Abstract
A Bacillus subtilis (MTCC9102) isolate was shown to produce significant amount of keratinase under optimized conditions in solid-state fermentation using Horn meal as a substrate. Optimized value for moisture, inoculum, and aeration were found to be 100% (v/w), 50% (v/w), and 150% (w/w), respectively, and the optimum nitrogen source was peptone and carbon source was dextrose. Maximum keratinolytic activity was observed at 48 h after incubation, and the optimum age (24 h) of inoculum was significant. The influence of cultivation temperature and initial pH of the medium on keratinase production revealed the optimum values for the temperature and pH as 37 °C and 7, respectively. Maximum keratinase activity of the crude extract was 15,972 U/mg/ml. These results indicate that this bacterial strain shows a high biotechnological potential for keratinase production in solid-state fermentation, and use of the horn meal as the substrate can be implemented for keratinous solid wastes management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The biological solid waste let out by the by-product industries is a matter of concern for all of us. Keratinous waste like horns, feather, nails, hoofs, scales, and wools are increasingly accumulating in the environment generated from poultry and meat processing plants, slaughterhouses, tanneries, and other industries. Horn meal is available in large quantities from animal by-product processing plants. These plants turn these wastes into an interesting low-cost keratin source for biotechnological applications [1]. Keratin protein present in keratinous waste do not degrade easily by commonly known proteases like trypsin, pepsin, and papain due to presence of disulfide bonds [2, 3]. The chemical processes [4, 5] can convert these keratinous wastes into useful materials, but again, chemical processing causes environmental pollution. To overcome these situations, microbial treatments are being considered with varying degree of success. Keratinases from Bacillus sp., specially Bacillus licheniformis and Bacillus subtilis, fungi, actinomycetes, and soil microorganisms have been studied for effective degradation of keratinous waste [6, 7]. Biodegradation by these organisms offer an improved method for utilization of these waste materials into useful products like keratin hydrolysate used for tanning [8] and keratinase, and related products have many applications [9], including B. licheniformis PWD-1, which can degrade the infectious form of prions in the presence of detergent and heat treatment [10].
Solid state fermentation (SSF) can use abattoir wastes, including keratinous waste, and convert them into value-added products. It is a low-energy-consuming and environmentally friendly process and greatly reduces pollution loads [11]. Use of low-cost natural material in SSF is advantageous for countries where these kinds of solid bio-waste are generated in abundance. Slaughterhouses in India discharge directly very large quantity of solid waste every year. Today, it has become necessary for us to properly dispose this waste to protect our environment. Technologists and scientists across the word have stimulated interest in converting waste materials into commercially valuable products.
SSF cultivates microorganisms on moist solid support, either on inert carriers or insoluble substrates that can, in addition, be used as carbon and energy source. SSF takes place in the absence and near absence of free water, thus being close to the natural environment to which microorganisms are adapted [12]. The aim of SSF is to bring the cultivated microorganisms into tight contact with the insoluble substrate and thus to achieve the maximum utilization of the substrate to get maximum growth and products under optimized conditions.
Keratinase or other proteins production by microbes is influenced by number of factors such as temperature, pH, the nature of carbon and nitrogen sources, aeration present in the medium, and various methods, including the optimization of culture conditions and medium composition to improve the enzyme yield [13, 14]. These factors have varied effects on different species. Scant information is available on the factors which control the synthesis and release of extracellular keratinases. Since the keratinase production varies from organism to organism, the study on the nutritional and environmental factors controlling the keratinase production from this highly potent strain of B. subtilis is required. We report SSF technique to produce keratinase using B. subtilis MTCC 9102 on horn meal as a substrate.
Materials and Methods
Microorganisms
The bacterial culture (new B. subtilis strain MTCC9102 was isolated in our laboratory from the horn meal shown to produce significant amount of keratinase in the culture medium, including horn meal powder by liquid state fermentation) was grown in nutrient broth (HI-Media Pvt. Ltd. Mumbai, India) overnight at 37 °C and was used as an inoculum (OD 0.6 at 600 nm) for SSF.
Substrates
Horn meal (prepared at CLRI) grinded to fine powder was used as a source of keratin.
Solis State Fermentation (SSF) and Optimization of Influential Parameters
To observe the substrate supports for keratinase production, a premix (100% w/w) of substrate powder and wheat husk was added to 2.5 ml of 50 mM phosphate buffer (pH 7.4) in Erlenmeyer flask followed by autoclaving at 121 °C, 15 psi pressure for 15 min and cooled to room temperature. The flasks were inoculated with 2.0 ml of 24-h grown bacterial culture (optical density at 600 nm between 0.59 and 0.61) under sterile conditions and incubated at 37 °C temperature. Keratinase production was investigated using basic culture parameters, which includes water quality, moisture content (30%, 50%, 100%, 150%, 200%, and 300%, v/w), inoculum size (10%, 30%, 50%, 100%, 150%, and 200%, v/w), co-carbon sources (dextrose, fructose, maltose, sucrose, and lactose at 10.0%, w/w), co-nitrogen sources (NH4Cl, (NH4)2SO4, NH4HCO3, yeast extract, beef extract, and peptone at 1%, w/w), and incubation periods (24, 36, 48, 72, 120, and 144 h). To optimize the effect of aeration on keratinase production, wheat husk (50%, 100%, 150%, 200%, 250%, 300%, 350% w/w) was studied. The constant amount (5 g) of substrate (horn meal) has been taken as the reference for the entire ratio, for instance, the meaning of 100% (v/w) moisture is the mixture of 5 g of substrate in 5 ml of phosphate buffer. Uninoculated flasks served as negative controls, where as the inoculated flasks without any co-carbon or co-nitrogen source served as positive controls. In each experiment, flasks were kept in triplicate under identical conditions to minimize error. Keratinase production was expressed as mean and standard deviations from the results obtained.
Keratinase Extraction from Fermented Matter
For isolation of keratinase produced under SSF, a known quantity of fermented matter was mixed with distilled water (1:10, w/v) by stirring on a magnetic stirrer for 20 min at room temperature (25 °C). The slurry was then squeezed through cheesecloth followed by centrifugation at 10,000 × g for 10 min at 4 °C to remove the insoluble matters. The clear supernatant was used for the keratinase assay, and the keratinase recovery was expressed as total units (U) of crude keratinase per milligrams per milliliter of the crude sample proteins.
Protein Estimation and Keratinase Assay
The total protein quantification was performed by Lowry’s method [15], and keratinase activity was assayed with azokeratin [16, 17] as a substrate with some modifications. An enzyme sample (0.5 ml) was incubated with 5 mg of azokeratin in 1.5 ml of phosphate buffer (pH 6.0) at 40 °C for 25 min, and the reaction was stopped by the addition of trichloroacetic acid (final concentration, 100 g/l). After centrifugation at 10,000 × g for 10 min, the absorbance of the supernatant fluid was determined at 440 and 595 nm. The average of both wavelengths was taken as the final OD. One unit of enzyme activity was the amount of enzyme that caused a change of absorbance of 0.01 at the average of 440 nm and 595 nm in 25 min at 40 °C.
Statistical Analysis
Results are represented as mean ± SD of at least three experiments. A probability level of P < 0.05 was considered statistically significant, and the analysis was done by Student’s t test.
Results
Effect of Initial Moisture Content of the Substrate and Moistening Agent on Keratinase Production
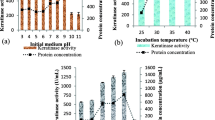
Initial moisture content and type of moistening agents of the medium have shown a great influence on keratinase production by B. subtilis MTCC-9102 strain. Out of different moistening agents, phosphate buffer (pH 7.4) gave maximum keratinase yield (Fig. 1).
With an increase in the initial moisture content of the substrate from 30% to 100%, keratinase production was concomitantly enhanced, but further increase beyond 100% resulted in a steady decline (Fig. 2).
Effect of Inoculum Size on Keratinase Production
As shown in Fig. 2, with an increase in inoculum size from 10% to 50%, keratinase production enhanced dramatically after 30%. Increasing the inoculum size between 50% and 200% resulted in the reduction in keratinase production by B. subtilis MTCC9102.
Effect of Co-carbon and Co-nitrogen Sources on Keratinase Production
As depicted in Fig. 3, optimum keratinase production was observed when SSF was carried out with dextrose (10%, w/w) as co-carbon source. Among the tested nitrogen compounds, supplementation of 1% (w/w) peptone gave optimum production followed by ammonium sulfate. Yeast extract and beef extract were found to be almost equally significant.
Effect of Aeration and Incubation Period
In all wheat husk compositions, the 150% aeration exhibited the peak keratinase production as shown in Fig. 4, followed by 200%. There is no significant production at 50% and sharp declination was found at 250%.
As shown in the Fig. 5, the peak value of keratinase production was observed at 48 h and least at 24 h with no significant activity at 72 h and others.
Keratinase Assay
The maximum keratinase activity was found to be 15,955 U/mg/ml of the total proteins present in crude samples under all optimized parameters (Fig. 5).
Discussion
The present investigation relates to a simple, novel, low-cost process for the high-level production of keratinase from B. subtilis using horn meal, a cheap source of carbon and nitrogen provided in SSF. Although some of solid substrates have been used for the production of bacterial keratinase, use of horn meal for keratinase production has been reported here for the first time in SSF. The major advantage of the present method of keratinase production is the possible commercial application of horn meal, which is available in large quantity and, at present, finds limited usage. Utilization of horn meal as fermentation substrates by microorganisms offers a low-cost microbial technology for obtaining proteolytic enzymes coupled with environment protection. The cost of horn meal production (large scale—above 100–150 tons/month) in India roughly comes to Rs 7–8/kg (manufacturer and exporter: P. Subbarj & Company, Chennai, India). Due to its large availability, horn meal is a potentially viable substrate for the keratinase production in SSF from B. subtilis MTCC9102.
To avoid the dilution factors by the optimization of moisture content and inocula size on the enzyme yield, we have expressed the enzyme activity in the terms of total unit (U) per milligram per milliliter of total crude proteins of the extract. To maintain the optimized moisture content at 100%, we used centrifuged inocula with appropriate dilution. The biochemical characterizations of crude enzyme were found to be same as previously reported by us [18], and the keratinase produced under optimized influential parameters has shown maximum enzyme activity of 15,955 U/mg/ml.
In most of the cases, the time required for the optimum keratinase production by bacteria or fungus may be as long as 48 h to 9 days [19, 20], but in the present study, the keratinase production by B. subtilis strain MTCC9102 on horn meal has demonstrated that optimum keratinase production occurred within a short duration (post 36 h–72 h of incubation), with optimum production within 48 h of incubation.
The major crucial factor in SSF system that influences the microbial growth and product yield is the initial moisture content of the substrate [21, 22]. Since growth of microbes and product formation takes place at or near the surface of moist solid substrate [23], maximum yield of keratinase requires optimization of the moisture content. For example, in the present study, after optimization of moistening agents, 100% initial moisture (phosphate buffer, pH 7.4) content of the substrate was found to be optimum for keratinase production by B. subtilis MTCC9102, whereas 50% and 150% initial moisture contents, respectively, although lesser, but was significant for keratinase production in SSF. Although the effect of phosphate on the keratinase production may not be ignored, it can be correlated that 50 mM phosphate buffer at 100% of the substrate was optimum for the keratinase production by B. subtilis MTCC9102. However, depending upon the type of microorganisms and type of substrates, the initial moisture content for optimum desired yield may vary. For example, Uyar and Baysal [24] reported 30% moisture level of wheat bran is optimum for alkaline protease production by Bacillus sp., whereas optimal moisture level was reported to be 74% with wheat bran for protease production by Pseudomonas sp. [25], and in our present study, 40% moisture level with reference to total solid matter (horn meal and wheat husk) was found to be optimum. A reduction in keratinase production with further increase in moisture content beyond 100% may be due to decrease in porosity and/or air content of the substrate causing interference with the microbial activity [26]. This is validated from our current optimization work of the aeration, which showed maximum keratinase production at 150% of wheat husk followed by at 200%.
The inoculum optimization parameter should also be given a proper consideration because it played a crucial role in enzyme production. In the present study, 50% of inoculum (optical density at 600 nm 0.59 ± 0.05) was found to be suitable for optimum keratinase production by B. subtilis MTCC9102 strain on horn meal, whereas Uyar and Baysal [24] reported a 20% and 25% (v/w) inoculum level requirement for the production of alkaline protease by a Bacillus sp. when grown on wheat bran and lentil husk, respectively, and these work support our results, which shows that 20% inoculum size with respect to solid matter is optimum. However, with the increase in inoculum level beyond 50%, the production of keratinase by B. subtilis MTCC9102 declined. This might be due to exhaustion of nutrients in the fermentation mash.
The choice of nitrogen and carbon sources has a major influence on the yield of enzymes. The findings from other laboratories suggested that different bacteria have different preferences for either organic or inorganic nitrogen for growth and enzyme production, although complex nitrogen sources are usually used for alkaline protease production [21, 27]. B. subtilis MTCC9102 strain used in the present study has shown a preference for organic nitrogen sources compared to inorganic nitrogen for keratinase production, but surprisingly, this strain gave ammonium sulfate as second preference, which we are unable to explain. In a sharp contrast to these observations, organic nitrogen sources like peptone and yeast extract were found to suppress the protease production of an alkaliphilic strain of Arthrobacter ramosus MCM B351 [28]. Chen et al. [29] described complete inhibition of the extracellular protease production from Geobacillus caldoproteolyticus strain SF03 in presence of glucose, a versatile source of carbon; however, present study shows that keratinase synthesis is enhanced when dextrose and other carbohydrates are supplied as co-carbon source to the fermentation medium. This observation is in accordance with the report of Prakasham et al. [21] describing no repressive effect of glucose on enzyme production by Bacillus sp. The data presented in the current study show that optimum keratinase production by B. subtilis MTCC9102 is supported when the optimized parameters were being combined together as shown in the results.
Conclusion
B. subtilis used in the present study could utilize horn meal (biological keratinous solid wastes) as low-cost waste residues for keratinase production in SSF. The keratinase produced by B. subtilis is free of undesirable flavor showing its advantages if used in leather, food, detergent, cosmetics, and pharmaceutical industries. The keratinase production in SSF by using horn meal or other keratin containing bio-wastes will help to treat this waste with cheap production of pharmacokinetic proteins.
References
Karthikeyan, R., Balaji, S., & Sehgal, P. K. (2007). Industrial application of keratins—A review. Journal of Scientific and Industrial Research, 66, 710–715.
Gortner, R. A., & Hoffman, W. F. (1941). L-cystine. Organic Syntheses, 1, 194–198.
Papadopoulos, M. C. (1986). The effect of enzymatic treatment on amino acid content and nitrogen characteristics of feather meals. Animal Feed Science and Technology, 16, 151–156. doi:10.1016/0377-8401(86)90058-1.
Corfield, M. C., & Robson, A. (1955). The amino acid composition of wool. The Biochemical Journal, 59, 62–68.
Parry, D. A. D., Crewthwr, W. G., Fraser, R. O. B., & MacRae, T. P. (1977). Structure of O-keratin structural implication of the amino acid sequence of the type I and type II chain segments. Journal of Molecular Biology, 113, 449–454. doi:10.1016/0022-2836(77)90153-X.
Manczinger, L., Rozs, M., Vagvolgyi, C. S., & Kevei, F. (2003). Isolation and characterization of a new keratinolytic Bacillus licheniformis strain. Word Journal of Microbiology and Biotechnology, 19, 35–39.
Thys, R. C. S., Lucas, F. S., Riffel, A., Heeb, P., & Brandelli, A. (2004). Characterization of a protease of a feather-degrading Microbacterium species. Letters in Applied Microbiology, 39, 181–186. doi:10.1111/j.1472-765X.2004.01558.x.
Balaji, S., Karthikeyan, R., ChandraBabu, N. K., & Sehgal, P. K. (2008). Microbial degradation of horn meal with Bacillus subtilis and its application in leather processing. Journal of American Leather Chemist Association, 103(3), 89–93.
Gupta, R., & Ramnani, P. (2006). Microbial keratinase and their prospective application: An overview. Applied Microbiology and Biotechnology, 70(1), 21–33. doi:10.1007/s00253-005-0239-8.
Langeveld, J. P. M., Wang, J. J., Van de Wiel, D. F. M., Shih, G. C., Garssen, G. J., Bossers, A., et al. (2003). Enzymatic degradation of prion protein in brain stem from infected cattle and sheep. The Journal of Infectious Diseases, 188(11), 1782–1789. doi:10.1086/379664.
Sandro, G., Pandy, A., Osaku, C. A., Rocha, S. N., & Soccol, C. R. (2003). Characterization and stability of proteases from Penicillium sp. produced by solid-state fermentation. Enzyme and Microbial Technology, 32, 246–251.
Holker, U., Hofer, M., & Lenz, J. (2004). Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Applied Microbiology and Biotechnology, 64, 175–186.
Ramnani, P., & Gupta, R. (2004). Optimization of medium composition for keratinase production on feather by Bacillus licheniformis RG1 using statistical methods involving response surface methodology. Biotechnology and Applied Biochemistry, 40(11), 191–196.
Anbu, P., Gopinath, S. C. B., Hilda, A., Priya, L. T., & Annadurai, G. (2005). Purification of keratinase from poultry farm isolate—Scopulariopsis brevicaulis and statistical optimization of enzyme activity. Enzyme and Microbial Technology, 36(5/6), 639–647.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Letourneau, F., Soussolte, V., Bressollier, P., Branland, P., & Verneuil, B. (1998). Keratinolytic activity of Streptomyces sp.S.K1-02: A Newisolated strain. Letters in Applied Microbiology, 26, 77–88.
Sangali, S., & Brandelli, A. (2000). Isolation and characterization of a novel feather—Degrading bacterial strain. Applied Biochemistry and Biotechnology, 83, 17–24.
Balaji, S., Senthil Kumar, M., Karthikeyan, R., Kumar, R., Kirubanandan, S., Sridhar, R., et al. Purification and characterization of an extracellular keratinase from a hornmeal-degrading Bacillus subtilis MTCC (9102). World Journal of Microbiology and Biotechnology, 24, 2741–2745.
Aikat, K., & Bhattacharyya, B. C. (2000). Protease extraction in solid-state fermentation of wheat bran by a local strain of Rhizopus oryzae and growth studies by the soft gel technique. Process Biochemistry, 35, 907–914.
Puri, S., Beg, Q. K., & Gupta, R. (2002). Optimization of alkaline protease production from Bacillus sp. by response surface methodology. Current Microbiology, 44, 286–290.
Prakasham, R. S., Subba Rao, C. h., & Sarma, P. N. (2006). Green gram husk—An inexpensive substrate for alkaline protease production by Bacillus sp. In solid-state fermentation. Bioresource Technology, 97, 1449–1454.
Ramachandran, A. K., Patel, K. M., Nampoothiri, F., Francis, G., & Pandey, A. (2004). Coconut oil cake—A potential ray material for the production of alpha-amylase. Bioresource Technology, 93, 169–174.
Pandey, A., Soccol, C. R., & Mitchell, D. (2000). New developments in solid-state fermentation. I. Bioprocesses and applications. Process Biochemistry, 35, 1153–1169.
Uyar, F., & Baysal, Z. (2004). Production and optimization of process parameters for alkaline protease production by a newly isolated Bacillus sp. Under solid-state fermentation. Process Biochemistry, 39, 1893–1898.
Chakraborty, R., & Srinivasan, M. J. (1993). Production of a thermo stable alkaline protease by a new Pseudomonas sp. by solid-substrate fermentation. Journal of Microbiology and Technology, 8, 7–16.
Gautam, P., Sahu, A., Pandey, A., Szackaes, G., & Soccol, C. R. (2002). Microbial production of extracellular phytase using polystyrene as inert support. Bioresource Technology, 83, 229–233.
Pandey, A., Soccol, C. R., Nigam, P., Brand, D., Mohan, R., & Roussos, S. (2000). Biotechnological potential of coffee pulp and coffee husk for bioprocess. Biochemical Engineering Journal, 6, 153–162.
Nilegaonkar, S. S., Kanekar, P. P., Sarnaik, S. S., & Kelkar, M. S. (2002). Production, isolation and characterization of extra cellular protease of an alkaliphilic strain of Arthrobacter ramosus, MCM B-351 isolated from the alkaline lake of Lonar, India. World Journal of Microbiology & Biotechnology, 18, 785–789.
Chen, X. G., Stabnikova, O., Tay, J. H., Wang, J. Y., & Tay, S. T. L. (2004). Thermoactive extracellular proteases of Geobacillus caldoproteolyticus, sp. nov, from sewage sludge. Extremophiles, 8, 489. —of Geobacillus 498.
Acknowledgments
The author Ramadhar Kumar acknowledges CSIR, Government of India for the financial assistance in the form of Junior Research Fellowship. S. Balaji gratefully acknowledges the financial support received from NMITLI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R., Balaji, S., Uma, T.S. et al. Optimization of Influential Parameters for Extracellular Keratinase Production by Bacillus subtilis (MTCC9102) in Solid State Fermentation Using Horn Meal—A Biowaste Management. Appl Biochem Biotechnol 160, 30–39 (2010). https://doi.org/10.1007/s12010-008-8452-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8452-4