Abstract
In the present work, a simple, fast, and highly sensitive chemiluminescence enzyme immunoassay for 17β-estradiol (E2) in environmental water samples was developed, using magnetic particles (MPs) labeled with secondary antibody as both the immobilization matrix and the separation tools. The specific anti-E2 polyclonal antibody (PcAb) was produced against a conjugate of estradiol–bovine serum albumin. The specificity of the anti-E2 antibody was studied. The results showed that the antibody did not cross-react with the structurally related endocrine-disrupting compounds, including estrone, ethinyl E2, estriol, E2-17-glucuronide, E2-3-sulfate-17-glucuronide, androstenedione, and dihydrotestosterone. The water samples were pretreated with solid-phase extraction using C18 cartridges for the removal of matrix effects. Several physicochemical parameters including the dilution ratios of E2-6–horseradish peroxidase conjugate and anti-E2 PcAb, immunoreaction time, volume of chemiluminescent substrate and MPs, chemiluminescence reaction time, and pH of assay solution were studied and optimized. At optimal experimental conditions, it was found that the proposed method exhibited high performance with detection limit of 2.0 pg/mL, linear range of 20–1,200 pg/mL, and total assay time of 45 min. Both inter- and intra-assay coefficient of variation were less than 10%. The average recoveries of three different spiked concentration samples ranged from 86.3% to 108%. The method was successfully applied to the determination of E2 in river, waste, and tap water, and showed a good correlation with the commercially available radioimmunoassay kit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, a number of chemicals including natural and synthetic steroid hormones, phytoestrogens, pesticides, surfactants, and polychlorinated biphenyls had entered into the environment [1] and interfered with normal endocrine function of both wildlife and humans. Among these, estrogen, 17β-estradiol (E2; 1,3,5 (10)-estratriene-3,17β-diol), has been found in many aquatic environments and defined as endocrine disruptor chemicals [2, 3]. Several adverse effects, including decreased sperm count, testicular [4], breast cancers [5], and prostate cancers [6], endangered the human life due to E2. Therefore, analyzing E2 from environmental samples is of growing importance.
The very low concentration of E2 in the aquatic environment has been reported [7, 8], requiring sophisticated methods for quantitation. During the last years, many efforts have been devoted to the development of analytical methodologies for the detection of E2 in environmental samples. Several protocols with high sensitivity and specificity based on gas chromatography–mass spectrometry [9], liquid chromatography-tandem mass spectrometry [1, 10], and ultra performance liquid chromatography-quadrupole time of flight mass spectrometry [10] have been proposed. However, these methods suffer from disadvantages in respect of intensive labor, expensive instrumentation, and requiring highly trained personnel, which prevent these methods from broader application, especially in the filed monitoring. In contrast, immunoassay methods are cost effective and easy to use, exhibiting good potential in widespread application. Up to now, the enzyme-linked immunosorbent assay (ELISA) [8, 10], radioimmunoassay (RIA) [11], fluoroimmunoassay (FIA) [12], and chemiluminescence enzyme immunoassay (CLEIA) [13] have been reported. ELISA has the unavoidable drawbacks of relatively long assay time resulted from long-term immunoreaction and color development. RIA suffers from the potentially harmful effect of the radioactive 125I to the operators, restricting its usage in specialized laboratories and, therefore, beyond field monitoring. The lanthanide labels in FIA are susceptible to outside interference, and the sensitivity of FIA is also limited. CLEIA has been widely used in environmental analysis due to its high sensitivity and wide dynamic range. Zhao and Lin [13] reported a microplate-based CLEIA for E2 in water by using magnetic particles. But the assay time was as long as 70 min, showing poor ability in the high-throughput screening. In addition, the volume of microplate well was limited, spattering of immunoreagents might occur during the washing, shaking, or separation process, resulting in errors in the analysis.
In recent years, due to the increasing number of clinical samples that have to be measured especially in combination with high-throughput screening, magnetic particles (MPs)-based immunoassay has been widely used, enabling separation of the bound and free proteins by application of a magnetic field [14–18]. Based on this, we reported a tube-based CLEIA for E2 derived from competitive reaction using magnetic particles (MPs) labeled with secondary antibody and horseradish peroxidase (HRP)-conjugated to E2. It was found that in such a homogenous system of a bigger volume, the mass transfer distance of analytes and reagents to the immobilized antibody is greatly reduced and, consequently, the antibody–antigen binding equilibrium can be achieved more rapidly than when antibodies are immobilized on the planar surface, such as microplate wells. The selective separation of captured proteins or immunocomplex from the bulk can be achieved efficiently by the application of a magnetic field. The reaction parameters of the present MPs-CLEIA were studied and optimized. The analytical characteristics were investigated. The detection limit was 2.0 pg/mL, linear range was 20–1,200 pg/mL, and total assay time was 45 min. Comparing with reported methods of Zhao and Lin [13], both the total assay time was decreased and the sensitivity was improved.
The development of our assays was initially designed to enable immunoassay with high sensitivity, high rapidity, and high-throughput screening ability for environmental water analysis. Applicability of the assay was evaluated through analyzing river, waste, and tap water samples. The methodological potential shown here might be readily available for the fabrication of MPs-based E2 assay that will be well accommodated in the fully automated immunoassay system that is being used in the on field water monitoring.
Experimental
Apparatus
A universal luminometer reader (chain-based flash in glow device from Berthold Technologies GmbH & Co. KG, Germany) with test tubes (10 mm diameter × 50 mm length) that could be placed into the luminometer was used for the particle-based chemiluminescence detection. The incubation and shaking procedures at 37°C were carried out at a thermostatic culture oscillator (ZHWY-100, Shanghai Zhicheng Analytical Instrument Manufacturing Co. Ltd., Shanghai, China). The magnetic separator was obtained from Beijing ChemClin Biotech. Co. Ltd. (Beijing, China). Data acquisition and treatment were performed with an integrated 16-bit microprocessor system.
Chemicals and Immunoreagents
The immunomagnetic microparticles coated with donkey anti-rabbit antibody, the commercial RIA kit for E2, and chemiluminescent substrate were all obtained from Beijing ChemClin Biotech. Co. Ltd. The solution of HRP-labeled E2 derivative (E2-6–HRP) and E2 antigen were obtained from Fitzgerald (USA). Bovine serum albumin (BSA) was from Sigma–Aldrich (St. Louis, MO, USA).
Buffers and Calibrators
Highly purified distilled and deionized water were used throughout. The washing solution was 0.05 mol/L phosphate buffer solution with 0.5% saline and 0.05% (w/v) Tween-20 (PBST), pH 7.38. The calibrator matrix was 0.05 mol/L phosphate buffer solution containing 1.0% (w/v) BSA and 0.5% (w/v) hydrolyzed gelatin, pH 7.38. The anti-E2 PcAb dilution buffer was 0.01 mol/L calibrator matrix solution containing 0.9% (w/v) NaCl, pH 7.38. Chemiluminescent substrate solution is luminol, H2O2, and p-iodophenol solution.
The E2 stock solution of 10 μg/mL was prepared in 50% methanol solution and stored at −20°C. For calibration, a serial dilution of the stock solution was prepared in fresh calibrator matrix for 0, 20, 60, 200, 600, and 1,200 pg/mL, designated as S0, S1, S2, S3, S4, and S5, correspondingly. The prepared calibrators were stored at 4°C.
Preparation and Purification of Anti-E2 PcAb
The complete antigen E2–BSA conjugate was prepared according to the previous methods [19]. The purified E2–BSA protein was injected into rabbits to produce the PcAb [20]. Briefly, two New Zealand white rabbits were immunized intravenously with 400 μg of the E2–BSA protein per rabbit and followed by a second immunization of 200 μg per rabbit 4 weeks later. After the second injection, three additional injections (200 μg protein per injection) were performed at 2-week intervals. Three weeks after the last injection, sera were collected and used to test the anti-E2 antibody. To purify the prepared anti-serum specific to E2 polyclonal antibody, IgG was fractionated from the rabbit anti-serum by precipitation with 40% saturated ammonium sulfate at 4°C. After desalinization, the flow-through was collected and stored at −20°C for the subsequent assay.
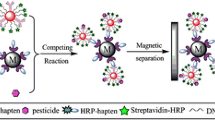
Magnetic Particle-based Chemiluminescence Enzyme Immunoassay (MPs-CLEIA)
Immunoassay procedures are presented in Fig. 1. First, 50 μL E2 calibrators or samples were added into the test tubes. Then 100 μL E2-6–HRP conjugate (dilution ratio of 1:4,000) and 100 μL rabbit anti-E2 PcAb (dilution ratio of 1:7,500) were added stepwise and incubated with gentle shaking at 37°C for 15 min. After the competitive reaction, 200 μL donkey anti-rabbit antibody-coated MPs were added and incubated at 37°C for another 10 min (capture time). Then the samarium–cobalt magnet was inserted under the test tube rack for separation. The antibody-coated MPs and any specific captured substances were attracted by the magnets to the bottom of the test tubes and unwanted substances remained in the solution and removed by gently tapping the test tubes against tissue paper. During the washing steps, 500 μL washing solution was added into the test tubes by placing them outside the magnet for five times, so the MPs were resuspended in the washing solution. Finally, 250 μL CL substrate solution was added, the mixture was incubated for 10 min at 37°C (in the dark), and the emitted photons were measured.
Radioimmunoassay (RIA)
The immunoassay procedures followed the details on the product introduction. Immunobeads coated with donkey anti-rabbit antibody was used as the solid phase. In short, 50-μL calibrators or water samples were added into the assigned test tubes. Then, 100 μL 125I tracer solution and anti-E2 PcAb were added to the bottom, respectively. The test tubes were incubated at 37°C for 50 min. After that, MPs were added to each tube, and the test tube rack was shaken to mix well and incubated at room temperature for 10 min. Finally, the deposited MPs were transferred to the clean tubes and placed into a gamma counter to measure the radioactive counts for 30 s.
Data Analysis
Standards and samples were measured in double tubes and CL intensity values were integrated. Standard curves were obtained by plotting CL intensity against the logarithm of analyte concentration and fitted to the equation of logitY − logX, in which the value of logitY was calculated according to the formula as follows:
S0 is the chemiluminescence intensity (in relative light unit, RLU) of zero calibrator, and S is the chemiluminescence intensity of other calibrators or samples. X means the analyte concentration.
Determination of Cross-reactivity
The cross-reactivity (CR) of anti-E2 PcAb was evaluated using several endocrine-disrupting compounds structurally related to E2. E2 concentration causing 50% inhibition (IC50) was used to calculate the cross-reactivity according to the equation:
Sample Preparation
The river water was collected from Qinghe River, Beijing. The waste water was collected from living area of Hangtian Cheng, Beijing. River and waste water samples were collected in brown glass bottles, stored at 4°C, and analyzed within 24 h. The tap water was obtained from our laboratory. The water samples and the spiked samples were pretreated with solid-phase extraction (SPE) because the excreted products of estrogens by human and animals were mainly glucuronic acid and sulfate, which could be degraded by bacteria and then reconverted to free steroids, exhibiting biological activity [19].
The SPE process was as follows: Firstly, 500-mL samples were filtered through a 0.45 μm GF/C glass microfiber filter. Then, the filtrate was adjusted to pH 7.0 for decreasing retention of humic acids on the sorbent [8]. After that, the filtrate was extracted with C18 cartridges using a visiprep system (Supelco). Prior to extraction, cartridges were conditioned sequentially with 5 mL of hexane, 5 mL of ethylacetate, 5 mL of methanol, and 10 mL of water. Then, samples were passed through the cartridges at a flow rate of 5.0 mL/min with the aid of a vacuum manifold. After the filtrate was loaded to the SPE cartridges, the cartridges were dried for 30 min under a gentle stream of nitrogen gas. The analytes was eluted using 6 mL of a mixture ethyl acetate–methanol (5:1, v/v). Finally, the elution was evaporated under a gentle stream of nitrogen gas and the residue dissolved in 500 μL of 50% methanol. For the MPs-CLEIA measurement, the final extract was diluted to a methanol content of 5% by 0.05 mol/L phosphate buffer solution containing 1.0% (w/v) BSA and 0.5% (w/v) hydrolyzed gelatin, pH 7.38.
Results and Discussion
Optimization of Immunoreaction Reagents
In general, the amounts of immunoreaction reagents are key parameter affecting the sensitivity and specificity of a competitive reaction. Therefore, in this experiment, the dilution ratios of anti-E2 PcAb and E2-6–HRP were investigated and optimized. As shown in Fig. 2, in the calibrator range of 20–1,200 pg/mL, when the dilution ratio of anti-E2 PcAb was lower than 1:7,500 (lower dilution ratio corresponds to higher concentration), the inhibition ratio, namely RLUS1/RLUS0, was high, indicating a low sensitivity. Empiristically, in a competitive reaction, the inhibition ratio of RLUS1/RLUS0 at about 85% means not only a good separation and even distribution of calibrator points on the dose–response curve but also a proper detection sensitivity. Therefore, a dilution ratio of 1:7,500 was selected for anti-E2 PcAb, corresponding to 86.2% inhibition. Meanwhile, for the dilution ratio of E2-6–HRP, when it was changed from 1:1,000 to 1:10,000, the RLUs decreased in the whole examination range, and 85.9% inhibition was obtained when using dilution ratio of 1:4,000, indicating a good detection limit. Thus, a dilution ratio of 1:4,000 was chosen for E2-6–HRP conjugate.
Optimization of the dilution ratios of anti-E2 PcAb and the HRP–E2 conjugate. a Optimization of dilution ratio of anti-E2 PcAb (i.e., 1:5,000, 1:6,000, 1:7,000, 1:7,500, 1:8,000, and 1:10,000). b Optimization of dilution ratio of HRP–E2 conjugate (i.e., 1:1,000, 1:2,000, 1:4,000, 1:6,000, 1:8,000, and 1:10,000). Detection conditions: immunoreaction time of 15 min, room temperature
Optimization of Physicochemical Parameters
The application of the proposed method to the environmental analysis requires the consideration of several physicochemical parameters such as immunoreaction time, volume of chemiluminescent substrate and MPs, chemiluminescence reaction time, pH, etc. The RLUs and inhibition ratio were also used to obtain the optimized values.
Optimization of Immunoreaction Time and Volume of Chemiluminescent Substrate
The immunoreaction time is one of the crucial parameters affecting the sensitivity of an immunoassay. Effect of immunoreaction time from 3 min to 30 min at 37°C was investigated and the results are shown in Fig. 3. As could be seen in Fig. 3, at each tested CL substrate volume, RLU increased with increasing immunoreaction time up to 15 min, indicating an equilibrium between antibody and antigen. Longer immunoreaction time resulted in RLU decreasing because disassociation of the immunocomplex. Therefore, immunoreaction time of 15 min was selected. Meanwhile, Fig. 3 also showed the variation of RLUs as a function of the volume of the chemiluminescent substrate. RLUs increased in the substrate volume range from 100 μL to 250 μL and reached a plateau at 250 μL. Thus, 250 μL CL substrate was chosen for the assay.
Optimization of Volume of MPs
The appropriate amount of MPs was critical for the sensitivity and homogeneity of the analytical system. It might lead to low sensitivity because excessive amount of MPs could absorb the emitted light inevitably. Therefore, the volume of MPs was investigated and the results are shown in Fig. 4. As can be seen in Fig. 4, RLU increased with the increasing volume of MPs and reached a maximum at 200 μL, corresponding to an inhibition of 85.4%. Larger volume of MPs brought decreased RLU and inappropriate inhibition. Therefore, the following experiment was executed with the 200 μL of MPs.
Chemiluminescence Reaction Kinetics
The chemiluminescence reaction time after adding chemiluminescent substrate to the solution containing immunocomplex was optimized according to the chemiluminescence kinetics curves, which was shown in Fig. 5. As could be seen, RLU increased with the increasing incubation time in the range of 0–10 min, kept a plateau in the range of 10–20 min, and then went down thereafter, indicating the sufficient interaction between substrate and enzyme. Hence, chemiluminescence reaction time of 10 min was chosen in the experiment.
Effect of pH
pH of solution is one of the most important impact factors affecting the sensitivity of an immunoassay, which was investigated and shown in Fig. 6. As can be seen in Fig. 6, RLUS0 showed a maximum at pH 7.4, and the ratio of RLUS1/RLUS0 also reached an appropriate inhibition at 86.4%. This phenomenon may be explained by the isoelectric point of the antibody. Therefore, pH 7.4 was chosen as the optimum condition in this experiment.
Calibration and Stability of the Calibrators
Under the optimal conditions, the dose–response curves for E2 with the proposed method are shown in Fig. 7. The linear ranges determined as the concentration causing 15–85% inhibition of the maximal RLU was 20–1,200 pg/mL.
Additionally, the stability of the calibrators was also studied as an indispensable parameter of the experiment. Therefore, the calibrators were stored at 37°C and 4°C for 3, 7, and 10 days, respectively. Finally, the chemiluminescence intensity was measured. The results showed that little change in RLU was observed after 3, 7, and 10 days storage, indicating enough stability of the calibrators in any experimental condition.
Detection Limit
Detection limit is an important factor that affects the reproducibility of an assay, especially for the samples with low E2 levels. Detection limit was defined as RLU signals for ten replicates of zero concentration (S 0) minus twice the standard deviation (S.D.) of the average. Detection limit obtained from the experiment was 2.0 pg/mL.
Precision
Samples with various E2 concentrations were prepared by spiking different amounts of E2 to normal water samples. A series of samples were measured ten times within one time to obtain the intra-assay precision. Inter-assay precision was calculated by comparing value for a series of samples included in three assay times (Table 1). The results showed that the inter- and intra-assay coefficients of variation (CV) were all below 10%, indicating high reproducibility of the proposed method.
Specificity
The specificity of the proposed MPs-CLEIA was evaluated using several endocrine-disrupting compounds that structurally related to E2 and commonly coexists with E2 in environmental water (Table 2). The results in Table 2 showed that anti-E2 PcAb did not cross-react with the tested compounds, including E1, EE2, E3, E2-17-glucuronide, E2-3-sulfate-17-glucuronide, androstenedione, and DHT, with CR lower than 3%, which was absolutely acceptable in the analysis.
Analysis of E2 in Water Samples
After being extracted by SPE, the recoveries of the water samples spiked at 0, 30, 100, and 200 pg/mL were analyzed five times by the proposed MPs-CLEIA. The average of recoveries was between 86.3% and 108%, as shown in Table 3.
In addition, E2 concentrations in environmental water samples were determined simultaneously by using the proposed method and a commercially available E2 RIA kit. Two immunoassay methods showed a good correlation. The results are shown in Fig. 8. It was found that the procedures were useful for the determination of E2 at low concentration, proving that the proposed MPs-CLEIA was suitable for the evaluation of E2 in environmental water.
The purpose of this work was to propose an immunoassay method capable of showing ability in high sensitivity detection and high-throughput screening. Therefore, the comparison of the proposed MPs-CLEIA with other immunoassay methods including microplate CLEIA, ELISA, FIA, and RIA for E2 detection in terms of detection limit and total assay time was performed. The results are shown in Table 4. As can be seen, although the proposed method was not the most sensitive, it still showed acceptable detection limit for the real water samples, as indicated by Ying et al. [7] and Hintemann et al. [8]. As to the total assay time, the proposed method showed good potential in the high-throughput screening with assay time of less than 45 min. The ELISA method [10] showed relatively long assay time in spite of best sensitivity. In addition, the low sensitivity of FIA method [12] prevents it from the real sample analysis in spite of its high rapidity.
Conclusions
A chemiluminescence enzyme immunoassay using magnetic particles was constructed to monitor E2 in environmental water samples. Based on the optimized SPE procedure, the easy and precise quantitation of E2 in environmental samples was achieved with detection limit of 2.0 pg/mL, a linear range of 20–1,200 pg/mL, and a total assay time of 45 min. Compared to other typical immunoassays, the sensitivity was improved and the assay time was reduced, thus providing a valid, simple, and rapid immunoassay for screening E2 in environmental water. If accommodated into an automated operation system, running MPs-CLEIA tests on site for environmental monitoring could be realized with good sensitivity and high throughput.
References
Ingrand, V., Herry, G., Beausse, J., & Roubin, M.-R. (2003). Journal of Chromatography. A, 1020, 99–104. doi:10.1016/S0021-9673(03)00770-2.
Kavlock, R. J., Daston, G. P., DeRosa, C., Fenner-Crisp, P., Gray, L. E., & Kaattari, S. (1996). Environmental Health Perspectives, 104(Suppl. 4), 715–740. doi:10.2307/3432708.
Rose, J., Holbech, H., Lindholst, C., Norum, U., Povlsen, A., & Korsgaard, B. (2002). Comparative Biochemistry and Physiology. C, 131, 531–539.
Jensen, T. K., Toppari, J., Keiding, N., & Skakkebaek, N. E. (1995). Clinical Chemistry, 41, 1896–1901.
Krambovitis, E., Hatzidakis, G., Hatzoglous, A., Romain, S., Durand, A., & Stefanakis, A. (1995). Clinical Chemistry, 41, 48–53.
Kanagaraj, P., Vijayababu, M. R., Ilangovan, R., Senthilkumar, K., Venkataraman, P., & Aruldhas, M. M. (2007). Clinica Chimica Acta, 377, 70–78. doi:10.1016/j.cca.2006.07.030.
Ying, G. G., Kookana, R. S., & Ru, Y. J. (2002). Environment International, 28, 545–551. doi:10.1016/S0160-4120(02)00075-2.
Hintemann, T., Schneider, C., Schöler, H. F., & Schneider, R. J. (2006). Water Research, 40, 2287–2294. doi:10.1016/j.watres.2006.04.028.
Ternes, T. A., Andersen, H., Daniel, G., & Bonerz, M. (2002). Analytical Chemistry, 74, 3498–3504. doi:10.1021/ac015717z.
Farré, M., Kuster, M., Brix, R., Rubio, F., Alda, M.-J. L., & Barceló, D. (2007). Journal of Chromatography. A, 1160, 166–175. doi:10.1016/j.chroma.2007.05.032.
Snyder, S. A., Keith, T. L., Verbrugge, D. A., Snyder, E. M., Gross, T. S., & Kannan, K. (1999). Environmental Science & Technology, 33, 2814–2820. doi:10.1021/es981294f.
Colli, I., Reder, S., Bucher, S., & Gauglitz, G. (2002). Biomolecular Engineering, 18, 273–280. doi:10.1016/S1389-0344(01)00111-3.
Zhao, L. X., & Lin, J.-M. (2005). Journal of Biotechnology, 118, 177–186. doi:10.1016/j.jbiotec.2005.02.023.
Zhu, D. B., Tang, Y. B., Xing, D., & Chen, W. R. (2008). Analytical Chemistry, 80, 3566–3571. doi:10.1021/ac0713306.
Zhu, D. B., Xing, D., Shen, X. Y., & Liu, J. F. (2004). Biochemical and Biophysical Research Communications, 324, 964–969. doi:10.1016/j.bbrc.2004.09.121.
Zhu, D. B., Xing, D., Shen, X. Y., Liu, J. F., & Chen, Q. (2004). Biosensors & Bioelectronics, 20, 448–453. doi:10.1016/j.bios.2004.02.029.
Zhou, X. M., Xing, D., Zhu, D. B., & Jia, Li. (2008). Electrochemistry Communications, 10, 564–567. doi:10.1016/j.elecom.2008.01.039.
Yan, G. H., Xing, D., Tan, S. C., & Chen, Q. (2004). Journal of Immunological Methods, 288, 47–54. doi:10.1016/j.jim.2004.02.006.
Majima, K., Fukui, T., Yuan, J., Wang, G., & Matsumoto, K. (2002). Analytical Sciences, 18, 869–874. doi:10.2116/analsci.18.869.
Li, Z. L., Wang, S., Lee, N. A., Allan, R. D., & Kennedy, I. R. (2004). Analytica Chimica Acta, 503, 171–177. doi:10.1016/j.aca.2003.10.026.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program, No. 2007CB714507) and National Nature Science Foundation of China (No. 20728505).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xin, TB., Wang, X., Jin, H. et al. Development of Magnetic Particle-based Chemiluminescence Enzyme Immunoassay for the Detection of 17β-estradiol in Environmental Water. Appl Biochem Biotechnol 158, 582–594 (2009). https://doi.org/10.1007/s12010-008-8356-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8356-3