Abstract
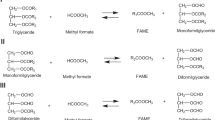
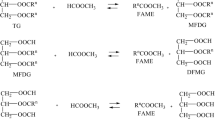
Biodiesel is a fatty acid alkyl ester that can be derived from any vegetable oil or animal fat via the process of transesterification. It is a renewable, biodegradable, and nontoxic fuel. In this paper, we have evaluated the efficacy of a transesterification process for rapeseed oil with methanol in the presence of an enzyme and tert-butanol, which is added to ameliorate the negative effects associated with excess methanol. The application of Novozym 435 was determined to catalyze the transesterification process, and a conversion of 76.1% was achieved under selected conditions (reaction temperature 40 °C, methanol/oil molar ratio 3:1, 5% (w/w) Novozym 435 based on the oil weight, water content 1% (w/w), and reaction time of 24h). It has also been determined that rapeseed oil can be converted to fatty acid methyl ester using this system, and the results of this study contribute to the body of basic data relevant to the development of continuous enzymatic processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel (fatty acid methyl esters) is an alternative and renewable energy source, the development of which is hoped to reduce global dependence on petroleum, as well as air pollution. Biodiesel generated from a variety of vegetable oils and animal fats has characteristics similar to those associated with petro-diesel, including viscosity, volumetric heating value, cetane number, and flash point [1–3]. Several processes have thus far been developed for the production of biodiesel via acid-, alkali-, and enzyme-catalyzed transesterification reactions [3–5]. In the enzymatic process employed for the production of fatty acid methyl ester from oils, several parameters have been shown to influence both yield and rate. These parameters include the reaction solvent utilized, the reaction temperature, the type and concentration of the alcohol, the quantity of enzyme in the reaction, the water content, and the mixing rate [6].
As compared to other catalyst types used in the production of biodiesel, enzymes have several advantages. They enable conversion under reaction conditions milder than those required for chemical catalysts. Moreover, in the enzymatic process, both the transesterification of triglycerides and the esterification of free fatty acids occur in one process step. However, lipase-catalyzed transesterifications induce a series of drawbacks. As compared to conventional alkaline catalysis protocols, reaction efficiency tends to be rather poor, and thus enzymatic catalysis generally necessitates significantly longer reaction times and higher enzyme amounts. The primary obstacle to the application of enzymes in industrial processes is their relatively high cost [7].
In our study, we conducted the enzyme-catalyzed methanolysis of rapeseed oil using Novozym 435, a well-known nonspecific lipase. Novozym 435 facilitates reactions between a wide variety of alcohols and is also a remarkably heat-tolerant enzyme [6, 8]. Watanabe et al. [9] previously reported that immobilized Candida antarctica lipase was inactivated in the presence of more than half the stoichiometric amount of methanol against total fatty acids in the oil. This disadvantage was surmounted by the utilization of three-step methanolysis, in which only one third of the total amount of methanol was added in each stage [7, 9].
Generally, alcoholysis using long-chain or branched alcohols proceeds efficiently even in solvent-free systems, whereas methanolysis tends to result in fairly low ester conversions. This has been generally attributed to the poor solubility of methanol in oils than that of long-chain or branched alcohols and the tendency of methanol to inactive enzymes [7]. In particular, the difficulty inherent to the dissolution of both hydrophobic and hydrophilic substrates in a common low-toxicity reaction solvent has been the principal limitation of biological synthesis protocols [6, 10, 11]. Depending on the type of lipase employed, a variety of solvents for oils and alcohols have been suggested, including petroleum ether, hexane, iso-octane, 1,4-dioxane, tert-butanol, ionic liquids, supercritical carbon dioxide, and several others [7]. In all of our experiments, t-butanol was employed as the reaction solvent, primarily because of its high substrate solubility, as well as the ease inherent to the separation of the product from the by-products, a consequence of its low boiling point [6]. In addition, tert-butanol has been shown to ameliorate the negative effects associated with excessive methanol [12].
In the enzymatic process utilized for the production of fatty acid methyl ester (biodiesel) from rapeseed oil, several factors can influence both the yield and rate. These factors include the reaction solvent, reaction temperature, reaction time, methanol/oil molar ratio, enzyme amount, and water content [7, 9, 12–14]. The initial step of this study involved the identification of factors likely to influence the conversion.
In this study, we have attempted to determine the optimal conditions for enzyme-catalyzed methanolysis of biodiesel production, using rapeseed oil with methanol and Novozym 435.
Materials and Methods
Materials
The Novozym 435 (Lipase B from C. antarctica, EC 3.1.1.3, a nonspecific lipase immobilized on macroporous acrylic resin, 1–2% water content, 10,000 propyl laurate units/g) was purchased from Novo Nordisk A/S (Bagsvaerd, Denmark). The t-butanol was purchased from Sigma-Aldrich (St. Louis, MO). The refined rapeseed oil originating from Jeju Island (South Korea) was supplied by Onbio (Bucheon, South Korea), and its characteristics are summarized in Table 1. Palmitic acid methyl ester, stearic acid methyl ester, oleic acid methyl ester, linoleic acid methyl ester, linolenic acid methyl ester, erucic acid methyl ester, and heptadecanoic acid methyl ester were obtained from Sigma-Aldrich and were chromatographically pure. Anhydrous methanol was acquired from Fisher Scientific. All other chemicals were of analytical grade, and the solvents were dried with molecular sieves (4Å, Yakuri Pure Chem., Japan) for 1day before use.
Lipase-catalyzed transesterification
Methanolysis was conducted in a 20mL reaction bottle, maintained at 40 °C in a rotary shaker at 260rpm. The initial weight of the rapeseed oil was 5g. To prevent direct contact between the lipases and the methanol drops, the methanol was mixed with 4mL tert-butanol and oil followed by the addition of lipases to the mixture. In all experiments without initial water content experiment, there was no set initial amount of water in reactant, with the exception of the water contained within the enzymes themselves.
To assess the effects of reaction temperature on enzymatic methanolysis, the reactions were conducted at 25–55°C, under the following conditions: the reactant was coupled with 5 of prepared rapeseed oil at a molar ratio of 3:1 with methanol and 5% (w/w) Novozym 435 based on the oil weight, for a reaction time of 2h. Mixing was conducted with a magnetic stirrer, which spun at approximately 200rpm. To determine the effects of enzyme amount on enzymatic methanolysis, 1–10% (w/w) Novozym 435 was added to the reactant, coupled with 5g of prepared rapeseed oil at a molar ratio of 3:1 with methanol and a reaction time of 24h at 40 °C in a rotary shaker at 260rpm. To determine the effects of the methanol/oil molar ratio on enzymatic methanolysis, a 1:1–6:1 molar ratio of methanol was added to the reactant, coupled with 5g of prepared rapeseed oil, with 5% (w/w) Novozym 435 and a reaction time of 24h at 40 °C in a rotary shaker at 260rpm. To determine the optimal initial water content and molecular sieve quantities for enzymatic methanolysis, 0–10% (w/w) water or 0–5% (w/w) molecular sieves were added to the reactant, coupled with 5g of prepared rapeseed oil at a molar ratio of 3:1 with methanol, with 5% (w/v) Novozym 435, for a reaction time of 24h at 40 °C in a rotary shaker at 260rpm. During the methanolysis, samples (100μL) were obtained from the reaction mixture at specified times. One hundred microliters of the sample, 400μL pyridine (served as solvent), and 500μL of the heptadecanoic acid methyl ester dissolved in pyridine (served as the internal standard) were measured precisely and mixed for gas chromatographic analysis. The results of the experiments were expressed as the mean values from at least two independent measurements.
Analytical Methods
Gas Chromatography for Fatty Acid Methyl Ester Analysis
The fatty acid methyl ester contents in the reaction mixture were determined using a Donam 6100 GC gas chromatograph (Donam, South Korea) equipped with an HP-INNOWAX capillary column (30m × 0.32mm × 0.5μm) and an flame ionization detector. The column oven temperature was maintained at 210 °C for analysis. The injector and detector temperatures were set to 250 and 250 °C, respectively. Helium was utilized as a carrier gas. The gas chromatography calibration was conducted via the analysis of standard solutions of palmitic acid methyl ester, stearic acid methyl ester, oleic acid methyl ester, linoleic acid methyl ester, linolenic acid methyl ester, and erucic acid methyl ester. The internal standard was diluted in pyridine, as were the reaction mixture samples. The conversion was expressed as the percentage of fatty acid methyl esters generated relative to the theoretical maximum quantity, based on the amount of original oils. In this paper, the conversion was expressed as the fatty acid methyl ester content or in accordance with conversion.
Results and Discussion
In the enzymatic process utilized herein for the production of fatty acid methyl ester from rapeseed oil, several factors are known to influence conversion. The initial step of this study involved the identification of factors likely to influence conversion. In this study, tert-butanol was applied in lipase-catalyzed methanolysis. tert-Butanol has been utilized previously in several enzymatic process, including sorbitan ester synthesis [6, 15]. It has also been confirmed that tert-butanol is inert in the Novozym 435-catalyzed methanolysis of rapeseed oil for the production of biodiesel [12].
In the solvent-free system utilized herein, involving the following reaction conditions: 5% (w/w) Novozym, 3:1 methanol/oil molar ratio, 260rpm rotary shaker speed, and 40 °C, the conversion was found to be extremely low (only 1.8% at 24h), owing principally to the toxicity of excessive methanol on lipase activity and the difficulty of mixing, whereas the conversion (70.9% at 24h) was increased as the result of the addition of tert-butanol as a reaction solvent into the reaction mixture (data not shown). This has been explained as reported previously by Li et al. [12] that the presence of tert-butanol could improve the solubility of methanol in the reaction mixture, and thus lipase retained a high level of activity with all methanol added for lipase-catalyzed methanolysis.
Effect of Reaction Temperature on the Methanolysis
Generally, alkali-catalyzed transesterification is conducted at near the boiling point of the alcohol, but enzyme-catalyzed transesterification is performed at a low temperature to prevent the loss of lipase activity [9]. The low reaction temperature was also found to be desirable, as the reaction temperature was closely related to the energy cost inherent to the process of biodiesel production [3].
The effect of temperature on rapeseed oil methanolysis was assessed in a temperature range of 25 to 55 °C under the following conditions: 5% (w/w) Novozym 435 as a catalyst, a methanol/oil molar ratio of 3:1, and a 2-h reaction time. As is shown in Fig. 1, conversions decline at reaction temperatures in excess of 40 °C. The conversions at 2h increase with increasing reaction temperatures until 40 °C and after that begin to decline. At a reaction temperature of 40 °C, the conversion was approximately 45.3%. This result is similar to the results reported by Chen et al. [14], which were obtained via the methanolysis of waste cooking oil using Rhizopus orzyae lipase; however, they reported that reaction temperatures in excess of 40 °C induced a reduction in the methyl ester yield caused by the loss of enzyme activity, which was induced by high temperatures. Furthermore, Jeong et al. [11] previously reported that the optimal reaction temperature of Novozym 435 in sorbitan ester synthesis is 40–43 °C. However, in this study, an optimal reaction temperature of 40 °C may have allowed for the maintenance of high lipase activity and low reactant viscosity during mixing. However, this remains unclear, and the subject will require further investigation.
Effect of Enzyme Amount on Methanolysis
Novozym 435 has been reported to significantly augment the methanolysis of vegetable oils [6, 9, 12]. In this study, the effects of enzyme quantity on the enzymatic methanolysis of rapeseed oil for biodiesel production with tert-butanol used as a solvent has been evaluated. Figure 2 shows the effects of enzyme concentration on rapeseed oil methanolysis at 40 °C at a methanol/oil molar ratio of 3:1 for 24h. The conversion was enhanced by increases in enzyme concentration. The highest conversion attained was approximately 88.5% at 24h with 10% (w/w) Novozym 435. However, Li et al. [12] previously reported that the methyl ester yield was increased with increasing amounts of lipase and that when the lipase amount reached 2% (w/w), a methyl ester yield of 90% could be achieved at 12h. In enzyme-catalyzed methanolysis, the optimal enzyme and its usage amounts were selected in terms of optimal concentration rather than high conversion conditions, as a consequence of the significant cost associated with higher enzyme amounts. Considering the enzyme cost inherent to the enzyme-catalyzed methanolysis of rapeseed oil, an enzyme amount of 5% (w/w) was utilized in the rest of the study.
Effect of Methanol/Oil Molar Ratio on Methanolysis
The stoichiometry of the methanolysis of oils requires 3mol of alcohol per 1mol of triglyceride to convert to 3mol of fatty acid methyl esters and 1mol of glycerol. The most significant factor in alkali/acid-catalyzed transesterification was the molar ratio of the alcohol and oil. As transesterification involves reversible and consecutive reactions, increases in the molar ratio of methanol will result in high conversions [3, 7]. However, in enzyme-catalyzed methanolysis, enzyme activity was shown to decrease in the methanolysis of oil as methanol concentration increased [13–14].
In this experiment, the effects of the methanol/oil molar ratio on rapeseed oil methanolysis oil were evaluated in a ratio range of 1:1 to 6:1, with a reaction temperature of 40 °C and 5% (w/w) Novozym 435 on the basis of oil weight as a catalyst for 24h, the results of which are indicated in Fig. 3. The conversion was enhanced in a linear fashion with increases in the methanol/oil molar ratio within a ratio range below 2:1. From the results of the methanol/oil molar ratio study, it was determined that when the molar ratio of methanol to oil was in a range of 2:1–5:1, high conversions were achieved, and no significant differences were detected with different methanol/oil molar ratios within this range. At methanol/oil ratios in excess of 6:1, the conversion was reduced. Considering the substantial operational stability of lipase, a methanol-to-oil molar ratio of 3 to 1 was utilized in the rest of the study.
Effect of Water Content and Molecular Sieve Amount on the Methanolysis
The activity of an enzyme in a nonaqueous reactant is known to be affected by the water content in the reactants. Additionally, the activity of the immobilized enzyme is reduced in aqueous systems, regardless of the immobilization method utilized [13].
In this study, the effects of the water content on the enzyme-catalyzed methanolysis of rapeseed oil was investigated via the addition of a small quantity of water to the reaction solution of methanol and rapeseed oil as substrates, tert-butanol as a solvent, and Novozym 435 as the enzyme. The reaction was conducted with the addition of water in a range from 0 to 10% (w/w) based on the weight of rapeseed oil, with a constant temperature of 40 °C and a reaction time of 24h. As is shown in Fig. 4, the highest conversion was approximately 76.1% at a water content of 1% (w/w). The conversion increased in a linear fashion with increases in the water content within a range of below 1% (w/w). At water content values in excess of 1% (w/w), the conversion was reduced in a linear fashion.
Figure 5 shows the effects of the application of molecular sieves as water absorbers on enzyme-catalyzed rapeseed oil methanolysis, using methanol as the substrate. The reaction was conducted via the addition of molecular sieves at a concentration of 0 to 10% (w/w) based on the weight of the rapeseed oil with a methanol/oil molar ratio of 3:1, a constant temperature of 40 °C, and reaction time of 24h. As can be observed in Fig. 5, the conversion evidenced no significant differences with the addition of molecular sieves within a range of 0–5% (w/w). With regard to our study, Jeong and Park [6] previously reported that the addition of molecular sieves was determined to generally inhibit sorbitan ester synthesis; however, neither the addition of water nor the dehydration of water with molecular sieves was found to be required for the enzymatic synthesis of sorbitan acrylate using Novozym 435, with the exception of the inherent water content of the enzymes.
Effect of Reaction Time on Methanolysis
In the process of biodiesel production, reaction temperature, methanol quantity, and reaction time were found to be significant operating parameters, which are closely associated with energy costs from an economic perspective [3]. Figure 6 shows the effects of reaction time on enzyme-catalyzed rapeseed oil methanolysis at the following conditions: 5% (w/w) Novozym, 3:1 methanol/oil molar ratio, and 40 °C. Within 10h, the reaction proceeded very fast and in a linear fashion. Rapeseed oil was converted at a rate greater than 67.7% within 12h and achieved an equilibrium state after approximately 24h.
However, Salis et al. [15] reported that solvent-free synthesis of oleic acid short-chain alcohols reached 100% of triolein conversion after only 6h at 40 °C by immobilized lipase of Pseudomonas cepacia. Furthermore, Kim [16] reported that the high conversion of 98.5% was possible at 45 °C of reaction temperature with 4:1 of methanol to oil molar ratio and 1% (v/v) methyl glucoside oleic polyester as an emulsifier using Novozym 435 in the presence of cosolvent. However, several researchers have reported that the conversion of vegetable oils to fatty acid methyl ester via alkali-catalyzed transesterification was achieved at a rate of above 90% within 5min with a sufficient molar ratio [2, 3, 17].
Conclusions
In this study, we have attempted to evaluate the efficacy of a technique for the production of the methyl ester of rapeseed oil via enzyme-catalyzed transesterifications using tert-butanol, a moderately polar organic solvent. We conducted experiments involving the alteration of several reaction conditions, including reaction temperature, methanol/oil molar ratio, enzyme amount, water content, and reaction time. The selected conditions for biodiesel production were as follows: reaction temperature 40 °C, Novozym 435 5% (w/w), methanol/oil molar ratio 3:1, water content 1% (w/w), and 24h of reaction time. Under these reaction conditions, a conversion of approximately 76.1% was achieved. Further studies are currently underway to determine a method by which the cost of fatty acid methyl ester production might be lowered, via the development of enzyme-catalyzed methanolysis protocols involving a continuous bioprocess.
References
Lang, X., Dalai, A. K., Bakhshi, N. N., Reaney, M. J., & Hertz, P. B. (2001). Bioresource Technology, 80, 53–62.
Jeong, G. T., Oh, Y. T., & Park, D. H. (2006). Applied Biochemistry and Biotechnology, 129–132, 165–178.
Jeong, G. T., & Park, D. H., et al. (2004). Applied Biochemistry and Biotechnology, 114, 747–758.
Freedman, B., Pryde, E. H., & Mounts, T. L. (1984). JAOCS, 61(10), 1638–1643.
Nelson, L. A., Foglia, T. A., & Marmer, W. N. (1996). JAOCS, 73(8), 1191–1195.
Jeong, G. T., & Park, D. H. (2007). Applied Biochemistry and Biotechnology, 136–140, 595–610.
Mittelbach, M., & Remschmidt, C. (2004). Biodiesel-the comprehensive handbook pp. 69–80. Vienna, Austria: Boersedruck Ges.m.b.H.
Virto, C., & Adlercreutz, P. (2000). Enzyme and Microbial Technology, 26, 630–635.
Watanabe, Y., Shimada, Y., Sugihara, A., & Tominaga, Y. (2001). Journal of the American Oil Chemists’ Society, 78, 703–707.
Castillo, E., Pezzotti, F., Navarro, A., & Lopez-Munguia, A. (2003). Journal of Biotechnology, 102, 251–259.
Jeong, G. T., & Park, D. H. (2006). Enzyme and Microbial Technology, 39, 381–386.
Li, L., Du, W., Liu, D., Wang, L., & Li, Z. (2006). Journal of Molecular Catalysis B: Enzymatic, 43, 58–62.
Iso, M., Chen, B., Eguchi, M., Kudo, T., & Shrestha, S. (2001). Journal of Molecular Catalysis B: Enzymatic, 16, 53–58.
Chen, G., Ying, M., & Li, W. (2006). Applied Biochemistry and Biotechnology, 129–132, 911–921.
Salis, A., Pinna, M., Monduzzi, M., & Solinas, V. (2005). Journal of Biotechnology, 119, 291–299.
Kim, H. S. (2003). Journal of the Korean Oil Chemists’ Society, 20, 251–258.
Jeong, G. T., & Park, D. H. (2006). Applied Biochemistry and Biotechnology, 129–132, 668–679.
Acknowledgements
This work is outcome of the Specialized Graduate School supported financially by the Ministry of Commerce, Industry and Energy (MOCIE).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, GT., Park, DH. Lipase-Catalyzed Transesterification of Rapeseed Oil for Biodiesel Production with tert-Butanol. Appl Biochem Biotechnol 148, 131–139 (2008). https://doi.org/10.1007/s12010-007-8050-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-8050-x