Abstract
The chit1 gene from the entomopathogenic fungus Metarhizium anisopliae, encoding the endochitinase CHIT42, was placed under the control of the CaMV 35S promoter, and the resulting construct was transferred to tobacco. Seventeen kanamycin-resistant transgenic lines were recovered, and the presence of the transgene was confirmed by polymerase chain reactions and Southern blot hybridization. The number of chit1 copies was determined to be varying from one to four. Copy number had observable effects neither on plant growth nor development. Substantial heterogeneity concerning production of the recombinant chitinase, and both general and specific chitinolytic activities were detected in leaf extracts from primary transformants. The highest chitinase activities were found in plants harboring two copies of chit1 inserts at different loci. Progeny derived from self-pollination of the primary transgenics revealed a stable inheritance pattern, with transgene segregation following a mendelian dihybrid ratio. Two selected plants expressing high levels of CHIT42 were consistently resistant to the soilborne pathogen Rhizoctonia solani, suggesting a direct relationship between enzyme activity and reduction of foliar area affected by fungal lesions. To date, this is the first report of resistance to fungal attack in plants mediated by a recombinant chitinase from an entomopathogenic and acaricide fungus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycoparasitic and entomopathogenic fungal species have been employed in the biological control of crop pests and diseases not only directly but also as a source of valuable genes to be introduced into plants [1, 2]. An important advantage of these biocontrol microorganisms is their specific mechanism to infect arthropods and phytopathogenic fungi, but not plants. An essential component of this system is the key step of penetration through host barriers in order to establish the infection process. In many cases, genes encoding a range of hydrolytic enzymes such as chitinases and β-glucanases that exert an active role in the early host–pathogen interaction have been studied aiming their antimicrobial activity [3–6]. The chitinase mechanism of action involves chitin degradation and cell wall disruption, leading to fungal cell lysis [7].
Most chitinase genes have been isolated from the mycoparasitic genus Trichoderma that produces endochitinases and exochitinases capable of generating high levels of nonspecific resistance to a broad spectrum of different pathogens [8, 9]. A single endochitinase gene from Trichoderma harzianum confers enhanced resistance to pathogens including the Deuteromycetes Alternaria alternata, Alternaria solani, Botrytis cinerea (leaf spot and fruit rot; [8]), the Basidiomycete Rhizoctonia solani (attacks the stem and root of tobacco and potato plants; [8]), and the Ascomycete Venturia inaequalis (apple scab disease; [10, 11]). The same gene was introduced into black spruce and hybrid poplar and conferred enhanced resistance to Melampsora medusae and Cylindrocladium floridanum, respectively [12]. Genetically transformed cotton with a similar endochitinase gene from Trichoderma virens showed increased resistance to infections by R. solani and A. alternata [13]. In addition to Trichoderma, a chitinase gene from another biocontrol fungus, Rhizopus oligosporus, has been introduced into tobacco plants conferring resistance to the grey mold pathogen B. cinerea [14]. More recently, heterologous expression in plants of a variety of chitinase genes from plants, fungi, bacteria, and virus were reported to enhance resistance to biotic stress and even to some abiotic stress [2, 7].
Even though Metarhizium anisopliae is one of the best studied entomopathogenic and acaricide fungus, with many chitinase genes and enzymes isolated and characterized [15–20], there are no reports available concerning their potential to confer plant resistance against arthropods or fungi. Besides being involved in the process of host penetration [21, 22], M. anisopliae chitinases are also thought to be implicated in fungal growth, which led us to test their capability to confer plant resistance against fungi. Here, we evaluated the ability of the full-length chit1 cDNA, which encodes the endochitinase CHIT42 (EC 3.2.1.14) from M. anisopliae to confer resistance against R. solani in transgenic tobacco plants. Production and activity of the recombinant enzyme are reported, examining the effects of the transgene on inheritance stability and plant vigor. In addition, selected transgenic plants were submitted to infection with the phytopathogen fungus, and disease symptoms were assessed under different conditions.
Materials and Methods
T-DNA Constructs and Plant Transformation
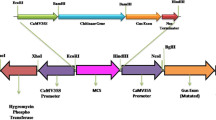
DNA manipulations were performed essentially as described in Sambrook and Russel [23]. The 1.7-kb DNA fragment containing the full-length cDNA chit1 from M. anisopliae was isolated from the plasmid pChit [17]. The insert, excised by SmaI/BamHI, was cloned into pMOG463 (Mogen, The Netherlands) cut with EcoRV and BamHI, positioning the chit1 gene between the Cauliflower Mosaic Virus (CaMV) 35S promoter and the nopaline synthase transcription terminator (nosT). The CaMV 35S-chit1-nosT cassette was excised by SacI and ligated into the binary vector pMOG402 (Mogen, The Netherlands). The recombinant binary plasmid was transferred to Agrobacterium tumefaciens LBA4404 by triparental matting [24]. Nicotiana tabacum cultivar SR1 “Little Havana” was transformed by the leaf disk method [25]. Independently produced transgenic plants harboring the CaMV 35S-chit1-nosT cassette were named chitplus. Control plants, named chitless, consisted of N. tabacum transformed with the unmodified T-DNA of pMOG402. Both T-DNA structures are shown in Fig. 1.
T-DNA constructs. A Chitplus construct containing the full-length chit1 cDNA from M. anisopliae. B Chitless construct, a control version T-DNA lacking the expression cassette CaMV 35S-chit1-nosT. LB T-DNA left border; RB T-DNA right border; nos P nos promoter; nos T nos terminator; nptII neomycin phosphotransferase II encoding gene (kanamycin selectable marker); CaMV 35S cauliflower mosaic virus leader sequence from 35S RNA. Primer positions of MachitFor and MachitRev and the probe (PEP) used for PCR and Southern blot analyses are shown by black-filled arrows and a fine broken line, respectively. Restriction endonuclease sites: SI SacI; EI EcoRI; PI PstI
Southern Blot Hybridization
Genomic DNA was prepared from leaf tissues by the CTAB method described by Doyle and Doyle [26] and quantified by DNAQuant Fluorometer Assay (GE Healthcare, USA) following manufacturer instructions. DNA (20 μg) samples were cleaved with EcoRI and electrophoretically separated on a 0.8% agarose gel. Probe labeling, hybridization, stringency washes, and detection were carried out according to Gene Images Random Prime Labeling and CPD Star Detection kit (GE Healthcare, USA). DNA blotting was probed with a 1,500-bp EcoRI-PstI DNA fragment containing the chit1 gene coding sequence [17] purified from agarose gels by GFX (GE Healthcare, USA).
PCR Analysis
Polymerase chain reactions (PCR) were performed using genomic DNA from leaves of 2-month-old primary putative transformants and 8-week-old progeny resulting from self-pollinated primary transgenics. DNA samples were prepared using Plant DNAzol Reagent (Invitrogen, USA), following the protocol supplied by the manufacturer and quantified as described for Southern blot. Reactions employed the specific primers to the chit1 gene coding region MachitFor (5′-GGAGGGTGGACGTGGTCAAC-3′) and MachitRev (5′-GCTGCCCCCAATCCCTTG-3′, Fig. 1). PCR components were as follows: 50 pmol of each primer, 2.5 U Taq DNA polimerase (CENBIOT Enzimas, Brazil), 10 mM Tris Cl pH 8.3, 5 mM KCl, 2 mM MgCl2, 200 μM dNTP, and 200 ng genomic DNA. A termocycler (PTC 100 MJ Research, USA) was programmed with an initial denaturation at 95°C for 5 min following 35 cycles at 94°C for 45 s, 47°C for 45 s, 72°C for 90 s, and a final extension step at 72°C for 10 min. Plants were evaluated for the presence of the 750 bp product by agarose gel electrophoresis. Results were submitted to statistic analysis using the double-tailed testing associated to binomial distribution as determined by Armitage and Berry [27].
Immunoblot Analysis
Leaf crude extracts were prepared and analyzed by SDS-PAGE as described in Memelink et al. [28]. Total protein was determined using the BCA Protein Assay Kit (Pierce, USA). After electrophoresis in a Mini-Protean II Cell (BioRad, USA), proteins were electroblotted onto nitrocelullose filters using the Mini-Transf-Blot Cell (BioRad, USA) apparatus. Immobilized proteins were probed with a polyclonal antibody specific to CHIT42 from M. anisopliae. The recombinant enzyme was visualized with the ECL Western Blot Detection and Analysis System (GE Healthcare, USA).
Chitinase Activity Assay
Total protein extracts from leaves were prepared according to Jefferson and Wilson [29] with the following modifications: protein samples were extracted with 0.1 M sodium phosphate buffer, pH 7.0, 10 mM EDTA, 0.1% (w/v) Sarcosyl, 0.1% (v/v) Triton X-100, and 10 mM β-mercaptoethanol. After centrifugation (10 min at 13,000 rpm), the supernatant was submitted to dialysis against 100 mM sodium phosphate buffer, pH 7.0, exceeding 100 times sample volume. Protein concentration was determined by the method of Bradford [30] using BSA as standard. Chitinolytic activity was measured using the synthetic substrates N,N′-diacetylchitobiose and N,N′,N″,N′″-tetracetylchitotetraose (Sigma, USA) using the procedures described in Reissig et al. [31]. One unit of chitinase activity was defined as the amount of enzyme that releases 1 μmol/min/mg N-acetylglucosamine (GlcNAc) of total soluble protein. For the substrate p-nitrophenyl-N-acetyl-β-d-glucosamine (Sigma, USA), chitinolytic activity was detected according to Yabuki et al. [32], and one unit of chitinase was defined as the amount of enzyme that releases 1 μmol/min/mg p-nitrophenol of total soluble protein.
Glycol-Chitin Zymograms
After the first dialysis step, leaf protein extracts were submitted to a second dialysis against 5 mM sodium phosphate buffer, pH 7.0, at an excess of 100 times the total sample volume. Chitinase activity gels were prepared as described by St. Leger et al. [33]. Chitinases from Streptomyces griseus (Sigma, USA) were used as molecular weight markers. Black lytic zones representing hydrolyzed chitin were examined under UV light.
Plant Resistance Assays Against R. solani
R. solani, a soilborne pathogen that causes damping off, seedling blight, and root rot, was isolated and kindly provided by Dr. A.T.S. Matsumura from the Department of Phytosanity, Agronomy Facult of UFRGS (Brazil). Primary transgenics and their progeny were tested using direct infection assays of leaves in agar dishes or fungus inoculation in soil, respectively. Infection experiments were conducted essentially as described by Lorito et al. [8], with the following modifications: tobacco plants were micropropagated on MS medium [34] for 1 month and leaves were cut and put on dishes containing 0.85% Phytoagar (Duchefa, The Netherlands) added of 8 mg/mL fungus mycelia. Dishes were incubated for 1 week at 28°C with a photoperiod of 16 h.
Results
Production of Transgenic Plants and Genetic Characterization
A total of 34 independently transformed tobacco plants (17 containing the M. anisopliae chit1 cDNA and named “chitplus” plants and 17 “chitless” plants) were obtained after A. tumefaciens leaf disk transformation and regeneration. The structures of both T-DNA versions are illustrated in Fig. 1. No distinguishable effects on plant growth, development, and fertility were observed either in chitplus or in chitless plants, with identical general phenotypes to wild-type plants throughout their life cycle (results not shown). All plants were tested for the presence of the chit1 transgene by preliminary PCR analysis. Figure 2a illustrates the result of the PCR analysis for two chitless and eight chitplus plants. The expected 0.75-kb fragment was detected only in chitplus transgenic plants. Southern blot hybridization of the transgenic plants was conducted with a 1.5-kb DNA probe representing an internal fragment of the chit1 coding region (Fig. 2b). Plant genomic DNA was cut with EcoRI, and each hybridization band corresponded to one different chit1 copy, since the integrated gene was cut once by EcoRI (see Fig. 1), and the other EcoRI site was localized in the plant genome. The position of the genomic site depends on the locus where the integration occurred. Since each integration event occurs independently and randomly, transgenic plants exhibited singular hybridization profiles. Hence, the chit1 transgene copy number was estimated as one in T12 and T21; two in T13, T22, T34, and T43; and four in T14. Surprisingly, no hybridization signals were detected in T32, suggesting that this plant has probably lost the chit1 transgene, maintaining the kanamycin resistance cassette that allowed its selection. Additionally, a DNA fragment of approximately 5 kb was observed in all plant genomic DNA samples, indicating that such signal is due to a plant chitinase gene sharing high sequence similarity with the chit1 gene from M. anisopliae. This DNA fragment was not detected in the fungus genomic DNA (Fig. 2b, lane Ma). Faint hybridization signals with high molecular weight are thought to result from partial cleavages of the genomic DNA and were not considered in the estimation of chit1 transgene copy number.
PCR and Southern blot analyses of chitless and chitplus transgenic plants. A PCR amplication of the fungal chit1 transgene using the MachitFor and MachitRev primers and template DNA from: two control chitless plants (C23 and C26), vector pMOG402 harboring the chitplus T-DNA (Pch), and eight chitplus transgenic plants (T12 to T43). A 0.75-kb product was detected in Pch and in chitplus plants, but not in chitless ones. B Southern blot analysis of EcoRI-digested genomic DNA extracted from M. anisopliae (Ma), wild-type tobacco (WT), two control chitless plants (C 23 and C 26 ), and eight chitplus plants (T 12 to T 43 ) probed with a 1.5-kb fragment of chit1 coding region (PEP). Except for one common signal shared with all plants, number of bands corresponded to the number of chit1 inserts
Recombinant Chitinase Production and Activity Levels in Leaf Protein Extracts from Transgenic Plants
Western blot analysis of leaf protein extracts revealed the presence of a 42-kDa band corresponding to the expected molecular mass of the chitinase CHIT42 in all chitplus plants tested, though individual transgenics showed differences in band intensities. As shown in Fig. 3a, the strongest signals were observed in protein extracts from T13 and T22 plants. The same band was not verified either in wild-type or in chitless plants.
Western blot and chitinase activity gels of leaf protein extracts from chitless and chitplus transgenic plants. Leaf soluble proteins (50 μg per lane) were extracted from wild-type (WT), chitless (C 23 and C 26 ), and chitplus (from T 12 to T 43 ) tobacco plants, separated by SDS-PAGE (12.5% for western and 12% for chitinase gels) and submitted to A western blot analysis, visualized with anti-CHIT42 polyclonal antibodies and to B, C chitinase activity gels, with black bands representing the hydrolysis of glycol-chitin, a substrate copolymerised in the polyacrilamide matrix. Estimated molecular weight of the chitinase (42 kDa) is indicated
Similar results were obtained through chitinase activity gels (Fig. 3b and c). No zones of hydrolytic activity were observed in wild-type and chitless plants. The 14 chitplus plants analyzed displayed clear differences in the area of glycol-chitin hydrolyzed by the enzyme, varying from plants not exhibiting any recombinant enzyme activity (T12, T132, T23, T25, T29, T33) to intermediate (T14, T21, T22, T27, T32) and strong signals (T13, T34, T43) of chitinolytic activity (Fig. 3b and c). These results support the idea that in some chitplus plants, CHIT42 was efficiently processed and the 35-amino-acid-residue signal peptide was accurately removed, allowing the production of the mature and active recombinant chitinase.
Specific chitinase activities were higher in chitplus than in wild-type or chitless plants for all substrates tested. Both exo- and endochitinase activities were significantly increased in chitplus plants, since the exochitinase-specific substrate p-nitrophenyl-N-acetyl-β-d-glucosamine was strongly hydrolyzed by the T21 (2.2050 U) and T22 (6.3601 U) protein extracts. Similar results were obtained for N,N′-diacetylchitobiose, and the highest values were also detected for the extracts T12 (1.2689 U) and T22 (6.1084 U). On the other hand, the endochitinase activity, derived from the hydrolysis of N,N′,N″,N′″-tetracetylchitotetraose, was evident in T32 (0.9731 U) and T13 (7.6461 U). Therefore, these results point to a preferential endochitinase-like mode of action for CHIT42 since differences between chitplus and controls were observed in a higher proportion for the assay using N,N′,N″,N′″-tetracetylchitotetraose as substrate (7.85-fold). Compared to the gel activity assay, these latter results indicate that the specific chitinase activity assays are more sensitive approaches, allowing to distinguish discrete amounts of chitinase even in leaf extracts showing very similar intensities as observed in the western blot analysis (T12, T14, T21, T32, T34, and T43) or no hydrolyzed regions in activity gels (T12). For T13, T22, T34, and T43 chitplus plants, positive signals found in western blots and in chitinase activity gels were confirmed by the chitinase specific activity assays, and these plants kept their position of top enzyme producing plants (Fig. 4).
Chitinase specific activity in leaf protein extracts. Leaf protein crude extracts were prepared from wild-type (WT), chitless (C 23 and C 26 ), and chitplus (T 12 to T 43 ) tobacco plants, and chitinase activity was assayed against four synthetic substrates A p-nitrophenyl-N-acetyl-β-d-glucosamine (pNP-GlcNAC), B N,N′-diacetylchitobiose (GlcNAC2), C N,N′,N″,N′″-tetracetylchitotetraose (GlcNAC4). Values are an average of three independent determinations. Bars labeled with the same letter are not significantly different according to Duncan’s test (α = 0.05)
Transgenic Plant Resistance to the Soilborne Pathogen R. solani
Four chitplus plants bearing the highest chitinase activities and one chitless plant were selected to test their resistance (or susceptibility) to R. solani. As shown in Fig. 5a, 1 week after inoculation, leaves of T22 and T43 chitplus plants presented typical symptoms of R. solani infection throughout the foliar area, mainly consisting of necrotic lesions, a severe reaction similar to that observed in leaves of the chitless C26 plant. On the other hand, T13 and T34 chitplus plants were completely resistant to the pathogen and appeared healthy after 1 week of infection, with the absence of chlorosis and necrotic lesions.
Resistance assay of transgenic plants to the phytopathogenic fungus R. solani. A Tobacco chitless (C26) and chitplus plants (T13, T22, T34, T43) were cultivated under aseptic conditions for 1 month, and leaves were removed and put into dishes containing 0.85% Phytoagar without mycelia (control) or added of 0.8 mg/mL of mycelia of the pathogen. Dark zones correspond to necrotic lesions, caused by fungal attack 1 week after inoculation (white arrows). b Four-month-old progenies of self-control (SWT), chitless (SC 26 ), and chitplus (ST 13 , ST 22 , ST 34 , ST 43 ) plants were grown on soil added of 8 g of R. solani mycelia. Highly damaged leaves with necrotic and chlorotic lesions were observed in SWT and SC26 (white arrows), with the best standing chitplus transgenics. Results are given 1 month after fungus inoculation
The descendants of these four T0 top chitinase producing plants were also submitted to infection by R. solani in soil (Fig. 5b). Wild-type (SWT) and the chitless plant SC26 were susceptive to the pathogen, developing typical necrosis lesions surrounded by a chlorotic halo. For chitplus segregant plants, on the other hand, disease symptoms, when present, were restricted to a few leaves (ST22), with most plants (ST13, ST34, ST43) remaining healthy even 1 month after fungus inoculation, with no observable lesions.
Analysis of T1 Plants for Transgene Inheritance Stability
The first self-pollinated generation of T13, T22, T34, and T43 chitplus plants, all harboring two copies of the chit1 transgene (Fig. 2b), were tested for the maintenance of a mendelian segregation pattern. As presented in Table 1, 67 T1 plants were submitted to PCR analysis altogether, and in 53, no significant deviations from the predicted ratio of chit1 presence (+) to chit1 absence (−) corresponding to 15:1 were observed. These results confirm the dihybrid segregation pattern of the chit1 transgene. The exact probability of finding such a proportion among descendants, at random, was calculated to be 0.8741, 0.9947, and 0.9907 for T13, T22, and T34, respectively. All values were above the significance level chosen for the test (α = 0.05). In contrast, only in one offspring, derived from T43, was the number of plants bearing the transgene found to be extremely reduced (two plants out of 14). The exact probability of such an event, at random, was highly significant (P ≪ 0.05).
Discussion
Transformation of Tobacco with a Biocontrol Fungus Chitinase
In this study, transgenic N. tabacum SR1 plants bearing a chitinase gene from the arthropod infective fungus M. anisopliae were generated by A. tumefaciens-mediated transformation. The presence of the transgene was confirmed by PCR and Southern blot analysis, revealing that the transgene copy number varied from 1 to 4 among plants. In all chitplus plants obtained, great heterogeneity concerning amounts of soluble-active chitinase CHIT42 was detected. Nevertheless, all activity levels were higher in chitplus plants, differing from chitless or wild-type plants. The analysis of descendants of double hemyzygous plants demonstrated that the transgene was inherited according to mendelian proportions in three independent progenies (whose parental individuals were T13, T22, and T43), whereas a significant deviation was found in one of them (derived from T43). Preliminary infection experiments in agar dishes revealed variable CHIT42 activity levels, reflecting in plant resistance to lesions caused by R. solani, ranging from fully resistant chitplus plants (T13 and T34) to plants as susceptible as the control (T22 and T43). However, soil infection assays showed that in all chitplus-descendant plants, resistance levels to R. solani were increased compared with the wild-type and chitless plants, even though less intense variations were observed.
Evaluation of Transgenic Tobacco for the Presence of Transgene and Recombinant Chitinase Levels
Strong degrees of variation on chitinase transgene expression among transgenic plants independently produced have been reported in other studies, including groundnut [35], cucumber [36], potato and tobacco [8], and creeping grass [37]. This pattern has been associated to intrinsic properties of the transformation protocols which may give rise to epigenetic silencing, co-suppression, position effects, and changes in transgene structure [38, 39].
Some correlation between the chit1 copy number and the chitinase activity levels in leaf protein extracts from chitplus plants could be noted. In general, four chitplus plants harboring two transgene copies (T13, T22, T34, and T43) expressed the highest amounts of chitinase specific activity. In those transgenic plants harboring 1, 3, and 4 chit1 copies, enzyme levels were much lower. No correlation between the number of copies, and the level of enzyme production was observed in transgenic plants transformed with chitinase genes from Trichoderma [8] or American elm [37].
Unspecific PCR products and hybridization signals detected in the wild-type, chitless, and chitplus plants are thought to be derived from plant chitinase genes which share a significant sequence similarity with chit1 from M. anisopliae. Such DNA fragments were not seen in the genomic DNA from the fungus. Indeed, chitinases constitute the main component of pathogenesis-related proteins involved in defense mechanisms against bacteria, fungi, and insects, widely spread throughout plant species [2, 40].
Moreover, these four independent chitplus plants (T13, T22, T34, and T43) were selfed, and the resulting segregation ratio was discovered not to differ significantly from the expected dihybrid pattern, which is 15:1 as predicted by two unlinked loci. This genetic evidence also corroborates transgene copy number estimated by Southern blot. A stable mode of inheritance for heterologous chitinase genes in plants has been described in rice [41] and tobacco [8]. Unlikewise, one deviation was verified for the progeny of the T43 plant, and the number of plants with chit1 integrated was surprisingly reduced, indicating that epigenetic rearrangements of T-DNA inserts may have occurred, leading to losses of these fragments. Inheritance instability was also reported by Deineko et al. [42] when testing the stability of the nptII marker gene in the descendants of transgenic tobacco plants. These authors described segregation deviations from the mendelian ratios in the progeny of double heterozygous plants [42].
The accurate cleavage of immature CHIT42 was suggested through western blot and chitinase activity gels because the expected band size of 42 kDa was detected in both experiments, indicating the removal of the 35-amino-acid signal peptide from the fungal enzyme. Lorito et al. [8] reported the correct post-translation processing of secretion signal peptides belonging to chitinases from T. harzianum and tomato when expressed in tobacco plants. Conversely, Bolar et al. [11] found an inefficient splicing in exochitinase transcripts from T. atroviride in apple plants.
No distinguishable effects on plant growth, development, and fertility were observed in chitplus plants, and their phenotypes were identical to chitless or wild-type plants throughout their life cycle. Even though similar results have been obtained by Lorito et al. [8] in tobacco, a negative correlation between the level of an endochitinase gene solely and endo- and exochitinase genes combined on growth of apple transgenic plants was registered in two previous papers by Bolar et al. [10, 11].
Antifungal Chitinase Activity in Planta against R. solani
All chitinase activity results shown in the present work suggested the rise in enzyme activity in chitplus plants was responsible for the improvement in plant resistance. T13 and T34 chitplus plants, expressing strong exo- and endochitinase activities, showed no necrotic or chlorotic lesions. Surprisingly, T22 and T43 exhibited a pattern of resistance not so high as predicted according to the great amount of specific chitinolytic activity. Overall, compared with parental resistance levels, the progeny of chitplus plants demonstrated a reduced degree of variation, with all plants exhibiting improved reactions against the fungal attack. Although some variation in response to infection has been reported, constitutive expression of chitinase in leaf tissues has been associated with high levels of plant resistance to pathogens [8, 11, 36, 41].
As far as we are aware, this is the first report of resistance to a fungal disease in plants mediated by a chitinase gene from an entomopathogenic and acaricide fungus. Further experiments will include challenge of transgenic tobacco plants with other pathogenic fungi and insects in order to evaluate possible resistance to a broader spectrum of pathogens, as well as the insertion of chit1 into economically relevant plant species such as potato, tomato, soybean, eucalyptus, barley, sugarcane, and rice.
References
Duo-Chuan, L. (2006). Review of fungal chitinases. Mycopathologia, 161, 345–360. doi:10.1007/s11046-006-0024-y.
Dana, M. M., Pintor-Toro, J. A., & Cubero, B. (2006). Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiology, 142, 722–730. doi:10.1104/pp.106.086140.
Lorito, M., Harman, G. E., Hayes, C. K., Broadway, R. M., Tronsmo, A., Woo, S. L., et al. (1993). Chitinolytic enzymes of Trichoderma harzianum—purification of chitobiosidase and endochitinase. Phytopathology, 83, 302–307. doi:10.1094/Phyto-83-302.
Lorito, M., Hayes, C. K., Di Pietro, A., Woo, S. L., & Harman, G. E. (1994). Purification, characterisation and synergistic activity of a glucan 1,3-beta-glucosidase and an N-acetyl-glucosaminidase from Trichoderma harzianum. Phytopathology, 84, 398–405. doi:10.1094/Phyto-84-398.
Lorito, M., Mach, R. L., Sposato, P., Strauss, J., Peterbauer, C. K., & Kubicek, C. P. (1996). Mycoparasitic interaction relieves binding of the Cre1 carbon catabolite repressor protein to promoter sequences of the ech42 (endochitinase-encoding) gene in Trichoderma harzianum. Proceedings of the National Academy of Sciences of the United States of America, 93, 14868–14872. doi:10.1073/pnas.93.25.14868.
Seidl, V., Huemer, B., Seiboth, B., & Kubicek, C. P. (2005). A complete survey of Trichoderma chitinases reveals three distinct subgroups of family 18 chitinases. FEBS Journal, 272, 5923–5939. doi:10.1111/j.1742-4658.2005.04994.x.
Dahiya, N., Tewari, R., & Hoondal, G. S. (2006). Biotechnological aspects of chitinolytic enzymes: a review. Applied Microbiology and Biotechnology, 71, 773–782. doi:10.1007/s00253-005-0183-7.
Lorito, M., Woo, S. L., Fernandez, I. G., Colucci, G., Harman, G. E., Pintor-Toro, J. A., et al. (1998). Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens. Proceedings of the National Academy of Sciences of the United States of America, 95, 7860–7865. doi:10.1073/pnas.95.14.7860.
Gokul, B., Lee, J. H., Song, K. B., Rhee, S. K., Kim, C. H., & Panda, T. (2000). Characterization and applications of chitinases from Trichoderma harzianum—a review. Bioprocess Engineering, 23, 691–694. doi:10.1007/s004499900138.
Bolar, J. P., Norelli, J. L., Wong, K. W., Hayes, C. K., Harman, G. E., & Aldwinckle, H. S. (2000). Expression of endochitinase from Trichoderma harzianum in transgenic apple increases resistance to scab and reduces vigour. Phytopathology, 90, 72–77. doi:10.1094/PHYTO.2000.90.1.72.
Bolar, J. P., Norelli, J. L., Harman, G. E., Brown, S. K., & Aldwinckle, H. S. (2001). Synergistic activity of endochitinase and exochitinase from Trichoderma atroviride (T. harzianum) against the pathogenic fungus (Venturia inaequalis) in transgenic apple plants. Transgenic Research, 10, 533–543. doi:10.1023/A:1013036732691.
Noël, A., Levasseur, C., Le, V. Q., & Seguin, A. (2005). Enhanced resistance to fungal pathogens in forest trees by genetic transformation of black spruce and hybrid poplar with a Trichoderma harzianum endochitinase gene. Physiological and Molecular Plant Pathology, 67, 92–99. doi:10.1016/j.pmpp.2005.09.010.
Emani, C., Garcia, J. M., Lopata-Finch, E., Pozo, M. J., Uribe, P., Kim, D. J., et al. (2003). Enhanced fungal resistance in transgenic cotton expressing an endochitinase gene from Trichoderma virens. Plant Biotechnology Journal, 1, 321–336. doi:10.1046/j.1467-7652.2003.00029.x.
Terakawa, T., Takaya, N., Horiuchi, H., Koike, M., & Takagi, M. (1997). A fungal chitinase gene from Rhizopus oligosporus confers antifungal activity to transgenic tobacco. Plant Cell Reports, 16, 439–443.
St. Leger, R. J., Cooper, R. M., & Charnley, A. K. (1991). Characterization of a chitinase and chitobiase produced by the entomopathogenic fungus Metarhizium anisopliae. Journal of Invertebrate Pathology, 58, 415–426. doi:10.1016/0022-2011(91)90188-V.
Pinto, A. S., Barreto, C. C., Schrank, A., Ulhoa, C. J., & Vainstein, M. H. (1997). Purification and characterization of an extracellular chitinase from the entomopathogen Metarhizium anisopliae. Canadian Journal of Microbiology, 43, 322–327.
Bogo, M. R., Rota, C. A., Pinto, H., Jr., Ocampos, M., Correa, C. T., Vainstein, M. H., et al. (1998). A chitinase encoding gene (chit1 gene) from the entomopathogen Metarhizium anisopliae: isolation and characterisation of genomic and full-length cDNA. Current Microbiology, 73, 221–225. doi:10.1007/s002849900368.
Kang, S. C., Park, S., & Lee, D. G. (1999). Purification and characterization of a novel chitinase from the entomopathogenic fungus Metarhizium anisopliae. Journal of Invertebrate Pathology, 73, 276–281. doi:10.1006/jipa.1999.4843.
Frazzon, A. P., Vaz Junior, I., Masuda, A., Schrank, A., & Vainstein, M. H. (2000). In vitro assessment of Metarhizium anisopliae isolates to control the cattle tick Boophilus microplus. Veterinary Parasitology, 94, 117–125. doi:10.1016/S0304-4017(00)00368-X.
da Silva, M. V., Santi, L., Staats, C. C., da Costa, A. M., Colodel, E. M., Driemeier, D., et al. (2005). Cuticle-induced endo/exoacting chitinase CHIT30 from Metarhizium anisopliae is encoded by an ortholog of the chi3 gene. Research in Microbiology, 156, 382–392. doi:10.1016/j.resmic.2004.10.013.
St. Leger, R. J., Charnley, K., & Cooper, R. M. K. (1986). Cuticle degrading enzymes of entopathogenic fungi: mechanisms of interaction between pathogen enzymes and insect cuticle. Journal of Invertebrate Pathology, 47, 117–125.
St. Leger, R. J., Joshi, L., Bidochka, M., Rizzos, M. J., & Roberts, D. W. (1996). Characterisation and ultrastructural location of chitinases from Metarhizium anisopliae, M. flavoviridae and Beauveria bassiana during fungal invasion of host (Manduca sexta) cuticle. Applied and Environmental Microbiology, 62, 907–912.
Sambrook, J., & Russel, D. W. (2001). Molecular cloning: a laboratory manual (3rd ed.). New York: Cold Spring Harbor Laboratory Press.
Peach, C. R. W., & Velten, J. (1994). Agrobacterium-mediated gene transfer to plant cells: cointegrate and binary vector systems. In S. B. Gelvin, R. A. Schilperoort & D. P. S. Verma (Eds.), Plant molecular biology manual, Section B1 (pp. 1–19). Dordrecht: Kluwer.
Horsch, R. B., Fry, J. E., Hoffmann, N. I., Eichholtz, D., Rogers, S. G., & Fraley, R. T. (1985). A simple and general method for transferring genes into plants. Science, 277, 1229–1231.
Doyle, J. J., & Doyle, J. L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissues. Phytochemical Bulletin, 19, 11–150.
Armitage, P., & Berry, G. (1987). Statistical methods in medical research (2nd ed.). London: Oxford University Press.
Memelink, J., Swords, K. M. M., Staehelin, L. A., & Hoge, J. H. C. (1994). Southern, northern and western blot analysis. In S. B. Gelvin, R. A. Schilperoort & D. P. S. Verma (Eds.), Plant molecular biology manual, Section F1 (pp. 1–23). Dordrecht: Kluwer.
Jefferson, R. A., & Wilson, K. J. (1994). The gus gene fusion system. In S. B. Gelvin, R. A. Schilperoort & D. P. S. Verma (Eds.), Plant molecular biology manual, Section B14 (pp. 9–33). Dordrecht: Kluwer.
Bradford, M. M. (1976). A rapid and sensitive method for quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. doi:10.1016/0003-2697(76)90527-3.
Reissig, J. L., Stromnger, L., & Leloir, L. F. (1955). A modified colorimetric method for the determination of N-acetylamino sugars. Journal of Biological Chemistry, 217, 959–966.
Yabuki, M., Mizushima, K., Amatou, T., Ando, A., Fuji, I., Shimada, M., et al. (1986). Purification and characterisation of chitinase and a chitobiase produced by Aeromonas hydrophila subesp anaraegenes A42. The Journal of General and Applied Microbiology, 32, 25–32. doi:10.2323/jgam.32.25.
St. Leger, R. J., Staples, R. C., & Roberts, D. W. (1993). Entomopathogenic isolates of Metarhizium anisopliae, Beauveria bassiana and Aspergillus flavus produce multiple extracellular chitinase isozymes. Journal of Invertebrate Pathology, 61, 81–84. doi:10.1006/jipa.1993.1014.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiologia Plantarum, 15, 473–497. doi:10.1111/j.1399-3054.1962.tb08052.x.
Rohini, V. K., & Rao, K. S. (2001). Transformation of peanut (Arachis hypogaea L.) with tobacco chitinase gene: variable response of transformants to leaf spot disease. Plant Science, 160, 889–898. doi:10.1016/S0168-9452(00)00462-3.
Kishimoto, K., Nishizawa, Y., Tabei, Y., Hibi, T., Nakajima, M., & Akutsu, K. (2002). Detailed analysis of rice chitinase gene expression in transgenic cucumber plants showing different levels of disease resistance to grey mold (Botrytis cinerea). Plant Science, 162, 655–662. doi:10.1016/S0168-9452(01)00602-1.
Chai, B., Maqbool, S. B., Hajela, R. K., Green, D., Vargas, J. M., Jr., Warkentin, D., et al. (2002). Cloning of a chitinase-like cDNA (hs2), its transfer to creeping bentgrass (Agrostis palustris Huds.) and development of brown patch (Rhizoctonia solani) disease resistant transgenic lines. Plant Science, 163, 183–193. doi:10.1016/S0168-9452(02)00069-9.
Finnegan, J., & McElroy, D. (1994). Transgene inactivation: plants fight back!. Biotechnologies, 12, 883–888. doi:10.1038/nbt0994-883.
Maqbool, S. B., & Christou, P. (1999). Multiple traits of agronomic importance in transgenic indica rice plants: analysis of transgene integration patterns, expression levels and stability. Molecular Breeding, 5, 471–480. doi:10.1023/A:1009634226797.
Gooday, G. W., Zhu, W. Y., & Donnell, R. W. (1992). What are the roles of chitinases in the growing fungus? FEMS Microbiology Letters, 100, 387–392. doi:10.1111/j.1574-6968.1992.tb05730.x.
Datta, K., Jumin, T., Oliva, N., Ona, I., Velazhahan Mew, T. W., Muthukrishnan, S., et al. (2001). Enhanced resistance to sheath blight by constitutive expression of infection-related rice chitinase in transgenic elite indica rice cultivars. Plant Science, 160, 405–414. doi:10.1016/S0168-9452(00)00413-1.
Deineko, E. V., Novoselya, T. V., Zagorskaya, A. A., Filipenko, E. A., & Shumnyi, V. K. (2000). Expression instability of the marker nptII gene in transgenic tobacco plants. Russian Journal of Plant Physiology: A Comprehensive Russian Journal on Modern Phytophysiology, 47, 394–399.
Acknowledgements
We thank Dr. A.T.S. Matsumura for providing the isolates of R. solani employed in this study, Dr. M.H. Bodanese-Zanettini for green house facilities, Dr. S.C. Jacques for her assistance with statistical analysis, and architect R. Pasquali for his help with the illustrations. This research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kern, M.F., Maraschin, S.d.F., Vom Endt, D. et al. Expression of a Chitinase Gene from Metarhizium anisopliae in Tobacco Plants Confers Resistance against Rhizoctonia solani . Appl Biochem Biotechnol 160, 1933–1946 (2010). https://doi.org/10.1007/s12010-009-8701-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8701-1