of HA. Therefore, medium supplementation with ions can lead to a higher production or MW of HA, depending on the quality of the product desired.
References
Chong, B. F., Blank, L. M., Mclaughlin, R., & Nielsen, L. K. (2005). Applied Microbiology and Biotechnology, 66, 341–351.
Zhang, F., Ding, X., Yang, L., & Kong, Z. (2006). Applied Microbiology and Biotechnology, 72, 168–172.
O’Regan, M., Martini, I., Crescenzi, F., De Luca, C., & Lansing, M. (1994). International Journal of Biological Macromolecules, 16, 283–286.
Shuler, M. L., & Kargi, F. (2002). Bioprocess engineering: basic concepts (2nd ed.). New Jersey, NJ: Prentice Hall.
Vogel, H. C., & Todaro, C. L. (1997). Fermentation and biochemical engineering handbook: principles, process design, and equipment (2nd ed.). New Jersey, NJ: Noyes Publications.
Deangelis, P. L. (2002). Glycobiology, 12, 9R–16R.
Petrová, P., Koca, J., & Imberty, A. (2001). European Journal of Biochemistry, 268, 5365–5374.
Winter, W. T., & Arnott, S. (1977). Journal of Molecular Biology, 117, 761–784.
Stern, R. (2003). Glycobiology, 13, 105R–115R.
Thompson, R. W., Gilbreath, R. L., & Bielk, F. (1975). Journal of Nutrition, 105, 154–160.
Tlapak-Simmons, V. L., Baron, C. A., & Weigel, P. H. (2004). Biochemistry, 43, 9234–9242.
White, D. (2000). The physiology and biochemistry of prokaryotes (2nd ed.). New York, NY: Oxford University Press.
Swann, D. A., Sullivan, B. P., Jamieson, G., Richardson, K. R., & Singh, T. (1990) Biosynthesis of hyaluronic acid. US Patent 4,897,349.
Cliquet, S., & Jackson, M. A. (1997). World Journal of Microbiology and Biotechnology, 13, 299–303.
Dische, Z. (1946). Journal of Biological Chemistry, 167, 189–198.
Balke, S., Hamielec, A., Leclkair, B., & Pearce, S. (1969). Industrial and Engineering Chemistry Product Reseasrch and Development, 8, 54–57.
Acknowledgements
The authors acknowledge CNPq and FAPESP for the financial support, Investiga Group (Campinas, São Paulo, Brazil) for the maintenance of the bacteria culture, Laboratory of Bioseparations for the availability of HPLC system, and Angela Klatil Ribeiro for the language review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pires, A.M.B., Eguchi, S.Y. & Santana, M.H.A. The Influence of Mineral Ions on the Microbial Production and Molecular Weight of Hyaluronic Acid. Appl Biochem Biotechnol 162, 2125–2135 (2010). https://doi.org/10.1007/s12010-010-8987-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-010-8987-z