Abstract
Batch pressure vessels commonly used for hydrothermal liquefaction have typical heating times in the range of 30 to 60 min. Thermodynamically, the complex set of reactions are path dependent, so that the heating rate can possibly affect yields and the composition of the resultant liquid products. It is postulated that the mode of heat transfer becomes an uncontrolled variable in kinetic studies and can seriously impact scale-up. To confirm this hypothesis and minimize these heat-transfer-related artifacts, we designed a batch pressure vessel equipped with an induction heating system, which allows the reduction of heat-up times by about two orders of magnitude to several seconds, compared to tens of minutes with standard pressure reactors. This system was used to study the direct liquefaction of corn stover and aspen wood with a pretreatment. The heating rate was found to have no significant effect on the composition of the liquid products. However, the liquid yields are dependent on the heating rate. Varying the cooling rate does not show obvious effects. The results confirm that the heating rate, as governed by the mode of heat transfer, is an important factor that needs to be considered during scale-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomass is the renewable energy source of choice for the production of liquid transportation fuels. Significant quantities of ethanol are currently being produced from corn and sugar cane via fermentation. Utilizing lignocellulosic biomass as a feedstock is seen as the next step toward significantly expanding ethanol production capacity. However, in addition to substantial technical challenges that still have to be overcome before lignocellulose-to-ethanol becomes commercially viable, any ethanol produced by fermentation has the inherent drawback that it needs to be distilled from a mixture, which contains 82% to 94% water. Instead, direct thermochemical conversion of lignocellulosic biomass to a bio-crude oil or fuel gases has been proposed as an alternative. This does not require distillation, thus resulting in less energy input per unit of energy output.
There are two typical thermo-chemical conversion processes: pyrolysis and liquefaction. During pyrolysis processes, the biomass feedstock is heated in the absence of air, forming bio-oils and a gaseous product, which then condenses. Liquefaction of biomass produces a water-insoluble bio-crude oil by using treatments at high pressure (50–200 atm) and low temperature (250–450°C). The results of previous biomass liquefaction research have been reviewed by several different authors [1–5]. The advantage of liquefaction is that the bio-crude oil produced by liquefaction is not miscible with water and has a lower oxygen content and therefore higher energy content than pyrolysis-derived oils [1, 6].
Among the treatment variables, the heating rate may have a significant impact on the production of liquid bio-oil [2, 7]. For example, depending on the heating rate, the pyrolysis process can be divided into three categories: (1) conventional pyrolysis (0.1–1°C/min), (2) fast pyrolysis (10–200°C/min), (3) flash pyrolysis(>1,000°C/min). For this paper, the effects of the heating rate and the cooling rate on high-pressure liquefaction were studied. The components of the liquid products from corn stover and aspen wood with a pretreatment were identified and compared.
Material and Methods
Materials
Air-dried corn stover provided by the Agricultural Utilization Research Institute was milled and then screened, and only the fraction less than 2-mm sieve was used in this research. Aspen pulping wood chips (Populus tremuloides) with a pretreatment, which were the intermediates of a biorefinery process, were provided by the Department of Bioproducts and Biosystems Engineering, University of Minnesota, St. Paul, Minnesota. Aspen wood chips that passed through a 1-in. sieve but not through a 0.5-inch sieve were used. Aspen wood chips were pretreated with dilute sulfuric acid to remove partial hemicellulose.
Biomass Analytical Procedures
Moisture and ash content of biomass were determined by the methods of LAP-001 and LAP-005, respectively, which are laboratory analytical procedures (LAPs) developed by the National Renewable Energy Laboratory. Structural analyses of the samples were carried out according to the ASTM E1758-01 standard test methods. The composition of all raw materials is listed in Table 1.
Apparatus and Process
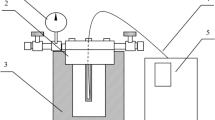
A 75-ml Parr high-pressure reactor (Parr Instrument, Moline, IL, USA) was used for liquefaction of the feedstock. The reactor consists of a reaction cylinder and a pressure gauge or valve assembly. An induction heating system, which allows the reduction of heat-up times by about two orders of magnitude, was customized by L. C. Miller, Monterey Park, CA, USA. For a typical run, 5 grams of feedstock and 45 ml distilled water were placed inside the cylinder. The cylinder was then sealed and purged through two valves on the reactor head with nitrogen gas at a flow rate of 80 ml/min for 5 min to remove air and prevent secondary reactions such as thermal cracking and repolymerization, and then the valves were closed. The reactor was heated to 350°C at a heating rate of about 140°C/min, and the desired temperature was held for 10 min. After completing the reaction, the cylinder was cooled down by soaking in the ice bath for 5 min. Gases were sampled into a gas bag for gas chromatography (GC) analysis later. The gas fraction was determined by measuring the weight difference of the reactor before and after gas sampling. The liquid, including both the water fraction and heavy oil fraction, was collected into a sample bottle for GC–mass spectrometry (MS) analysis later. The procedure for separating aqueous, water insoluble, and solid phases in the liquid is shown in Fig. 1. The aqueous phase was filtered to separate the water solution and the water insoluble fraction. The water insoluble fraction and the wall of the pressure reactor were washed with 10 ml acetone three times and then separated through filtration. The total amount of the acetone solution containing heavy oil and the aqueous phase were referred to as the liquid fraction. The acetone insoluble fraction was air-dried at room temperature for 48–72 h to a constant weight to yield the solid residue. Constant weight is defined as less than ± 0.01 g change in the weight upon 12 h of redrying.
The yield percentage of each fraction from liquefaction is defined as
All experiments and analysis were performed in triplicate.
Heating Rates and Cooling Rates
The heating rate was controlled by adjusting the output of the induction heater. The fast and slow heating rates were approximately 140 and 14°C/min, respectively. A 500-W band heater was used to mimic the conventional heating process. The conventional heating rate using the band heater was approximately 5°C/min.
For fast cooling, the reactor was cooled to room temperature by soaking in the ice bath for 5 min. For slow cooling, the reactor was left at room temperature for 2 h until it reaches room temperature. The fast- and slow-cooling rates were approximately 66 and 5.5°C/min, respectively.
Chemical Analysis
A Perkin–Elmer Auto-system gas chromatograph (GC), which houses a 30-m, 0.53-mm (ID) fused silica capillary column (Carboxen 1010 Plot, Supelco), was used to analyze the gaseous samples from the liquefaction process. Temperature programmed step heating was performed as follows: 40°C for 1.7 min, increase by 40°C/min until 220°C, and leave at 220°C for 1.8 min. Argon was the carrier gas at a flow rate of 20 ml/min. Two detectors were used for gas analysis: a flame ionization detector (FID) for carbon-bearing species and a thermal conductivity detector (TCD) for H2. Uncertainties in reported concentrations are estimated to be within ±5% [8].
Chemical compositions of the liquid products were identified using a Varian 3 GC/mass spectrometer with a HP-1 capillary column. The GC was programmed to hold the temperature at 40°C for 0.5 min and then increased by 10°C/min to 300°C and, finally, to hold with an isothermal for 10 min. The injector temperature was 300°C, and the injection size was 1 μl. The flow rate of the carrier gas (helium) was 0.6 ml/min. The ion source temperature was 230°C for the mass selective detector. The compounds were identified by comparison with the NIST Mass Spectral Database.
Results and Discussion
Effect of Heating Rate and Cooling Rate on Liquefaction of Corn Stover
Corn stover was used as the raw material. The reaction mixture was heated to 350°C and 20 MPa at a heating rate of about 140°C/min, 14°C/min, and 5°C/min, respectively, and the final temperature was held for 10 min. Table 2 and Fig. 2a show that the yield of liquid products obtained from separation of liquefaction products increased with increasing heating rate. When the heating rate increased from 5°C/min to 140°C/min, the liquid yield increased drastically from 51–53% to 69–71% of the total biomass, but less gaseous products were generated, and the yield of solid residue also decreased from 18–20% to ~9% of the total mass.
The gaseous phase from liquefaction of corn stover primarily consisted of hydrogen (H2), carbon monoxide (CO), methane (CH4), and carbon dioxide (CO2), which were 2.6–4.8%, 8–27%, 1.1–2.8%, and 68–85% of the total gaseous products, respectively.
Under the current reactor configuration, the liquid yield from the liquefaction of corn stover can be calculated from the data given in Table 2. The liquid yield as a function of the heating rate (°C/min) can be calculated from
The liquid yields calculated by using Eq. 1 are also given in Fig. 2a. Equation 1 represents the correlation obtained by means of regression analysis. The correlation coefficient is 0.9181.
Compared with the fast cooling process, the slow-cooling process does not show obvious effects on the profile of liquefaction products and gaseous products (Table 2). The possible reason is that the system may have reached equilibrium conditions after 10-min liquefaction.
Effect of Heating Rate and Cooling Rate on Liquefaction of Pretreated Aspen Chips
While liquefying the pretreated aspen, the reaction mixture was also heated to 350°C and 20 MPa at a heating rate of about 140°C/min, 14°C/min, and 5°C/min, respectively. The result of liquefying the pretreated aspen is similar to that of corn stover. From Table 3 and Fig. 2b, it is clear that the yield of liquid products obtained from the separation of liquefaction products increased with increasing heating rate. When the heating rate increased from 5°C/min to 140°C/min, the liquid yield increased from 50.3% to 72–74% of the total biomass, and the yield of solid residue decreased from 24.7% to 6–7% of the total mass. The cooling process did not show obvious effects on the profile of liquefaction products and gaseous products from the pretreated aspen.
Under the current reactor configuration, the liquid yield of the pretreated aspen chips as a function of the heating rate (°C/min) can be calculated from
The liquid yields calculated by using Eq. 2 are also given in Fig. 2b. Equation 2 represents the correlation obtained by means of regression analysis. The correlation coefficient is 0.9358. However, Eqs. 1 and 2 are only valid for the experiments within the extreme range of the heating rates between 5°C/min and 140°C/min while maintaining other variable parameters to be constant, including reactor type and dimensions, biomass conditions, etc.
Typical heating times in batch pressure vessels commonly used for thermal-chemical conversion are in the range of 30 to 60 minutes. Thus, an artifact is inadvertently introduced in kinetic studies, as the mix of reaction products is path dependant. To minimize these heat transfer artifacts, we designed a batch pressure vessel equipped with an induction heating system, which allows the reduction of heat-up times by about two orders of magnitude, from tens of minutes to several seconds, compared to standard pressure reactors. During the process of slow heating, liquefaction may involve more side reactions and result in a higher yield of solid residue but less liquid fuel. However, varying the cooling rate did not show obvious effects, as equilibrium conditions may already be reached. So, it suggests that the heating rate and mode of heat transfer are important factors, which need to be considered during scale-up.
GC–MS Analysis of the Liquid Products
The components of the liquid products from various biomasses were determined by GC–MS analysis. The mass to charge ratios of the components were checked against the mass spectral library published by NIST. The peak areas shown on the GC spectra were calculated and are given in Table 4. The percentage values indicate the proportions of individual compounds in the liquid and do not represent the actual concentration of these compounds.
Within the GC–MS detectable range, the composition difference of liquid products from various origins is able to be identified. Liquid products from corn stover contain 4-ethyl-phenol, 1-(4-hydroxy-3,5-dimethoxyphenyl)-ethanone, desaspidinol, 2-hydroxy-3-methyl-2-cyclopenten-1-one, 2,5-hexanedione, and 1-hydroxy-2-propanone, and these compounds are barely detectable in the liquid products from the pretreated aspen. The liquid products from the pretreated aspen also contain unique compounds, such as 3-methyl-2-cyclopenten-1-one, 3-methyl-phenol, and 2-methoxy-4-propyl-phenol. Besides, 2,6-dimethoxy-phenol in the liquid products of the pretreated aspen is markedly higher then that of corn stover. Therefore, the composition of liquid products from liquefaction is dependent on biomass species.
With this liquefaction platform, the heating rate was found to have no effect on the individual compound of the liquid products (GC–MS data not shown), but it did affect the yield of liquefaction.
Conclusions
In this study, we found that during thermo-chemical liquefaction of biomass, the relative proportion of the resulting solid, liquid, and gaseous fractions was dependent on the heating rate; however, variations in the heating rate did not significantly affect chemical composition of the liquid products. The composition of the liquid products is more dependent on the biomass species used for liquefaction. Varying the cooling rate did not show obvious effects on the ratio of liquid to solid to gas nor on the composition of the liquid products. These results imply that, when trying to maximize liquid yield during biomass liquefaction, it is critical to design the full-scale system for high heating rates and optimal heat transfer.
References
Huber, G. W., Iborra, S., & Corma, A. (2006). Chemical Reviews, 106, 4044–4098.
Denirbas, A. (2004). Energy Sources, 26, 715–730.
Yu, F., Ruan, R., Lin, X., Liu, Y., Fu, R., Li, Y., et al. (2006). Applied Biochemistry and Biotechnology, 130, 563–573.
Elliott, D. C., & Schiefelbein, G. F. (1989). Abstracts of papers of the American Chemical Society, 198, 55–FUEL.
Elliott, D. C., Beckman, D., Bridgwater, A. V., Diebold, J. P., Gevert, S. B., & Solantausta, Y. (1991). Energy & Fuels, 5, 399–410.
Goudriaan, F., van de Beld, B., Boerefijn, F. R., Bos, G. M., Naber, J. E., van der Wal, S., et al. (2001). In A. V. Brigdwater (Ed.), Progress in thermochemical biomass ConVersion 2 (pp. 1312–1325). Oxford, UK: Blackwell Science.
Boocock, D. G. B., & Porretta, F. (1986). Journal of Wood Chemistry and Technology, 6, 127–144.
Fu, Q., Sherwood Lollar, B., Horita, J., Lacrampe-Couloume, G., & Seyfried, Jr., W. E. (2007). Geochimica et Cosmochimica Acta, 71, 1982–1998.
Acknowledgment
The University of Minnesota Initiative for Renewable Energy and the Environment (IREE) is gratefully acknowledged for its financial support. The authors would like to thank collaborators: Dr. Shri Ramaswamy, Dr. Ulrike W Tschirner, Dr. Vernon R Eidman, Dr. Steve Polasky, Dr. Waleed Wafa Al-Dajani, and Dr. Huajiang Huang. The authors also would like to thank Dr. Qi Fu for assistance with GC and Dr. Fei Yu for comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, B., von Keitz, M. & Valentas, K. Thermal Effects on Hydrothermal Biomass Liquefaction. Appl Biochem Biotechnol 147, 143–150 (2008). https://doi.org/10.1007/s12010-008-8131-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8131-5