Abstract
The agricultural residues, wheat bran and rice hulls, were used as substrates for cellulase production with Trichoderma sp 3.2942 by solid-state fermentation. Microwave irradiation was employed to pretreat the substrates in order to increase the susceptibility. Although the highest cellulase yield was obtained by the substrates pretreated by 450 W microwave for 3 min, pretreatment time and microwave power had no significant effect on cellulase production. The initial reducing sugar content (RSC) of substrates was decreased by microwave irradiation, but more reducing sugars were produced in later fermentation. Alkali pretreatment combined with microwave pretreatment (APCMP) of rice hulls could significantly increase cellulase yields and reducing sugar. The maximum filter paper activity, carboximethylcellulase (CMC)ase, and RSC were increased by 35.2%, 21.4%, and 13%, respectively, compared with those of untreated rice hulls. The fermented residues could produce more cellulase and reducing sugars than fresh rice hulls after they were treated by APCMP. The increased accessibility of the substrates by microwave pretreatment was mainly achieved by rupture of the rigid structure of rice hulls. However, for alkali pretreatment and APCMP, delignification and removal of ash played very important roles for increasing the acceptability of substrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulose composes more than 60% of plant biomass produced on earth, which mainly consists of three components: cellulose, hemicellulose, and lignin [1]. Cellulose may be hydrolyzed by cellulosic enzymes to produce glucose that can be further fermented to ethanol and some other products. In the bioconversion of cellulose to ethanol, cellulase is employed for the saccharification of the glycan in either separate hydrolysis and fermentation or simultaneous saccharification and fermentation [2]. Therefore, stable supply of cellulase is also a key factor for the progress of cellulosic ethanol.
Filamentous fungi are the major source of cellulases and hemicellulases [3, 4]. The fungi-based production of cellulase can be carried out either by a submerged culture process or solid-state fermentation. Although the submerged fermentation has advantages such as reproducible enzyme activities in each batch, ease of contamination control, labor saving, and so on; the production cost is relatively high because of the high energy requirement, expensive medium composition, and low enzyme concentration [5]. In solid-state fermentation, the microorganisms grow under conditions closer to their natural habitats so they may be more capable of producing certain enzymes and metabolites which usually will not be produced or will be produced only with low yield in a submerged fermentation [6]. Furthermore, the substrates used in solid-state fermentation are always agricultural wastes, which can reduce the fermentation cost [7].
It has been known that cellulase is a complex mixture of enzyme proteins with different specificities to hydrolyze glycosidic bonds [8]. The hydrolysis of cellulose requires the synergistic action of endo-β-1, 4-glucanase (EC 3.2.1.4), exo-β-1, 4-glucanase (EC 3.2.1.91), and β-glucosidase. The latter is important as it provides a selective hydrolysis of cellobiose to glucose [3, 8]. Furthermore, cellulase is an inducible enzyme, and cellulose is the best inducer [5]. However, the crystallinic structure and lignin existence in cellulosic materials prevent attack by microorganisms. The growth of fungi is too slow in untreated raw materials for commercial production of cellulase. Therefore, a pretreatment process is necessary in order to make the lignocellulosic substratres more susceptible to the growth of fungi and the hydrolytic action of cellulases. Being similar to the pretreatment process for increasing the enzymatic digestibility of lignocellulosic biomass, the purpose of the pretreatment for solid-state fermentation is to remove lignin and hemicellulose, reduce cellulose crystallinity, and increase the porosity of the materials [9]. A series of methods have been developed to pretreat lignocellulosic materials for enzymatic hydrolysis, including physical pretreatment, physicochemical pretreatment (steam explosion), ammonia fiber explosion, CO2 explosion, and chemical pretreatments, all of which have been well described in some review articles [9–13]. These methods also can be employed to pretreat the substrates for cellulase production by solid-state fermentation. Proper pretreatment of the substrates, such as pulverization, steam explosion, acid hydrolysis, alkali soaking, and organosolv pretreatment can promote the growth of the microorganisms and induce more cellulase [5, 14–16].
As microwave heating is a kind of volumetric and rapid heating technique with high efficiency and no temperature gradient, it has been used for organic reactions and the pretreatment of lignocellulosic materials [16–21]. Compared with some other physicochemical pretreatment methods, such as steam explosion, microwave irradiation seems to be less energy consumption with lower complexity apparatus. When the lignocellulosic materials were pretreated by microwave irradiation or microwave-assisted pretreatment in the presence of water, their enzymatic susceptibility were increased. In present work, microwave irradiation was employed to pretreat the substrates for cellulase production in order to obtain some preliminary data for further investigation.
Materials and Methods
Microorganism
Trichoderma sp 3.2942 was purchased from China General Microbiological Culture Collection Center and used for solid-state fermentation. It was cultured on potato glucose agar slants at 30 °C for 72 h and stored at 4 °C when spores were formed.
Solid Substrates
Wheat bran and rice hulls obtained from Hunan and Hebei province in China were used as substrates for fermentation. Rice hulls were milled to 1–2 mm particle size. The substrates consisted of 21 g wheat bran, 9 g rice hulls, and 30 g mineral salts solution in a 500-ml beaker, according to the previous optimized experimental results. The composition of mineral salts solution was: (NH4)2SO4 10 g, KH2PO4 3 g, CaCl2 0.5 g, MgSO4 0.5 g, and 1,000 ml deionized water.
Inocula
The liquid medium for culturing inocula was obtained by boiling the mixture of 10 g wheat bran and 100 ml deionized water for 20 min. After the mixture was filtered, the medium was gained by filtrate being added enough deionized water until the liquid volume was 100 ml. Then, the medium was sterilized at 115 °C for 15 min and used for culturing inocula in shake flasks for 24 h at 30 °C and 130 rpm.
Pretreatment
The solid substrates mixed with mineral salts solution was put in a 500-ml beaker covered by preservative film. The beaker was then put into a common microwave oven for pretreatment. Alkali pretreatment combined with microwave pretreatment (APCMP) was just used for pretreating rice hulls. In APCMP, the rice hulls was soaked in 4 wt.% NaOH solution at 40 °C for 24 h with liquid-to-solid ratio of 2.5:1. The mixture was then heated by 450 W microwave for 3 min. The pretreated rice hulls was washed with water until neutral and dried at 105 °C. Subsequently, 9 g of pretreated hulls and 21 g fresh wheat bran were mixed with 30 g mineral salts solution for fermentation.
Culture Condition
Following heat sterilization (121 °C for 40 min), the solid medium was inoculated with 6 ml inocula liquid. Then, the fermentation was conducted at 30 °C for 120 h in an incubator with 95% relative humidity. The pH of the substrate was not controlled in the fermentation. For comparison, control experiments using untreated substrates for fermentation were conducted simultaneously in each batch of cultivation. At least duplicate test was performed for each experiment.
Enzyme Extraction
Fresh moldy substrates were soaked in pH 4.8 acetic acid buffer solution (1:10, w/v) for 3 h at 30 °C. Then, the solution was filtered and centrifuged at 4,000 rpm for 15 min. The liquor was quickly analyzed for cellulase activity and reducing sugar concentration.
Enzyme Activity
Saccharifying cellulase activity and endo-β-1,4-glucannase activity were assayed according to the method recommended by Ghose [22] and expressed as international units (IU), using filter paper and 1% CMC, respectively. One international unit of cellulase activity (FPA) is the amount of enzyme that forms 1 μmol glucose (reducing sugars as glucose) per minute during the hydrolysis reaction. One international endocellulase unit (CMCase) is that amount of enzyme that forms 1 μmol glucose equivalents per minute from CMC. Reducing sugar was determined by the dinitrosalicylic acid method. For comparison, the FPA and CMCase were expressed as the amount of enzyme produced from per gram of dry substrates (IU/g dry substrates, IU/gds) respectively.
Analysis of Polysaccharides, Lignin, and Ash
The contents of polysaccharides, acid-insoluble lignin, acid-soluble lignin, ash, and acetyl group of rice hulls were determined according to the NREL LAP-002 (Determination of Carbohydrates in Biomass by High Performance Liquid Chromatography), NREL LAP-003 (Determination of Acid-Insoluble Lignin in Biomass), NREL LAP-004 (Determination of Acid-Soluble Lignin in Biomass), NREL LAP-005 (Standard Method for Ash in Biomass), and NREL LAP-017 (Determination of O-Acetyl Groups in Biomass by High Performance Liquid Chromatography) methodology, respectively [23].
Statistical Analysis of Experimental Data
For determining the significance of the effects of factors on the response variables, analysis of variance was employed to analyze the experimental data, and Matlab 6.5 software was used for the analysis.
FTIR and SEM
FIIR
IR spectra were recorded by using a NICOLET 560 FTIR spectrometer (Nicolet Company, USA). The dried samples were embedded in KBr pellets with an approximate concentration of 1 mg/100 mg KBr. The spectra were recorded in the absorption band mode in the range of 4,000–400 cm−1.
SEM
The rice hulls were fixed on the conductive glue and covered with gold sputter coater. Then micrographs of the biomass specimen were taken using an HRSEM JSM7401 scanning electron microscope (JEOL, Japan).
Results and Discussion
Comparison of the Cellulase Production Induced by Two Different Pretreated Substrates
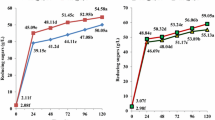
Wheat bran contained more nutrients such as starch and protein than rice hulls but less cellulose and lignin. It has been found that wheat bran is much easier to utilize by microorganisms than rice hulls [24]. However, significant loss (more than 90%) of reducing sugar was found in the experiments after wheat bran was pretreated by microwave irradiation. Therefore, while using microwave to pretreat the substrates, two schemes can be selected. One is to pretreat the mixed substrates and further use it for fermentation; the other is to only pretreat rice hulls and further mix it with fresh wheat bran for fermentation. The former substrates were denoted as pretreated mixed substrates (PMS), and the latter as pretreated rice hulls substrates (PRHS). For comparing the effectiveness of these two pretreatment schemes, we investigated the cellulase production courses induced by these two substrates, as shown in Fig. 1. All of the substrates showed maximum FPA at the 48th–60th hour and maximum CMCase and reducing sugar content (RSC) at the 36th–48th hour. After 60-h incubation, the FPA was significantly decreased probably due to the inactivation of cellulase complex. However, the strain still grew on the substrates by utilizing the residual reducing sugar as carbon source. From Fig. 1, it can be known that the maximal FPA and CMCase induced by PMS and PRHS were both higher than those by the untreated substrates; nevertheless, there was no significant difference between the yields of cellulase induced by PMS and PRHS. The initial RSC of PMS was lower than those of PRHS and untreated materials, but it was significantly increased and reached higher acme at the 24th hour. It indicated that the PMS were more accessible for enzymatic hydrolysis, which was in accordance to some literatures [18, 20, 25]. Similarly, the accessibility of pretreated substrates was increased for growth of T. sp 3.2942 and cellulase production. All of these facts demonstrated that microwave irradiation could enhance the bioconversion of substrates to some extent.
It was interesting to find from the time courses of fermentation that the maximum FPA and CMCase did not occur at the same fermentation time. The maximum FPA appeared at around 48th or 60th hour, while the maximum CMCase appeared earlier, at around 36th hour. Since FPA measures the synergistic activity of the total cellulase complex and the CMCase assay measures endocellulase activity [3], it reflected that the hydrolysis of cellulose was the synergism work of the cellulase groups.
Effect of Pretreatment Time
As the pretreatment process was conducted only with preservative film covering the beaker, moisture loss was unavoidable. If pretreatment time was too long, the substrates would be charred. For comparing the influence of pretreatment on maximum FPA and CMCase, the mixed substrates were pretreated by 450 W of microwave for 0, 3, 6 and 9 min respectively. After pretreatment, the substrates were cooled down to room temperature and added with enough deionized water until the moisture content in the substrates was 50% (w/w). Then, the mixture was used for fermentation. The maximal FPA and CMCase during fermentation were summarized in Table 1.
It can be seen that all the pretreated substrates gave more cellulase production. The maximum FPA was obtained when the substrates was pretreated for 3 min, but prolonging pretreatment time over 3 min did not benefit the increase of FPA and CMCase. However, the maximal CMCase was induced by the substrates pretreated for 9 min. It further reflected that the substrates had different ability to induce the production of different cellulase groups. Although all the pretreated substrates showed much higher increases of RSC, no exact relation was found between pretreatment time and maximum increase of RSC.
Effect of Microwave Power
For fixed pretreatment time, the degree of heating was increased when microwave power was increased. In order to compare the effect of microwave power in pretreatment process on cellulase production, 0, 300, 450, 550, and 700 W of microwave were respectively employed for pretreating the mixed substrates for 3 min. These pretreated substrates were then added with enough water and used for fermentation. The maximum FPA and CMCase were also found to appear at the 48th–60th hour and 36th hour, respectively. As shown in Table 2, both FPA and CMCase were increased to some extent for the substrates pretreated by 300–550 W microwave, while the substrates pretreated by 700 W microwave gave the similar enzyme production to that of untreated substrates. It was found that the moisture loss was increased with microwave power. The temperature in the center of substrates after pretreatment was increased from 95 to 110 °C when microwave power increased from 300 to 700 W. The highest maximum FPA and CMCase were induced by 450 W microwave-pretreated substrates. However, no significant difference (p > 0.1) was found for 300, 450, and 550 W microwave pretreatments. The untreated substrates had the highest initial RSC. Increasing microwave power caused more loss of initial reducing sugar, but the maximum RSC during the fermentation were increased when substrates were pretreated by 300 and 450 W microwave. It further confirmed that the susceptibility of substrates was enhanced by microwave pretreatment.
Xiong et al. found that microwave irradiation also had influence on the crystallinity of cellulose. The crystallinity index (N-O′KI) of cellulose I was decreased first and increased later when the microwave power was increased from 128 to 750 W. The lowest N-O′KI was obtained at microwave power of 360 W. Increasing microwave power to 750 W caused a significant increase of cystallinity [26]. On the other hand, when microwave power was higher than a certain value, the substrates were probably carbonized with degradation of sugars. Therefore, the phenomenon that the cellulase production, and RSC were decreased when microwave power was increased to 700 W might be explained by these facts.
Effect of APCMP
Alkali pretreatment is an effective method for increasing the enzymatic hydrolysis of lignocellulosic biomass. The mechanism of alkali hydrolysis is believed to be saponification of intermolecular ester bonds crosslinking xylan hemicelluloses and other components, such as lignin and other hemicellulose [9]. It was found that when rice hulls were pretreated by alkali or microwave, both of the maximum FPA and CMCase were increased. Therefore, we further investigated the APCMP in order to further increase cellulase production. Wheat bran is much easier to digest by the fungi [24], while alkali treatment of wheat bran caused significant loss of original sugars. Therefore, alkali pretreatment was only used for pretreating rice hulls. Then, the pretreated rice hulls were washed, dried, and added to the substrates for cellulase production. The time courses of FPA, CMCase, and RSC are shown in Fig. 2. It was clear that the pretreated substrates induced significantly higher FPA, CMCase, and RSC. Compared with untreated substrates, the APCMP pretreated substrates and alkali-pretreated substrates gave 35.3% and 12.2% higher FPA, and 21.4% and 9.0% higher CMCase, respectively. The RSC in the pretreated substrates were also found to be greatly increased especially at the 36th hour. A series of repeated experiments also confirmed the increase of cellulase and reducing sugar production, indicating the effectiveness of APCMP for increasing the susceptibility of substrates.
It has been known that alkali pretreatment can cause solubilization and redistribution of lignin and modifications in the crystalline state of the cellulose [10]. These reactions increased the porosity of substrates and promote the growth of mycelium. Subsequent microwave irradiation strengthened the pretreatment action. Furthermore, rice hulls had a high ash content, which manly consists of SiO2. Its dissolving in alkali solution during alkali pretreatment probably also benefited the cellulase production. More details will be discussed in later section.
Recycle of Fermented Residues
When fermentation was finished, the fermented residue still can be used for cellulase production, but the residue became dark green due to the presence of fungi biomass and spores. It was found that the residue could not be directly used for the second batch of fermentation unless it was treated. Therefore, the residue were collected together and divided into three parts. The first part was treated by APCMP, washed with water, and dried at 105 °C; the second one was washed with water and dried at 105 °C; the third one was directly dried at 105 °C without washing. Subsequently, these treated residues were mixed with fresh wheat bran and used for fermentation. The fresh rice hulls mixed with fresh wheat bran was selected as control sample. The maximum FPA, CMCase, and RSC were summarized in Table 3. It can be seen that the maximum FPA and RSC for sample 1# or 2# was greatly increased compared with those of control one. However, the unwashed residues seemed to inhibit FPA and reducing sugar production probably because of the presence of cell biomass and some metabolic products in the substrates which prohibited the growth of the microorganisms. It was also found that the growth of the fungi was significantly lagged on 3# substrates. On the other hand, the water-washed residue gave a satisfied cellulase and reducing sugar production, although the maximum FPA and RSC were somewhat lower than those of alkali-microwave-pretreated sample. The significant increase of RSC for sample 1# further confirmed the effectiveness of APCMP for improving the accessibility of substrates.
A general flow scheme and mass balance for cellulase production using wheat bran and rice hulls by solid-state fermentation can be drawn as that shown in Fig. 3. Rice hulls was pretreated by APCMP and mixed with fresh wheat bran and mineral salts solution then used for fermentation. The fermented residues can be directly recycled after being washed and dried. However, for large-scale production of cellulase, more details should be investigated, especially for the mass balance of each component and estimation of energy consumption, etc.
Compositions of Untreated and Treated Rice Hulls
Table 4 shows the percentage of solid recovered after pretreatment and the compositions of untreated and treated rice hulls. Microwave pretreatment obtained a high percentage of solid recovered, while alkali pretreatment, and APCMP pretreatment had much lower ones. It indicated that alkali pretreatment dissolved a large part of rice hulls. One of the most important substances dissolved in the alkali solution is ash. The ash content in the fresh rice hulls was as high as 15.3%, but it decreased to less than 3% after alkali or APCMP pretreatment. It was found that the ash components, especially silicon in rice hulls act as another physical barrier, protecting the plant from enzymatic hydrolysis and fungal degradation. Removal of silicon can benefit the increase of acceptability of the substrates [27]. Alkali pretreatment can dissolve a significant part of silicon, and microwave pretreatment also removes a part of acid-insoluble ash [28]. From Table 4, it also can be seen that the glucan and xylan content were both increased after pretreatment, but the lignin content was somewhat decreased for alkali-pretreated and APCMP-pretreated samples. Delignification also can benefit the enzymatic hydrolysis of biomass [29], so the fact that more yields of cellulase and reducing sugar were induced by alkali-pretreated and alkali-microwave-pretreated substrates also can be partially attributed to the removal of silicon and lignin. Alkali pretreatment could significantly remove the silicon and lignin, while this action could be further strengthened by microwave irradiation in APCMP pretreatment.
On the other hand, deacetylation was observed in alkali and APCMP pretreatment; but no such action was found in the microwave pretreatment. It illustrated that removal of acetyl group was caused by the alkali. Acetyl group is believed to inhibit the interaction between enzymes and cellulose. When the hydroxyl groups of cellulose were substituted by acetyl groups, the productive binding of cellulase to cellulose could not be formed through hydrogen bonds, which decreases the enzymatic hydrolysis of the substrates [30]. Therefore, deacetylation also could be helpful to the increase of substrates acceptability.
FTIR Spectra and SEM of Rice Hulls
In order to further investigate the change of chemical structure of rice hulls, FTIR spectra were performed to analyze the samples as shown in Fig. 4. Corresponding absorption band and assignment are summarized in Table 5. It can be known that after alkali pretreatment, the absorption bands at 1,732 cm−1 disappeared, which was assigned to the unconjugated C=O stretching in acetyl group of hemicellulose. The disappearance of this band indicated the occurrence of deacetylation in alkali pretreatment, which was verified in previous work. The band at 897 cm−1 was strengthened after alkali pretreatment, indicating that cellulose content was increased after pretreatment, which was ascribed to alkali delignification and removal of some ash components. However, no difference was found between the spectra of alkali-pretreated sample and alkali-microwave-pretreated sample. It illustrated that no significant change of chemical components happened during microwave pretreatment of rice hulls, and the increased susceptibility of substrates by microwave irradiation must be primarily attributed to the physical change of the substrates. Therefore, SEM was used for further studying the surface morphology of the rice hulls, as shown in Fig. 5. It is clear that the structure of untreated rice hulls exhibited rigid and ordered structure, but after microwave pretreatment, some cracks appeared. Similarly, the samples treated by alkali and APCMP became rough and porous. These cracks and porous structure helped the mycelia grow inside to the biomass and utilize cellulose as carbon source to synthesize cellulase. Furthermore, the rupture of rigid structure enhanced the acceptability of cellulase to cellulose, which enhanced the yield of reducing sugar.
Referring to the pretreatment mechanism, it was proposed that microwave pretreatment seemed to be similar to steam explosion [18, 20]. However, the steam explosion is typically initiated at a temperature of 160–260 °C (corresponding pressure 0.69–4.83 MPa) for several seconds to a few minutes before the material is exposed to atmospheric pressure [9]. The heating procedure of biomass is from outside to inside of the materials. A high pressure was needed to destroy the compact cell wall structure. Contrarily, in microwave treatment the cellulosic materials are heated internally—that is, the water, cellulose, hemicellulose, and the other low molecular compounds such as the organic acid contained in the cellulosic materials absorb the microwave as the kinetic energies when the polar molecules and their neighboring clusters are forced to orient to the specific direction. Water molecules have the strongest absorbability of microwave irradiation because of their stronger polarity and will, therefore, be heated most quickly. This makes the rate of the thermal motion of water molecules more rapid than that of water-swelled chain segments of cellulose and chain segments of cellulose crystal regions. Therefore, the water molecules moving rapidly collide with the chains cellulose, creating damage in the crystal region, and reducing the total crystal region of cellulose [26]. This damage will increase the susceptibility of microwave-pretreated substrates to enzymatic or microbial degradation. Moreover, some chemical reactions, such as the breaking of the linkages between cellulose and cellulose, hemicellulose and lignin, cellulose and hemicellulose, probably happened in microwave irradiation but could not be well reflected from the FTIR spectra. Further investigation on the crystallinity would be helpful for understanding the pretreatment mechanism.
Conclusions
When the substrates were pretreated by microwave irradiation under atmospheric pressure, the maximal FPA, CMCase, and RSC were increased. Although the most cellulase was produced by the substrates pretreated by 450 W microwave for 3 min, pretreatment time and microwave power had no significant effects on cellulase production. The initial RSC of substrates was decreased by microwave irradiation, but more reducing sugars were induced in later fermentation. Alkali pretreatment combined with microwave pretreatment of rice hulls could significantly increase the yields of cellulase and reducing sugar. Alkali pretreatment partly removed acetyl group and lignin and dissolving significant part of ash, increasing the susceptibility of rice hulls, while microwave irradiation further strengthened the effectiveness of pretreatment. After being pretreated by alkali and microwave or only being washed by water, the fermented residues could induce more cellulase and reducing sugars than the fresh rice hulls. Further experimental results indicated that the increase the accessibility of substrates by microwave pretreatment was mainly achieved by rupture of the rigid structure of the biomass. However, for alkali pretreatment and APCMP, delignification and removal of ash played very important roles for increasing the acceptability of substrates.
References
Tengerdy, R. P., & Szakacs, G. (2003). Biochemical Engineering Journal, 13, 169–179. doi:10.1016/S1369-703X(02)00129-8.
Balata, M. H., & Öz, B. C. (2008). Pror. Energy Combust. Sci., 34, 551–573. doi:10.1016/j.pecs.2007.11.001.
Duff, S. J. B., & Murray, W. D. (1996). Bioresource Technology, 55, 1–33. doi:10.1016/0960-8524(95)00122-0.
Gusakov, A. V., Salanovich, T. N., Antonov, A. I., Ustinov, B. B., Okunev, O. N., et al. (2007). Biotechnology and Bioengineering, 97, 1028–1038. doi:10.1002/bit.21329.
Cen, P. L., & Xia, L. M. (1999). Advances in Biochemical Engineering/Biotechnology, 65, 68–92.
Jecu, L. (2000). Industrial Crops and Products, 11, 1–5. doi:10.1016/S0926-6690(99)00022-9.
Mekala, N. K., Singhania, R. R., Sukumaran, R. K., et al. (2008). Applied Biochemistry and Biotechnology, 151, 122–131. doi:10.1007/s12010-008-8156-9.
Bhat, M. K., & Bhat, S. (1997). Biotechnology Advances, 15, 583–620. doi:10.1016/S0734-9750(97)00006-2.
Sun, Y., & Cheng, J. (2002). Bioresource Technology, 83, 1–11. doi:10.1016/S0960-8524(01)00212-7.
Hendriks, A. T. W. M., & Zeema, G. (2008). Bioresource Technology, 100, 10–180. doi:10.1016/j.biortech.2008.05.027.
Mosier, N., Wyman, C. E., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., et al. (2005). Bioresource Technology, 96, 673–686. doi:10.1016/j.biortech.2004.06.025.
Taherzadeh, M. J., & Karimi, K. (2008). International Journal of Molecular Sciences, 9, 1621–1651. doi:10.3390/ijms9091621.
Yang, B., & Wyman, C. E. (2008). Biofuels Bioprod. Biorefin, 2, 26–40.
Chahal, D. S. (1985). Applied and Environmental Microbiology, 49, 205–210.
Shin, C. S., Lee, J. P., Lee, J. S., & Park, S. C. (2000). Applied Biochemistry and Biotechnology, 84–86, 237–245. doi:10.1385/ABAB:84-86:1-9:237.
Zheng, G. J., Zhou, Y. J., Zhang, J. A., Cheng, K. K., et al. (2007). Journal of Wood Chemistry and Technology, 27, 65–71. doi:10.1080/02773810701486675.
Azuma, J. I., Tanaka, F., & Koshijima, T. (1984). Journal of Fermentation Technology, 62, 377–384.
Ooshima, H., Aso, K., & Harano, Y. (1984). Biotechnology Letters, 6, 289–294. doi:10.1007/BF00129056.
Miura, M., Kaga, H., Sakurai, A., Kakuchi, T., & Takahashi, K. (2004). Journal of Analytical and Applied Pyrolysis, 71, 187–199. doi:10.1016/S0165-2370(03)00087-1.
Kitchaiya, P., Intanakul, P., & Krairiksh, M. (2003). Journal of Wood Chemistry and Technology, 23, 217–225. doi:10.1081/WCT-120021926.
Hu, Z., & Wen, Z. (2008). Biochemical Engineering Journal, 38, 369–378. doi:10.1016/j.bej.2007.08.001.
Ghose, T. K. (1987). Pure and Applied Chemistry, 59, 257–268. doi:10.1351/pac198759020257.
NREL.Standard Biomass Analytical Procedures-Laboratory Analytical Procedures (LAPs). Available from http://www.nrel.gov/biomass/analytical_procedures.html
Chen, S. F., Zhao, L., & Liu, D. H. (2004). Food Ferment. Ind., 30, 8–12.
Mondal, K., Roy, I., & Munishwar, N. G. (2004). Biocatalysis and Biotransformation, 22, 9–16. doi:10.1080/10242420310001634971.
Xiong, J., YE, J., Liang, W. Z., & Fan, P. M. (2000). J. South China Univ. Techonl., 28, 84–89.
Řezanka, T., & Sigler, K. (2008). Phytochemistry, 69, 585–606. doi:10.1016/j.phytochem.2007.09.018.
Ma, H., Liu, W. W., Chen, X., Wu, Y. J., & Yu, Z. L. (2009). Bioresource Technology, 100, 1279–1284. doi:10.1016/j.biortech.2008.08.045.
Zhao, X. B., Wang, L., & Liu, D. H. (2008). Journal of Chemical Technology and Biotechnology (Oxford, Oxfordshire), 83, 950–956. doi:10.1002/jctb.1889.
Pan, X. J., Gilkes, N., & Saddler, J. N. (2006). Holzforschung, 60, 398–401. doi:10.1515/HF.2006.062.
Acknowledgement
This work was supported by National Basic Research Program of China (973 Program; No. 2004CB719700). The author also much appreciate Dr. Qiang Zhang in Beijing Key Laboratory of Green Chemical Reaction Engineering and Technology, Tsinghua University for his help with SEM study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, X., Zhou, Y., Zheng, G. et al. Microwave Pretreatment of Substrates for Cellulase Production by Solid-State Fermentation. Appl Biochem Biotechnol 160, 1557–1571 (2010). https://doi.org/10.1007/s12010-009-8640-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8640-x