Abstract
In this study, we introduced a new strategy, feeding d-glucose, to overproduce extracellular 5-aminolevulinic acid (ALA) in the recombinant Escherichia coli. We investigated that the d-glucose concentration is dependent on extracellular ALA production. The results indicated that increasing d-glucose concentration in bacteria culture enhanced final cell density and ALA yield and simultaneously decreased the activities of ALA synthase (ALAS) and ALA dehydratase (ALAD); then, the inhibitory effect of d-glucose on ALAS activity was relieved with the metabolism of d-glucose. when 4.0 g/L d-glucose was added at late exponential phase; 1.46 g/L ALA was achieved in shaking culture, which is 47% or 109% higher than the ALA yields with 30 mM levulinic acid of ALAD inhibitor or no inhibitor. In jar fermenter, final extracellular ALA concentration reached 3.1 g/L by feeding with d-glucose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
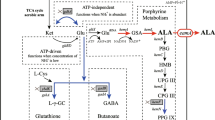
Aminolevulinic acid (ALA) is generally known as an essential precursor of tetrapyrrole biosynthesis, such as porphyrins and vitamin B12 [1, 2]. Recently, it has received considerable attention as a selective and biodegradable herbicide and insecticide and growth-promoting factor for plants [3, 4] and photodynamic therapy for the treatment of various kinds of cancers [5, 6].
ALA can be synthesized through two pathways [7]. One is the C4 pathway present in mammals, birds, yeast, and purple non-sulfur photosynthetic bacteria. The other is the C5 pathway, which occurs in higher plants, algae, and in many bacteria including Escherichia coli. ALA is tightly regulated by ALA synthase (ALAS; in the C4 pathway) and glutamate transfer RNA (tRNA) ligase and glutamyl tRNAglu reductase (in the C5 pathway). In these pathways, ALA dehydratase (ALAD) catalyzes the asymmetric formation of one molecule of porphobilinogen (PBG) from two molecules of ALA.
For the high production of ALA, it is essential to elevate cell density and ALAS activity and simultaneously inhibit ALAD activity. There are two categories of ALAD inhibitors, analogs to substrate and products. Levulinic acid (LA), as substrate analog, induces high yields of ALA in various organisms [8, 9]. It is known that the aldehyde group of d-glucose can react with amino groups of proteins resulting in a Schiff base conjugate formation. This process can promote the cross-linking of proteins through ε-NH2 group of an active-site lysine residue or hydroxylysine of ALAD.
Many studies have reported that primary metabolism, such as anabolism and catabolism (fermentation and respiration), varied with the excess supply of oxygen or carbon source [10–12]. Recently, Xie et al. have reported that the presence of d-glucose greatly repressed the activity of ALAS from Rhodobacter sphaeroides and ALA yield reached 5.2 g/L under the optimal condition [13].
In this work, we investigated the effect of d-glucose on the activity of ALAS from Agrobacterium radiobacter and the influence of d-glucose, as an economical and non-toxic inhibitor of ALAD, on the yields of extracellular ALA in the recombinant E. coli BL21(DE3) pET28a-hemA [14].
Materials and Methods
Bacterial Strain and Plasmid
BL21(DE3) [FompT hsdS B (r B − m B −) gal dcm (DE3)] and pET28a [Kan r His•Tag T7•Tag T7/lac promoter] were purchased from Novagen. Recombinant E. coli BL21(DE3)pET28-A.R-hemA harboring the hemA gene from A. radiobacter was created in previous work [14].
Cultivation Conditions
E. coli BL21(DE3)pET28-A.R-hemA was cultivated in the Luria–Bertani medium (10.0 g tryptone per liter, 5.0 g yeast extract per liter, and 10.0 g NaCl per liter, pH 7.0) with 30 mg kanamycin per liter as inoculant. The primary culture was inoculated by 20:1 dilution with precursor supplementaries (10 g succinate per liter, 2 g glycine per liter). Then, inhibitors (LA, d-glucose) of ALAD were under investigation. Cells were cultured at 37 °C in 50-mL fermentation medium at 200-rpm orbit rotation or in a 5-L jar fermenter (KF-5L, KFC, Korea) containing 3 L of fermentation medium; 0.05 mM isopropyl β-d-thiogalactopyranoside was added to the fermentation broth 2 h after inoculation; then, the temperature was set to 28 °C. The fermenter was operated at 400-rpm orbit rotation with an air flow rate of 3.0 L/min. And pH value was automatically controlled at 6.5 by 4 M NaOH and 0.2 M H2SO4.
Analyses
Cell concentration was determined off-line by measuring the optical density at 600 nm (OD600). ALA was analyzed by the method described by Mauzerall and Granick [15]. The reaction was performed with 2-mL supernatant of the culture, 1 mL 1 M sodium acetate buffer (pH 4.6), and 0.5 mL acetyl acetone and heated up in boiled water bath (100 °C) for 15 min. After cooling, 2 mL of the reaction mixture was mixed with 2-mL freshly prepared modified Ehrlich's reagent (1 g p-dimethyl-aminobenzaldehyde in 42 mL glacial acetic acid and 8 mL 70% perchloric acid). After sitting at room temperature for 30 min, the absorbance at 554 nm of the mixture was measured by a UV/vis spectroscopy (Utrospec. 3300, Amersham Biosciences).
Preparation of Cells and Cell Extracts
Cultures (30 mL) were harvested by centrifugation at 10,000×g for 10 min at 4 °C. The cells were washed with 50 mM potassium phosphate buffer (pH 7.0). The pellet was resuspended in 3 mL of the same buffer then was stored at −20 °C for further treatment. Cells were lysed by an ultrasonic cell crasher (Scientz Biotechnology Co., Ltd., Ningbo). The debris was removed by centrifugation at 10,000×g for 10 min at 4 °C. The supernatant was removed for immediate examination of enzymatic activity and protein electrophoresis. Protein concentration was measured using a pierce bicinchoninic acid protein assay kit (Sigma. St. Louis, MO, USA).
Assay of ALAS
ALAS activity was determined by the method described by Burnhan [16].The reaction mixture contained 50 mM Tris–HCl (pH 7.0), 20 mM MgCl2, 0.1 M glycine, 0.1 mM pyridoxal phosphate, 0.2 mM succinyl-CoA and cell extracts. After 10 min, 300 μL of the reaction was transferred to an Eppendorf vial with 150 μL of 10% trichloroacetic acid. The vial was centrifuged (5 min at 10,000×g, 4 °C), and 300 μL of the supernatant was transferred to another Eppendorf vial containing 400 μL 1 M sodium acetate (pH 4.6) and 35 μL acetyl acetone. This mixture was heated in boiled water bath (100 °C) for 15 min. After cooling to room temperature, 700-μL freshly prepared modified Ehrlich's reagent was added. The absorbance at 554 nm of this mixture was measured 5 min after adding Ehrlich's reagent. One unit of ALAS activity was defined as the amount of enzyme needed to produce1 μmol ALA in 1 min at 37 °C.
Assay of PBG and ALAD Activity
The concentration of PBG in the culture was analyzed using the method by Mauzerall and Granic [15]. ALAD activity was determined by the method described by Mitchell and Jaffe [17]. One unit of ALAD activity was defined as the amount of enzyme that produced 1 μmol of PBG in 1 h at 37 °C.
SDS-PAGE Analysis
The proteins of cell extracts were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). A 12% (w/v) separation slab gel was prepared by the method of Laemmli [18]. Proteins were stained with Coomassie brilliant blue G.
Results and Discussion
Effects of d-glucose on the Activity of ALAS and ALA Production
Initial d-glucose concentration is an important operational parameter for cell growth and ALA production. The results of our previous research showed that d-glucose efficiently inhibited the ALAS activity (Fig. 1) [19]. The soluble ALAS protein expressions were analyzed by SDS-PAGE electrophoresis (Fig. 2); the results showed that ratio of soluble ALAS to total soluble protein of the strain decreased as the initial concentration of d-glucose increased, which indicated that the repression of gene expression by d-glucose may be one of the main reasons for the inhibition of ALAS activity, which needs to be investigated further.
The data also indicated that higher initial concentration of d-glucose led to higher yields of ALA at the end of stationary phase (Fig. 3), suggesting that the inhibitory effect of d-glucose on ALAS activity possibly is relieved with the metabolism of d-glucose. It is well known that d-glucose is metabolized into succinic acid by tricarboxylic acid cycle during aerobic metabolism. Succinic acid, as precursor of ALA biosynthesis, could elevate the yields of ALA [19, 20].
d-Glucose Affects the Activity of ALAD and the Yield of ALA
Inhibition of ALAD activity is essential for the high production of ALA. It is well known that LA is a competitive inhibitor of ALAD in various organisms [9, 21]. In this work, we investigated whether d-glucose could replace LA as inhibitor of ALAD and improve the yield of extracellular ALA in recombinant E. coli BL21 (DE3) pET28a-A.R-hemA culture.
Firstly, the adding time of d-glucose and LA was optimized. As shown in Fig. 4, the optimal adding time was at the end of the exponential cell growth phase (at 8 h of cell cultivation) for both inhibitors, which is consistent with the results reported [9, 21]. Then, the d-glucose and LA concentration dependencies of ALA accumulation were investigated (Table 1).
As shown in Table 1, d-glucose and LA could both improve the extracellular ALA yields, which agreed with the results of Choi et al. [9], Qin et al. [19], Lee et al. [21], and Akihiko et al. [22]. Thirty, 40, or 50 mM LA enhanced the yields of extracellular ALA to nearly the same levels, suggesting that ALAD was not sensitive to this inhibitor beyond 30 mM. As shown in Fig. 5, the inhibition of ALAD activity was directly related to the concentration of d-glucose. With 1 g/L d-glucose, only 50% of the activity of ALAD remained. When concentration of d-glucose reached to 5 g/L, 90% of ALAD activity was eliminated. These data indicated that the inhibitory effect of low concentration of d-glucose on ALAD activity was stronger than on the activity of ALAS.
Simultaneously, d-glucose increased the accumulation of ALA in media with shorter culture time compared to the culture using LA as inhibitor of ALAD, which indicated that d-glucose could replace LA as an effective inhibitor of ALAD. The effect of d-glucose on inactivation of ALAD might be the differential affinity of the d-glucose for lysine groups or near the active site and the hydrophobicity of the active-site environment [23, 24] and the side effects of the free radicals formed by d-glucose autoxidation on the enzyme activity [25]. On the other hand, low concentration of d-glucose induced higher qALA production than high concentration of d-glucose did in shake flask. This suggests that low concentration of d-glucose can lead to faster synthesis rate of ALA. Our results showed that, though d-glucose repressed activities of both ALAS and ALAD (i.e., repressing both the synthesis and degradation of ALA), low-concentration d-glucose may favor the overall reaction balance to ALA synthesis.
Cultivation in a Stirred-Tank Fermenter
Considering the distinction between tank fermenter and shake-flask culture on aspects such as aeration, agitation, and pH (controlled in fermenter and not in flask), we redid the above experiments in a tank fermenter to check any possible discrepancies.
According to the results above, the optimal initial concentration of d-glucose in the medium should be 2.0 g/L. And the optimal concentrations of two categories of precursors (succinate and glycine) were 10.0 g/L and 2.0 g/L, respectively, based on our previous results [20]. One batch was done without any inhibitor supplement; the second batch was with 30 mM LA, and third batch was with 1.0 g/L d-glucose at the end of exponential phase (Fig. 6).
Fermentation medium was: 5 g/L yeast extract, 10 g/L tryptone, 5 g/L KH2PO4, 2 g/L d-glucose, 2 g/L glycine, 10 g/L succinic acid, and 30 mg/L kanamycin. pH was controlled at 6.5. Both inhibitors of ALAD (LA, d-glucose) are supplied at 8 h of fermentation, respectively.
Both inhibitors, 30 mM LA and 1.0 g/L d-glucose, increased cell density and the yields of extracellular ALA because they were both used as carbon source for cell growth and inhibitors of ALAD. The measurements indicated that d-glucose and glycine were consumed completely in the broth after 12 h of fermentation (data unlisted).
Based on the results above, a new fed-batch strategy was employed. We continuously supplied a mixture containing 50 g/L d-glucose and 10 g/L glycine at 30-mL/h flow rate from the end of exponential phase (Fig. 7). By fed-batch process, the concentration of extracellular ALA reached 3.1 g/L, which is higher than LA as the inhibitor of ALAD [19].
Due to low concentration of d-glucose provided, no obvious accumulation of acetic acid was found. Because d-glucose can be used as inhibitor of ALAD and good carbon source for cell growth, it was obvious that when d-glucose was supplied, the high cell density was achieved and the yields of ALA reached 3.1 g/L, indicating that d-glucose can completely replace LA as inhibitor of ALAD.
Fermentation medium was: 5 g/L yeast extract, 10 g/L tryptone, 5 g/L KH2PO4, 2 g/L d-glucose, 2 g/L glycine, 10 g/L succinic acid, and 30 mg/L kanamycin. pH was controlled at 6.5.
Conclusion
d-glucose as an important carbon source can increase cell density in low-concentration range. d-glucose also metabolizes into succinic acid, a precursor of ALA, which can elevate the yields of ALA. Our results indicate that d-glucose inhibits the activity of both ALAS and ALAD; however, the ALAD is more sensitive than ALAS to d-glucose inhibition, leading to the accumulation of ALA in culture. Given the non-toxicity and lower cost of d-glucose compared to LA and the highest yield of 3.1 g/L extracellular ALA induced by flowing low concentration of d-glucose, this work provides persuading evidence to use d-glucose rather than LA as ALAD inhibitor for commercial manufacture of ALA.
References
Beale, S. I., & Weinstein, J. D. (1990). Biosynthesis of heme and chlorophylls, pp. 287–391. New York: McGraw-Hill.
Vladimir, Y. B., Demain, A. L., & Zaitseva, N. I. (1997). Critical Reviews in Biotechnology, 17, 21–37. doi:10.3109/07388559709146605.
Sasikala, C. H., Ramana, V., & Raghuveer, R. P. (1994). Biotechnology Progress, 10, 451–459. doi:10.1021/bp00029a001.
Hotta, Y., & Watanabe, K. (1999). Chemical Regulation of Plants, 34, 85–96.
Chen, H. M., Chen, C. T., Yang, H., Lee, M. I., Kuo, M. Y., Kuo, Y. S., et al. (2005). Journal of Oral Pathology & Medicine, 34, 253–256. doi:10.1111/j.1600-0714.2004.00267.x.
Tobias, J. B., Marius, B., Saulius, B., Zita, K., Wolfgang, B., Ronald, S., et al. (2007). Journal of Photochemistry and Photobiology B, Biology , 87, 174–182. doi:10.1016/j.jphotobiol.2007.03.008.
Jordan, P. M. (1991). Biosynthesis of tetrapyrroles, vol. 19: New comprehensive biochemistry, pp. 1–24. Amsterdam: Elsevier.
Sasaki, K., Ikeda, S., Nishizawa, Y., & Hayashi, M. (1987). Journal of Fermentation Technology, 65, 511–515.
Choi, C., Hong, B. S., Sung, H. C., Lee, H. S., & Kim, J. H. (1999). Biotechnology Letters, 21, 551–554. doi:10.1023/A:1005520007230.
Vemuri, G. N., Eiteman, M. A., & Altman, E. (2002). Journal of Industrial Microbiology & Biotechnology, 28, 325–332. doi:10.1038/sj.jim.7000250.
Lee, D. H., Jun, W. J., Shin, D. H., Cho, H. Y., & Hong, B. S. (2005). Bioscience, Biotechnology, and Biochemistry, 69, 470–476. doi:10.1271/bbb.69.470.
Alan, J. B., Keith, C., David, H., Karl, I., & Karen, S. (2002). Journal of Bacteriology, 184, 1685–1692. doi:10.1128/JB.184.6.1685-1692.2002.
Xie, L., Hall, D., Eiteman, M. A., & Altman, E. (2003). Applied Microbiology and Biotechnology, 63, 267–273. doi:10.1007/s00253-003-1388-2.
Liu, X. X., Lin, J. P., Qin, G., & Cen, P. L. (2005). Chinese Journal of Chemical Engineering, 13, 522–528.
Mauzerall, S., & Granick, S. (1956). Journal of Biological Chemistry, 219, 435–442.
Burnham, B. F. (1970). Methods in Enzymology, 17A, 195–204. doi:10.1016/0076-6879(71)17179-0.
Mitchell, L. W., & Jaffe, E. M. (1993). Archives of Biochemistry and Biophysics, 300, 169–177. doi:10.1006/abbi.1993.1024.
Laemnli, U. K. (1970). Nature, 227, 680–685. doi:10.1038/227680a0.
Qin, G., Lin, J. P., & Liu, X. X. (2006). Journal of Bioscience and Bioengineering, 102, 316–322. doi:10.1263/jbb.102.316.
Chung, S. Y., Seo, K. H., & Rhee, J. I. (2005). Process Biochemistry, 40, 385–394. doi:10.1016/j.procbio.2004.01.024.
Lee, D. H., Jun, W. J., Kim, K. M., Shin, D. H., Cho, H. Y., & Hong, B. S. (2003). Enzyme and Microbial Technology, 32, 27–34. doi:10.1016/S0141-0229(02)00241-7.
Akihiko, A., Hitoshi, F., Katsumi, N., & Yoshinori, N. (2000). Journal of Bioscience and Bioengineering, 89, 176–180. doi:10.1016/S1389-1723(00)88733-2.
Echelard, Y., Dymetryszyn, J., Drolet, M., & Sasarman, A. (1988). Nucleotide sequence of the hemB gene of Escherichia coli K12. Molecular & General Genetics, 214, 503–508. doi:10.1007/BF00330487.
Brownlee, M. (1994). Glycation and diabetic complications. Diabetes, 43, 836–841.
Jain, S., & Palmer, N. (1997). The effects of oxygen radicals metabolites and vitamin E on glycosylation of proteins. Free Radical Biology & Medicine, 22, 593–596. doi:10.1016/S0891-5849(96)00377-2.
Acknowledgments
We gratefully acknowledge Meng, F. J., Ph.D., for revising the manuscript. This study was supported by the Scientific Research Fund of the Zhejiang Provincial Education Department (no. 20070617).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X.X., Wang, L., Wang, Y.J. et al. d-glucose Enhanced 5-Aminolevulinic Acid Production in Recombinant Escherichia coli Culture. Appl Biochem Biotechnol 160, 822–830 (2010). https://doi.org/10.1007/s12010-009-8608-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8608-x