Abstract
Objectives
Microbial production of biopolymers is typically associated with high viscosity and suitable mixing plays an important role in their production. Due to the nature of Streptococcus strains in high production of lactic acid and consequently high consumption of NaOH, which is associated with increased viscosity and reduced mixing caused by hyaluronic acid production, the injected NaOH accumulates and causes cells loss, and decreases in quantity and quality of the produced hyaluronic acid.
Results
In this study, the effect of increasing dilution of media culture of Streptococcus zooepidemicus fed-batch culture during pH control by NaOH on mixing time, volumetric oxygen transfer coefficient, and increasing hyaluronic acid production in a 2-L fermenter were studied. The results showed that significant increasing dilution causes reduction mixing time, remarkable improvement volumetric oxygen transfer coefficient, hyaluronic acid production enhancement from 6.6 to 8.4 g/L, and diminution the consumption of NaOH.
Conclusion
Dilution of media culture of S. zooepidemicus fed-batch culture by the pH controlling agent achieved one of the highest amounts of hyaluronic acid that was reported recently. This method does not require any automatic control and can be used at a low cost to produce other soluble extracellular biopolymers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyaluronic acid as a high value-added bio-product is one of the most widely used biopolymers in industry and medicine. Hyaluronic acid also referred to in some texts as hyal, is a non-branched (linear) polysaccharide that is a repeating compound of the glucuronic acid (GlcUA) and N-acetyl glucosamine subunits that are interconnected with 1, 3 beta and 1, 4 beta glycosidic bonds and their molecular weight varies from 104 to 107 Da. It has remarkable viscoelastic properties influenced by its polymeric and polyelectrolyte properties and can absorb water up to 1000 times its volume. In the human body, hyaluronic acid is found in hyaluronate salt and at high concentrations in the skin, cord and vitreous fluid are founded. HA has unique physical and chemical properties due to its special structure, and its viscoelastic and rheological properties are its distinctive features. Although the network structure formed by the polymer HA solution does not have covalent cross-linking bonds between molecules, it still has elastic and viscous characteristics, that is, viscoelasticity. Under the action of shearing force, the viscosity of the solution decreases significantly with increasing shear rate and is characterized by non-Newtonian fluid (Huang and Chen 2019). Generally, there are two methods of extraction from animal origin and bacterial fermentation for hyaluronic acid production. Hyaluronic acid is successfully produced on an industrial scale by Streptococcus zooepidemicus.
The high viscosity resulting from the production of HA in the culture medium limits access to higher concentrations and molecular weights. Because of its high viscosity, the uniform mixing and oxygenation of the medium poses a serious challenge. So appropriate mixing and oxygenation play an important role in high production of HA by creating a homogenous environment and uniform oxygenation. Investigation of the effects of mixing rate, aeration rate, and dissolved oxygen have shown that in aerobic medium compare to anaerobic production, molecular weight and production were higher (Chong and Nielsen 2003; Duan et al. 2008). Under aerobic conditions, bacterial metabolism changes from lactate production to acetate, formate, and ethanol, resulting in more ATP being produced. The expression of hyaluronan synthase in S. zooepidemicus under oxygen conditions is ninefold higher than in anaerobic conditions because oxygen induces expression of HasD gene and its enzymes secretion and consequently increases acetone cycle activation (Chong and Nielsen 2003).
In a study, the amount of dissolved oxygen was kept constant at different levels, and it was found that by increasing the oxygen content from 0 to 50%, the molecular weight of the product could increase by up to 90% and a further increase in oxygen resulted in a decrease in molecular weight (Duan et al. 2008). The reduction in mass transfer and mixing in high viscosity processes are due to these reasons (Schugerl 1981): reduction of the mass transfer due to turbulence reduction, the formation of larger bubbles and reduction of special contact area, the more speed of the bubble coalescence and faster reaching to their final volume, forced reduction of mixer speed due to high generated heat from high mixer speed at high viscosity, presence small bubbles with low oxygen content and high residence time, decrease molecular diffusion and increase the thickness of the boundary layer between the gas and the liquid. Other factors that can reduce the production of hyaluronic acid are the formation of layers of polysaccharides around the cell, which reduce the penetration of nutrients into the cell, and the high accumulation of lactic acid around the cell also reduces the quantity and quality of the product.
The high viscosity of the medium limits oxygen transfer and reduces HA productivity. One way to overcome this limitation is to reduce viscosity by chemical and physical methods. In a study, the effects of hydrogen peroxide and ascorbate were investigated as factors that reduce the molecular weight of HA and consequently reduce the viscosity. After the addition of these substances at the cellular growth stage, the molecular weight decreased from 1300 to 80 kDa and the product production increased from 5 to 6.5 g. Soluble oxygen also increased from 1% saturated air to 10%, indicating the effect of reduced viscosity on dissolved oxygen content (Long et al. 2009). However molecular weight loss diminishes product value, which is one of the major disadvantages of using such methods.
Increased homogeneity of media culture and oxygen solubility have led to increased production of some extracellular polysaccharides such as HA and xanthan. By too much increasing the stirrer speed, in addition to rising power consumption, due to the stress applied to the cell and the HA polymer decrease the molecular weight and concentration of HA. On the other hand, by decreasing the mixer speed due to lack of oxygen and non-uniformity of the medium the similar results with the excessive increase stirring speed are obtained.
Another way to reduce the viscosity is to increase dilution by chemostat cultivation, which is not easily achieved due to the instability of the HA-producing phenotype at high dilution rates. The increasing dilution can be achieved by continuous cultivation while in this system the goal is to achieve higher productivity, there is an increased risk of contamination and low productivity (Seviour et al. 2011).
One of the other ways to increase production is to increase dissolved oxygen by using oxygen vectors. Oxygen vectors are hydrocarbon compounds in which the solubility of oxygen is 10–20 times that of water. These compounds should not have any negative interaction with the cell and should not be problematic during the purification process and can in some cases be used as a cell energy source. Among these compounds can be called n-dodecane and perfluorodecalcine (PFC), which has been investigated the effect of the latter material on increasing hyaluronic acid production and has led to increased efficiency (Liu et al. 2009a, b). In another study, an increase of 5% of n-dodecane in the medium in the growth phase, resulted in a 3.6-fold increase in kla and 30% yield (Lai et al. 2012). Complicating the purification process is the disadvantages of using such methods.
Most studies have used 5 M NaOH to control pH (Liu et al. 2009a, b; Zakeri and Rasaee 2016; Amado et al. 2017; Vazquez et al. 2015; Izawa et al. 2010). Due to the nature of Streptococcus strains in high production of lactic acid and consequently high consumption of NaOH, especially at the end of the production stage, which is also accompanied by increased viscosity and reduced mixing effect, it causes accumulation sodium and cell viability reduction and ultimately reduce the quantity and quality of the product.
Therefore, in order to prevent this problem and reduce mixing, lower NaOH concentrations were used to increase the dilution of the culture medium, its effect on reducing mixing time and increase the volumetric oxygen transfer coefficient and HA production was investigated.
Materials and methods
The S. zooepidemicus strain mutated at this center was used as the HA producing strain. The mutation was done by a serial selection of S. zooepidemicus which was exposed to ultraviolet (UV) light and N-methyl-N′-nitro-N-nitroguanidine. Cultivation was performed in a 2-L B.Braun Biostat B fermenter with two Rushton 6 blades and the ratio of the blade diameter to the diameter of the tank is 0.5. The composition of the culture medium was hydrolyzed casein 20, yeast extract 20, primary glucose 30, sodium chloride 1.5 and magnesium sulfate 0.6 g/L in 900 mL deionized water. The inoculum was prepared in a 200 mL Erlenmeyer flask containing 50 mL from the culture medium and incubated at 37 °C and 150 rpm and was added at 5% with OD600 = 0.5. 100 mL of 30% glucose was gradually fed into the medium from the middle of the growth phase (time 8 h). The pH of the medium was fixed at 7 and the temperature was 37 °C during culture. Minimum oxygen content was set at 5% with different aeration values during cultivation. NaOH at concentrations of 0.5, 2 and 5 M was used to adjust the pH and each experiment was repeated twice with each concentration.
Measurement methods
Carbazole method was used to evaluate the amount of HA produced. The principle of this method is to measure the amount of glucuronic acid present in the HA structure. In this method, the HA solution is first boiled in concentrated sulfuric acid. This results in the breakdown of HA into smaller components and then the carbazole reagent is added to the medium. This reagent binds to glucuronic acid units and produces color by heat and the intensity of this dye is read by means of a spectrophotometer and the concentration of HA is obtained by comparison with standard glucuronic acid. The detailed method of measurement is described in the article (Zakeri et al. 2017). The viscosity was measured with the RVDV-II + Pro Brookfield Viscometer. The time to reach 95% complete mixing from the start of the injection was also considered as the mixing time (the time the pH sensor response was fixed).
The volumetric oxygen mass transfer coefficient was determined using the dynamic method. It consists of aeration until the dissolved oxygen concentration is stabilized; then suspending the oxygen supply generating a decrease in concentration due to the respiration rate of the microorganisms present in the reactor.
The next step, is evaluate the increase of dissolved oxygen concentration in the medium with respect to the time, represented by Eq. (1).
where C is the dissolved oxygen concentration, x ∗ O2 is the respiration rate, KLa is the oxygen transfer coefficient, C dt shows the concentrations changes versus time. Determine the constant KLa for the reactor and proposed conditions, is possible plotting C vs {(\(\frac{\mathrm{dCL}}{\mathrm{dt}})+\) x*O2}, where the negative inverse of the slope is the volumetric oxygen transfer coefficient (Viloria et al. 2017).
Results
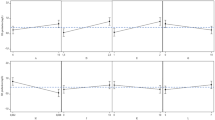
Figure 1 shows the time variations of mixing time at different concentrations of NaOH. Due to better mixing and mass transfer at higher NaOH dilutions, the mixing time is also reduced. Approaching the end of the production phase, we see a gradual decrease in mixing time due to heterogeneous environmental conditions in terms of mass transfer of available nutrients and oxygen to the cell and a decrease in the molecular weight of hyaluronic acid produced by environmental stresses such as stirring, oxygen radicals and hyaluronidase production.
Figure 2 shows the time variations of the volumetric oxygen transfer coefficient at different NaOH concentrations. According to the viscosity results, it was predicted that by increasing the dilution of NaOH, the amount of mixing and the volumetric oxygen transfer coefficient would increase, which confirms the obtained results. The results obtained in this study are in good agreement with the relationship obtained by long et al. in the production of hyaluronic acid.
N is the agitation speed (/s), VS is the superficial air velocity (m/s), and η is the broth viscosity (mPa·s).
The correlation model is in good agreement with the experimental data and have an average deviation of ± 12% (Liu et al. 2009a, b).
Figure 3 shows the changes in the viscosity of media culture at different concentrations of the NaOH. As predicted, by decreasing the dilution of NaOH, the viscosity of the medium increases, resulting in reduced mixing and mass transfer, resulting in lower final product yields and, due to anaerobic conditions, more cell energy spent on lactic acid production. The decrease in the amount of caustic soda consumed in Fig. 4 also illustrates this. The reduction in the amount of NaOH consumed in more dilutions indicates that cell metabolism is more spent producing hyaluronic acid than lactic acid. This increase is due to better mixing of the fluid and better mass transfer of nutrients and oxygen.
The effect of increasing dilution on the amount of yield and the end product is shown in Fig. 5. The results indicate that with the decrease in NaOH concentration, the yield of hyaluronic acid decreased, but as the final volume increased with increasing NaOH dilution, the final product increased. The final product content at 0.5 M NaOH concentration is 8.4 g, which is increased by 6.6 g compared to usual conditions (using 5 M NaOH).
Discussion
The use of NaOH with more dilution is a simple, inexpensive, and practical method that can decrease the mixing time and increase the oxygen and nutrient mass transfer and thus increase production in microbial production of extracellular polysaccharides. In the competition for carbon to convert to lactic acid or hyaluronic acid, the increased mixing and oxygen available to the cell, produce more energy and consequently more product. Other methods of production enhancement require the re-designing of the fermenter and mixer or the use of materials that are costly and difficult to purification. The highest number reported in the production of hyaluronic acid was 6–7 g/L and applying this method can be achieved by higher than 8 g/L of the final product. The benefits of this method include less reduction of product and cell degradation due to NaOH point accumulation during injection and no need for automatic control of dilution during fermentation by other methods. Another point to note is that, unlike most bio-products such as proteins where the higher concentration of the product is desirable in the purification process, due to the high viscosity of the HA, it is necessary to dilute it in the purification process, which is eliminated by this method.
References
Amado IR, Vázquez JA, Pastrana L, Teixeira JA (2017) Microbial production of hyaluronic acid from agro-industrial by-products: molasses and corn steep liquor. Biochem Eng J 117:181–187
Chong BF, Nielsen LK (2003) Aerobic cultivation of Streptococcus zooepidemicus and the role of NADH oxidase. Biochem Eng J 16:153–162
Duan XJ, Yang L, Zhang X, Tan WS (2008) Effect of oxygen and shear stress on molecular weight of hyaluronic acid produced by Streptococcus zooepidemicus. J Microbiol Biotechnol 18(4):718–724
Huang G, Chen J (2019) Preparation and applications of hyaluronic acid and its derivatives. Int J Biol Macromol 125:478–484
Izawa N, Hanamizu T, Sone T, Chiba K (2010) Effects of fermentation conditions and soybean peptide supplementation on hyaluronic acid production by Streptococcus thermophilus strain YIT 2084 in milk. J Biosci Bioeng 109(4):356–360
Lai Z, Abdul Rahim R, Arbakariya B, Rosfarizan M (2012) Biosynthesis of high molecular weight hyaluronic acid by Streptococcus zooepidemicus using oxygen vector and optimum impeller tip speed. J Biosci Bioeng 114(3):286–291
Liu L, Du G, Chen J, Wang M, Sun J (2009) Comparative study on the influence of dissolved oxygen control approaches on the microbial hyaluronic acid production of Streptococcus zooepidemicus. Bioprocess Biosyst Eng 32(6):755–763
Liu L, Yang HQ, Zhang DX, Du GC, Chen J, Wang M, Sun J (2009) Enhancement of hyaluronic acid production by batch culture of Streptococcus zooepidemicus via the addition of n-dodecane as an oxygen vector. J Microbiol Biotechnol 19(6):596–603
Long L, Guocheng D, Jian C, Yang Z, Miao W, Jun S (2009) Microbial production of low molecular weight hyaluronic acid by adding hydrogen peroxide and ascorbate in batch culture of Streptococcus zooepidemicus. Biores Technol 100:362–367
Schügerl K (1981) Oxygen transfer into highly viscous media in reactors and reactions. Springer, Berlin, Heidelberg, pp 71–174
Seviour RJ, McNeil B, Fazenda ML, Harvey LM (2011) Operating bioreactors for microbial exopolysaccharide production. Crit Rev Biotechnol 31:170–185
Vázquez J, Pastrana L, Piñeiro C, Teixeira J, Pérez-Martín R, Amado I (2015) Production of hyaluronic acid by Streptococcus zooepidemicus on protein substrates obtained from Scyliorhinus canicula discards. Mar Drugs 13(10):6537–6549
Viloria AC, Ardila KS, Mendez DA, Rojas IC, Moreno NC (2017) Volumetric oxygen mass transfer coefficient determination and hydrodynamic optimization of polyhydroxyalkanoate production with vegetal oil as carbon source. Chem Eng Trans 57:1303–1308
Zakeri A, Rasaee MJ (2016) Identification of wild type Streptococcus zooepidemicus and optimization of culture medium and fermentation conditions for production of hyaluronic acid. Biosci Biotechnol Res Asia 13(1):189–198
Zakeri A, Rasaee MJ, Pourzardosht N (2017) Enhanced hyaluronic acid production in Streptococcus zooepidemicus by over expressing HasA and molecular weight control with niscin and glucose. Biotechnol Rep 16:65–70
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saharkhiz, S., Babaeipour, V. The dilution effect of media culture on mixing time, Kla O2, and hyaluronic acid production in S. zooepidemicus fed-batch culture. Biotechnol Lett 43, 2217–2222 (2021). https://doi.org/10.1007/s10529-021-03192-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-021-03192-0