Abstract
Nonylphenol is an aromatic organic compound that has an estrogen-like effect and has a negative effect on the human endocrine system. A method has been developed for the competitive determination of nonylphenol using magnetic particles, rabbit antiserum, nonylphenol conjugate with soybean trypsin inhibitor (STI) and biotin. The principle of the analysis is the formation of immune complexes on the surface of magnetite particles due to covalent immobilization of protein G through the oriented immobilization of polyclonal antibodies from rabbit serum during a competitive reaction between the free analyte (nonylphenol) and the bound one (as part of the nonylphenol-STI-biotin conjugate) for the binding sites of specific antibodies. The detection of formed immune complexes is proposed to be carried out using a streptavidin-polyperoxidase conjugate, which makes it possible to achieve a nine-fold gain in the level of the analytical signal. The developed ELISA using magnetite particles allows us to achieve a detection limit of nonylphenol at the level of 3.8 ng/mL, which is 14.5 times lower in comparison with the classic competitive ELISA (55 ng/mL). Based on the results of the experimental work, the optimized volume of the test sample was 500 μL, which makes it possible to concentrate low-contaminated samples by 17 times.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pollution of the environment by toxic compounds poses a serious threat to both human health and natural objects. Aquatic ecosystems, in particular, face a great risk [1–3]. Industrial waste, agricultural effluent, and improper waste disposal lead to water pollution and reduce the quality of consumed water, which, in turn, result in the development of various diseases [4–6].
Endocrine disruptors, such as nonylphenol (NP), are a growing concern among a variety of aquatic toxicants. Nonylphenol is the end product of the degradation of non-ionic surfactants commonly used in textiles, agriculture, and paper industry. One of the main sources of NP is industrial wastewater, from which it goes into aquatic ecosystems [7, 11]. NP levels can be as high as 0.98 μg/kg in waters, up to 37.3 mg/m3 in surface water, and 3.85 mg/m3 in groundwater [12].
NP is highly hydrophobic, thus it can accumulate in human and animal bodies, disrupt the functioning of endocrine, reproductive and nervous system, and also increase the risk of tumor development [13–15]. The maximum allowable concentrations of NP in waters are set in the range of 0.3 to 2 μg/L [16]. Thus, control of NP content in water bodies is an important task.
To detect NP, such methods as gas chromatography, liquid chromatography, and mass spectrometry are usually used. However, those methods are characterized as laborious, time-consuming, and requiring expensive equipment [17–20]. Moreover, in natural objects, NP is often represented as a mixture of isomers with different branched structures, which makes it difficult to identify peaks on chromatograms and reduces the accuracy of determination. Therefore, simple, fast, and highly sensitive methods for detecting NP are required.
Alternatives for NP detection are immunochemical methods, for example, enzyme-linked immunosorbent assay (ELISA) [21, 22], fluorescence polarization immunoassay [23], and flow injection analysis [24, 25]. They are characterized by speed and convenience, high reproducibility, automation capability, and low cost. The development of immunochemical methods requires the production of highly specific antibodies due to the high heterogeneity of NP isomers [26, 27].
In this study, we proposed ELISA based on magnetite particles (MP) for NP detection. Since the NP content in the samples may be low, the concentration of the sample is justified. The use of MP makes it possible to simultaneously separate unbound components and concentrate the analyte, which increases the sensitivity and accuracy of the analysis.
The aim of the study is to develop ELISA for NP with the use of magnetic concentrations of samples and the separation of immune complexes containing horseradish peroxidase as a label.
MATERIALS AND METHODS
Chemical reagents. Nonylphenol, soybean trypsin inhibitor (STI), gelatin, dimethyl sulfoxide (DMSO), Tween-80, biotinamidohexanoyl-6-aminohexanoic acid N-hydroxysuccinimide ester, bovine serum albumin (BSA), conjugate of streptavidin and a polymerized horseradish peroxidase, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), formaldehyde (Sigma-Aldrich, USA). Antiserum against NP was obtained by immunizing rabbits with the NP-BSA conjugate according to the method described in [26]. We also used polyclonal goat antibodies against rabbit immunoglobulins, labeled with horseradish peroxidase, streptococcal protein G (IMTEK, Russia), a ready-to-use 3,3',5,5'-tetramethylbenzidine (TMB) peroxidase substrate based with H2O2 (Immunotech, Russia), methanol (Fluka, Switzerland) and carboxylated magnetite particles (Magsphere, UK).
Synthesis of hapten-protein conjugate. Nonylphenol was conjugated with soybean trypsin inhibitor using a method based on the Mannich reaction [26] to obtain the NP-STI conjugate. NP was dissolved in DMSO, obtaining a solution with a concentration of 33 mg/mL. 10 mg of STI was added to 1 mL of 0.1 M carbonate buffer (pH 10.0). Then, 60 μL of NP solution was added to the protein solution. After that, 100 μL of 35% formaldehyde was added, followed by incubation with stirring for 30 min at room temperature. The reaction mixture was left in a thermostat for 5 days at 37°C. The preparation was purified by dialysis against 10 mM phosphate buffer solution with 0.1 M sodium chloride, pH 7.4. The resulting conjugate was stored at 4°C.
Biotinylation of a hapten-protein conjugate. The NP-STI conjugate was mixed with biotinamidohexanoyl-6-aminohexanoic acid N-hydroxysuccinimide ester in a molar ratio of 1 : 20 and incubated for 2 hours at room temperature with stirring. The reaction product was dialyzed against 50 mM phosphate buffer (PB, pH 7.4) using Amicon Ultracel 10K centrifuge filters (Millipore, USA).
Preparation of a magnetic immunosorbent MP-protein G-IgG. 20 μL of a magnetic particle solution (1 mg/mL) was mixed with 143 μL of EDC solution (7 mg/mL), the resulting mixture was adjusted to 1 mL using 50 mM PBS (pH 7.4) and incubated for 15 min with stirring. Next, protein G was added to the resulting solution at an initial concentration of 100 μg/mL and incubated for 1 hour at room temperature with stirring. After that, 25 μL of 10% BSA was added, incubated for another 10 min with stirring, and the conjugate was purified by washing three times with PB using an external magnet. Then, 10.5 μL of antiserum against NP was added to 1 mL of the resulting conjugate, incubated for 1 hour at room temperature with stirring, and washed as described above. The resulting conjugate was stored at 4°C.
Competitive ELISA for NP. The NP-STI conjugate (1 μg/mL) in PB was added to the microplate wells (Corning Costar, USA) and incubated overnight at 4°C, and then washed three times with 50 mM PBS containing 0.05% Tween-80 (PBST). Next, 150 μL of PBS with 0.1% gelatin was added to the wells of the microplate and incubated for 30 min at 37°C, after which the microplate was washed with PBST. To prepare NP solutions, a stock methanol solution with a concentration of 1 mg/mL was used. Solutions of NP with different concentrations (from 100 μg/mL to 0.01 ng/mL) in a methanol:water mixture (1 : 4) were added to the wells, and then antiserum was added at a dilution of 1 : 5000 in PBS with 0.1% gelatin. After incubation for 1 hour at 37°C and washing the plate with PBST, an anti-species antibody-peroxidase conjugate (1 : 3000) was added and incubated for 45 min at 37°C with vigorous stirring. The microplate was washed 4 times with PBST, a solution of TMB substrate (100 μL) was added to the wells, and after 15 min of incubation at room temperature, the reaction was stopped by adding 50 μL of 0.1 M H2SO4.
The optical density of the peroxidase reaction product was measured at 450 nm using an EnSpire multimode plate reader (Perkin Elmer, USA).
Competitive ELISA for NP using MP. NP solutions were added to Eppendorf tubes at concentrations from 200 to 0.78 ng/mL in 500 μL of a methanol : water mixture (1 : 4), after which an immunosorbent based on MP was added (final concentration 28 μg/mL) and incubated 15 min. The immunosorbent was washed three times with PBST and 0.1% BSA using an external magnet. Next, the NP-STI-biotin conjugate (5 μg/mL) was added, incubated for 15 min, and washing was repeated. Then, the conjugate of streptavidin with polyperoxidase (1 : 1000) was added to the resulting solutions, incubated for another 10 min and washed as described above. At the final stage, 100 μL of TMB substrate was added to the tubes; after 15 min, MP were precipitated using an external magnet, the supernatant was collected, transferred to the wells of a microplate, and the reaction was stopped by adding 50 μL of 0.1 M H2SO4.
The optical density of the peroxidase reaction product was measured as described above.
Processing of ELISA results. The dependence of the signal–optical density (y)–on the antigen concentration (x) was approximated using the Origin program version 9.0 (OriginLab, USA) using a four-parameter sigmoid function:
where a is the maximum signal, b is the minimum signal, c (or IC50) is the inflection point, the antigen concentration that inhibits 50% of antibody binding, d is the slope of the curve at point c.
Based on the obtained function, the detection limit of the antigen was determined according to the 3σ criterion.
RESULTS AND DISCUSSION
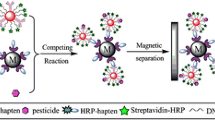
Principle of analysis. The proposed approach consists of the competitive interaction of native (contained in the sample) and conjugated antigen with a carrier protein and biotin with specific antibodies in combination with the interaction of biotin with streptavidin conjugated with a peroxidase tag. Concentration of the formed immunocomplexes from the reaction medium is carried out by adding an immunosorbent based on magnetic particles, followed by separation using an external magnet. Recording the level of peroxidase activity reflects the antigen content in the sample.
These processes are shown in Fig. 1. The protein G, which can interact with immunoglobulins (IgG) in rabbit serum, is covalently immobilized on the MP, resulting in the formation of MP-protein G-IgG complex. In these conditions, Fab fragments of antibodies on the surface of MP become open and available to interact with competitor compounds—unbound NP and NP-STI-biotin conjugate. In cases where NP is present in the sample, it interacts with antibodies and occupies the binding sites. When NP is absent in the sample, NP-STI-biotin conjugate binds with antibody. At the next stage, high-affinity binding of biotin with streptavidin takes place during addition of conjugate of streptavidin with polymerized horseradish peroxidase (Stp-pHRP) resulting in the formation of MP-protein G-IgG-NP-STI-biotin-Stp-pHRP complex. Peroxidase label provides the oxidation of the substrate (TMB) in the presence of hydrogen peroxide with the development of the colored product.
Synthesis and characterization of hapten-protein conjugate. The conjugation of NP with STI was performed using Mannich reaction in the molar ratio of 30 : 1. During the reaction, carbon of formaldehyde forms a methylene group embedded in the ortho position relative to phenolic hydroxyl. This group in the conjugate is the link between the amino group of the protein and NP. The obtained conjugate was characterized by spectrophotometry (Fig. 2). The absorption maximum of native NP is at 278 nm, forming a characteristic shoulder of the protein peak in the spectrum of hapten-protein conjugate. The calculated concentration of the conjugate by protein was 7.4 mg/mL.
Characterization of immunoreagents in competitive ELISA for NP. To evaluate the reactivity of antiserum to NP, ELISA with immobilized NP-STI conjugate in microplate wells was used [28]. The optimal dilution of antiserum was selected at 1 : 10 000, which corresponds to an optical density of 1.0 in the absence of free hapten. Further, the dependence of the signal on NP concentration was obtained in competitive ELISA (Fig. 3). The detection limit of NP amounted to 55 ng/mL, and the working range was from 200 to 1700 ng/mL. Thus, antiserum and hapten-protein conjugate have shown functional activity. Analytical performance was compared with the characteristics of developed MP-based ELISA.
Synthesis of MP-protein G-IgG conjugate. The magnetic particles used in this work are coated with a polymer with carboxylic groups. This surface modification allowed protein G to be immobilized covalently by activating the carboxyl groups. Further addition of rabbit antiserum (1 : 10 000), allowed to obtain a reagent with the direct orientation of IgG molecules.
The synthesized conjugates were characterized using transmission electron microscopy. According to the obtained data, the average diameter of the particles was 286 ± 6 nm.
Optimization of ELISA based on MP. To perform the proposed scheme of analysis with magnetic concentration, the obtained NP-STI conjugate was biotinylated with further verification of its immunochemical activity. Since NP-STI-biotin conjugate is bifunctional and must interact with both immunoglobulins in antiserum and streptavidin, the verification consisted of testing these interactions. To do so, antiserum in dilutions from 1 : 1000 to 1 : 1 000 000 was added to microplate wells with immobilized NP-STI and NP-STI-biotin conjugates (3 μg/mL), following the addition of anti-species antibodies labeled with horseradish peroxidase and registration of optical signals generated by enzyme label in immune complexes. According to the obtained data, the NP-STI conjugate before and after biotinylation was functionally active (Fig. 4). The reactivity of biotin was verified in the same procedure using streptavidin-horseradish peroxidase conjugate (Stp-HRP). As can be seen from Fig. 5a, binding of NP-STI-biotin with Stp-HRP was detected at different dilutions of the latter; however, the optical density is low. Therefore, the alternative conjugate of streptavidin with polymerized horseradish peroxidase (Stp-pHRP) was used to increase signal. Replacement with a conjugate with an increased number of enzyme tags made it possible to increase the optical density value by nine times, while maintaining a low level of nonspecific signal (Fig. 5b). Thus, we confirmed the reactivity of the NP-STI-biotin conjugate and also selected the necessary reagent dilution: 1 : 1000 for Stp-pHRP and 1 : 13 000 for Stp-HRP (Fig. 5).
The NP-STI-biotin conjugate concentration was selected varying it from 5 µg/mL to 0.6 µg/mL. The selection criterion was to achieve the highest optical density. The conjugate concentration of 5 µg/mL was chosen (Fig. 6).
At the next stage, the optimal concentration of immunosorbent based on MP was determined. For this, the interaction of the obtained conjugate (concentration from 250 to 9 µg/mL) with goat polyclonal antibodies against rabbit IgG labeled with horseradish peroxidase was registered. Figure 7 shows the dependence of optical density at 450 nm on MP concentration. The concentration of immunosorbent, corresponding to an optical density of 1.0 (coinciding with traditional ELISA), was 28 µg/mL.
ELISA for NP with magnetic concentration. NP detection was performed taking into account the conditions for ELISA with the use of MP. NP was prepared in aqueous solutions with 20% methanol due to the low solubility of the analyte in water. This concentration of methanol doesn’t lead to antibody inactivation or loss of antigen-binding capacity [29–31]. The content of methanol at the competitive stage was equal for traditional competitive ELISA and for ELISA based on MP (Fig. 8). NP limit of detection amounted to 3.8 ng/mL, and the working range was from 6.3 to 33 ng/mL (IC50 = 14.2 ng/mL). Thus, ELISA modification allowed to reduce the limit of detection 14.5 times (from 55 to 3.8 ng/mL). The use of complexes of MP with antibodies helps to maintain the reactivity of reagents and concentrate the analyte, which allows detection of its low concentrations.
The last stage of development was the determination of the sample volume, which was optimal to provide concentration without loss of analysis sensitivity. For this purpose, samples containing equal amounts of NP were prepared in different volumes of solvent (from 0.1 to 5.0 mL). All stages of the analysis were performed using chosen concentrations of the reagents in small volumes (30 µL), excluding the competitive stage. According to the obtained dependence of the optical density on the sample volume (Fig. 9), the maximum signal is achieved when the sample is concentrated to 0.5 mL. Thus, the use of modified magnetic particles allows the sample to be concentrated 17 times, which is highly important for samples with a low content of analyte.
The approach presented in this study is interesting for several reasons. Carrying out the analysis in microtubes minimizes the non-specific interaction of NP compared with microplates of different sorption capacities. The direct orientation of rabbit serum immunoglobulins during the interaction of their Fc-fragments with protein G provides more effective competitive interaction with both free NP and in the composition of hapten-protein conjugate. The use of high-affinity biotin-streptavidin interactions, as long as polymerized peroxidase is used, reduces the limit of detection of the analyte. The obtained limits of detection for NP are in accordance with the established water content standards [12], which proves the suitability of the developed approach for determining the required low NP concentrations. Finally, the use of MP as carriers of immune complexes ensures the concentration of diluted samples and the detection of trace amounts of NP.
REFERENCES
Evans, A.E.V., Mateo-Sagasta, J., Qadir, M., Boelee, E., and Ippolito, A., Curr. Opin. Environ. Sustain., 2019, vol. 36, pp. 20–27.
Zamora-Ledezma, C., Negrete-Bolagay, D., Figueroa, F., Zamora-Ledezma, E., Ni, M., Alexis, F., and Guerrero, V.H., Environ. Technol. Innov., 2021, vol. 22, p. 101504. https://doi.org/10.1016/j.eti.2021.101504
Fang, W., Peng, Y., Muir, D., Lin, J., and Zhang, X., Environ. Int., 2019, vol. 131, p. 104994. https://doi.org/10.1016/j.envint.2019.104994
Fuller, R., Landrigan, P.J., Balakrishnan, K., Bathan, G., Bose-O', ReillyS., Brauer, M., et al., Lancet Planet. Health, 2022, vol. 6, no. 6, pp. e535–e547.
Palani, G., Arputhalatha, A., Kannan, K., Lakkaboyana, S.K., Hanafiah, M.M., Kumar, V., and Marella, R.K., Molecules, 2021, vol. 26, no. 9, p. 2799. https://doi.org/10.3390/molecules26092799
Babuji, P., Thirumalaisamy, S., Duraisamy, K., and Periyasamy, G., Water, 2023, vol. 15, no. 14, p. 2532. https://doi.org/10.3390/w15142532
Bhandari, G., Bagheri, A.R., Bhatt, P., and Bilal, M., Chemosphere, 2021, vol. 275, p. 130013. https://doi.org/10.1016/j.chemosphere.2021.130013
Gałązka, A. and Jankiewicz, U., Microorganisms, 2022, vol. 10, no. 11, p. 2236. https://doi.org/10.3390/microorganisms10112236
Morin-Crini, N., Lichtfouse, E., Liu, G., Balaram, V., Ribeiro, A.R.L., Lu, Z., et al., Environ. Chem. Lett., 2022, vol. 20, no. 4, pp. 2311–2338.
Chen, Y., Yang, J., Yao, B., Zhi, D., Luo, L., and Zhou, Y., Environ. Pollut., 2022, vol. 310, p. 119918. https://https://doi.org/https://doi.org/10.1016/j.envpol.2022.119918
Hong, Y., Feng, C., Yan, Z., Wang, Y., Liu, D., Liao, W., and Bai, Y., Environ. Chem. Lett., 2020, vol. 18, no. 6, pp. 2095–2106.
Careghini, A., Mastorgio, A.F., Saponaro, S., and Sezenna, E., Environ. Sci. Pollut. Res., 2015, vol. 22, no. 8, pp. 5711–5741.
Jardak, K., Drogui, P., and Daghrir, R., Environ. Sci. Pollut. Res., 2016, vol. 23, no. 4, pp. 3195–3216.
Lu, D., Yu, L., Li, M., Zhai, Q., Tian, F., and Chen, W., Chemosphere, 2021, vol. 275, p. 129973. https://doi.org/10.1016/j.chemosphere.2021.129973
Noorimotlagh, Z., Mirzaee, S.A., Martinez, S.S., Rachon, D., Hoseinzadeh, M., and Jaafarzadeh, N., Environ. Res., 2020, vol. 184, p. 109263. https://doi.org/10.1016/j.envres.2020.109263
Directive 2013/39/eu of the European parliament and of the council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy.
Shih, H.-K., Shu, T.-Y., Ponnusamy, V.K., and Jen, J.-F., Anal. Chim. Acta, 2015, vol. 854, pp. 70–77.
Vargas-Berrones, K., Díaz de León-Martínez, L., Bernal-Jácome, L., Rodriguez-Aguilar, M., Ávila-Galarza, A., and Flores-Ramírez, R., Talanta, 2020, vol. 209, p. 120546. https://doi.org/10.1016/j.talanta.2019.120546
Aparicio, I., Martín, J., Santos, J.L., Malvar, J.L., and Alonso, E., J. Chromatogr., A, 2017, vol. 1500, pp. 43–52.
Yin, H.-L. and Zhou, T.-N., Chinese J. Anal. Chem, 2022, vol. 50, no. 8, p. 100112. https://https://doi.org/https://doi.org/10.1016/j.cjac.2022.100112
Céspedes, R., Skryjová, K., Raková, M., Zeravik, J., Fránek, M., Lacorte, S., and Barceló, D., Talanta, 2006, vol. 70, no. 4, pp. 745–751.
Matsui, K., Kawaji, I., Utsumi, Y., Ukita, Y., Asano, T., Takeo, M., and Kato, D.-I., and Negoro, S., J. Biosci. Bioeng., 2007, vol. 104, no. 4, pp. 347–350.
Yakovleva, J.N., Lobanova, A.Y., Shutaleva, E.A., Kourkina, M.A., Mart’ianov, A.A., Zherdev, A.V., Dzantiev, B.B., and Eremin, S.A., Anal. Bioanal. Chem., 2004, vol. 378, no. 3, pp. 634–641.
Ermolaeva, T.N., Dergunova, E.S., Kalmykova, E.N., and Eremin, S.A., J. Anal. Chem., 2006, vol. 61, no. 6, pp. 609–613.
Badea, M., Nistor, C., Goda, Y., Fujimoto, S., Dosho, S., Danet, A., Barcelo, D., Ventura, F., and Emneus, J., Analyst, 2003, vol. 128, no. 7, pp. 849–856.
Mart’ianov, A.A., Zherdev, A.V., Eremin, S.A., and Dzantiev, B.B., Int. J. Env. Anal. Chem., 2004, vol. 84, no. 13, pp. 965–978.
Mart’ianov, A.A., Dzantiev, B.B., Zherdev, A.V., Eremin, S.A., Cespedes, R., Petrovic, M., and Barcelo, D., Talanta, 2005, vol. 65, no. 2, pp. 367–374.
Berlina, A.N., Komova, N.S., Serebrennikova, K.V., Zherdev, A.V., and Dzantiev, B.B., Eng. Proc., 2023, vol. 48, no. 1, p. 9. https://doi.org/10.3390/CSAC2023-14919
Berlina, A.N., Ragozina, M.Y., Gusev, D.I., Zherdev, A.V., and Dzantiev, B.B., Chemosensors, 2023, vol. 11, no. 7, p. 393. https://doi.org/10.3390/chemosensors11070393
Kuang, H., Liu, L., Xu, L., Ma, W., Guo, L., Wang, L., and Xu, C., Sensors, 2013, vol. 13, no. 7, pp. 8331–8339.
Kato, M., Ihara, Y., Nakata, E., Miyazawa, M., Sasaki, M., Kodaira, T., and Nakazawa, H., Food Agric. Immunol., 2007, vol. 18, nos. 3–4, pp. 179–187.
Funding
This study was financially supported by the Russian Science Foundation (grant no. 22-13-00293).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Berlina, A.N., Barshevskaya, L.V., Serebrennikova, K.V. et al. Development of Microplate Immunoenzyme Determination of Nonylphenol with Magnetic Sample Concentration. Appl Biochem Microbiol 60, 496–502 (2024). https://doi.org/10.1134/S0003683824603536
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683824603536