Abstract
In order to increase the yield of biodiesel and reduce its cost, it is appropriate to apply a process of interesterification of vegetable oil with carboxylate esters. No glycerol is formed during this process, and the triacylglycerols formed during the process improve the low-temperature properties of biodiesel. The aim of the study was to investigate the interesterification process of rapeseed oil with methyl formate using a biotechnological method. The 15 enzyme preparations were tested and Lipozyme RM IM was found to be more effective. The optimal conditions of enzymatic interesterification were determined. Molar ratio of methyl formate to rapeseed oil, process duration, and amount of catalyst were optimized. The yield of rapeseed oil methyl esters increases with increasing duration and excess of methyl formate. Optimal catalyst concentration in the process of interesterification of rapeseed oil with methyl formate is 15%. A yield of rapeseed oil methyl esters of 67.9% at a process temperature of 20 °C was obtained with a molar ratio of methyl formate to oil of 32:1 and a process time of 40 h. As the duration of the process increases, the amount of triformylglycerol in the reaction mixture increases, and 4.86% of the desired reaction product, triformylglycerol, is formed within 40 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With rising energy costs and declining fossil fuel resources, the use of renewable energy sources, including biofuels from biomass, is increasing in various sectors of industry. Diesel engines use biodiesel, which is produced by transesterification of vegetable oil with methanol. The use of biodiesel is important from an environmental point of view: it does not contain sulfur compounds, and the emissions of harmful components such as carbon monoxide, unburnt hydrocarbons, polycyclic aromatic hydrocarbons, and particulate matter are significantly reduced compared to usage of mineral diesel. In addition, biodiesel is characterized by rapid biodegradability.
The process of transesterification of triglycerides is catalytic; the most commonly used alkaline catalysts in the industry are potassium or sodium hydroxide. Fatty acid methyl esters are obtained as the main product, together with the by-product technical glycerol, which accounts for about 10% of the biodiesel amount. Technical glycerol contains about 80–90% pure glycerol, the rest consists of fatty acid esters, soap residues, water, and methanol.
With the increase in biodiesel production, the amount of technical glycerol on the market is increasing, which is causing problems in its sale and use. Technical glycerol cannot be used in common applications such as the food, cosmetics, pharmaceutical, and paper industries where pure glycerol is used. Refining of technical glycerol requires high material and energy costs, so new efficient ways for the application of technical glycerol are being sought. In order to solve the problems of glycerol utilization, the research of biodiesel synthesis technologies, during which glycerol is not formed, has been started. This is achieved in place of the transesterification of triglycerides with methanol performing the process of interesterification of triglycerides with carboxylate esters. The glycerol is not formed in this process; instead, glycerol derivatives are obtained—esters of glycerol and organic acids, which are highly soluble in biodiesel [1]. Triacylglycerol (TAG) has been found effective for improving the low-temperature properties of biodiesel and has been proposed for use as a depressant for biodiesel [2,3,4]. Researchers have used TAG as an additive to biodiesel, but it is possible to apply such a process when this compound is co-produced with biodiesel, avoiding the formation of free glycerol [5,6,7]. The interesterification process of triglycerides results in a mixture of fatty acid alkyl esters (biodiesel) and esters of glycerol and organic acids of low molecular weight with good low-temperature properties, avoiding the separation of the glycerol phase from biodiesel. In this way, biodiesel yield is increased by nearly 10%. Depending on the efficiency of the process, mono-, di-, or triacylglycerides are formed. The interesterification process, like the transesterification process, is catalytic; both chemical and enzymatic catalysts can be used to increase the rate of interesterification. Recently, there has been a growing interest in the application of biotechnological processes in various industries. The use of enzymes in industrial processes increases because they are eco-friendly, do not generate greenhouse gases, and have reduced the demand for energy in industries. Enzymes are remarkably effective in versatile reactions, such as hydrolysis, esterification, transesterification, alcoholysis, and C–C bond formation. Enzymes have been recently used as a potential biocatalyst in a large number of biotechnological sciences; more specifically, these include dairy products (cheese recovery, flavor enhancement, and the production of enzyme-modified cheese (EMC), detergents, pharmaceuticals (ibuprofen, naproxen), chemicals, agriculture products (pesticides, insects), and oil chemistry (fat and oil hydrolysis, and the synthesis of bio-detergents) [8]. Enzymes act as a good catalyst; therefore, its production and utilization may be a better alternative to chemical catalysts [9]. Immobilized enzymes are commonly used in both industry and research. Immobilization improves selectivity of enzymes, repeatability, and variation of substrates [10, 11]. Immobilized enzymes have increased contact with reagents, improved enzyme stability, and prevented ejaculation [12,13,14,15]. Immobilized enzymes have a larger surface area, are more stable and easier to separate from the substrate, and can be reused [16, 17]. Enzymes have been immobilized on various carriers such as porous silicon, pectin, cellulose substrate, activated carbon, alginate, and chitosan to increase the stability and reusability of lipases [18,19,20,21]. With the development of nanotechnology, nanomaterials are used as favorable carriers for enzyme immobilization [Liu and Dong, 2020]. Immobilization on magnetic nanoparticles has numerous advantages, including high surface area, good stability for oxidation, and high biocompatibility.
The possibilities of using enzymatic preparations—lipases for transesterification and interesterification of oils—are widely studied. Lipases (triacylglycerol acyl hydrolases, EC 3.1.1.3) comprise a common family of enzymes produced by animals, plants, and microorganisms. Enzymes with excellent catalytic properties have been well studied as catalysts because of their unique physicochemical behavior [22, 23]. Lipases as catalysts in the interesterification process: lipases are capable of recognizing many different substrates, and they can catalyze hydrolysis, acidolysis, esterification, transesterification, and amination reactions. Lipase molecules have active sites made up of serine, aspartate, or glutamate, and histidine covered by a polypeptide chain (lid), which isolates it from the reaction medium. In contact with hydrophobic surfaces become adsorbed on it, the active site is exposed and the open form of lipases develops and activity increases.
Various types of vegetable oils such as rapeseed oil [24, 24], soybean oil [25], sunflower oil [26], and waste canola oil [27] were used for experiments of interesterification. The use of non-edible vegetable oil, such as non-edible soybean oil [28], waste cooking oil used in food production [29], algae oil [30], and others, has also been investigated.
Researchers have used methyl, ethyl, propyl, and butyl acetates as acyl receptors for interesterification studies. They noted that methyl and ethyl acetates have a greater prospect. When using higher molecular weight and longer-chain carboxylate esters for the interesterification of triglycerides, the efficiency of the process is lower [3]. The same trend is observed in the transesterification process: transesterification with longer-chain alcohols is more complex, so the most commonly used short-chain alcohol is methanol. It is probable that the methyl ester of formic acid could be used effectively in the interesterification process as well, but so far there are insufficient data on the efficiency of the use of methyl formate for interesterification of triglycerides and optimal process conditions. The use of the methyl formate chosen for our research is attractive for several reasons. Interesterification of oil with methyl formate does not lead to the formation of the glycerol phase, while at the same time increasing the biofuel yield. The methyl formate is easily synthesized at chemical industry enterprises from methanol, which can be obtained from raw materials of biological origin; the fuel obtained in this way has a lesser negative impact on the environment.
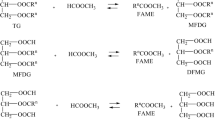
Like the transesterification of triglycerides, the interesterification process takes place in three stages (Fig. 1). Using methyl formate as an acyl receptor in the first step, one molecule of fatty acid methyl ester (conventional biodiesel) and one molecule of monoformyldiglicerides (MFG) are formed, in the second step, another molecule of biodiesel and diformylmonoglyceride (DFG) is formed by reacting monoformylglyceride with methylformate, and in the third step, triformylglycerol (TFG) and biodiesel are formed (Fig. 1).
Studies on the use of methyl formate in the synthesis of biofuels using rapeseed oil and a chemical catalyst (28.6% sodium methoxide in methanol) have been initiated. Synthesis at 27 °C with a molar ratio of catalyst to oil of 0.16:1, molar ratio of methyl formate to oil of 18:1, and a reaction time of 60 min gave a yield of 88.3% biodiesel [31]. The authors tried to perform the research using biochemical catalysts but did not get positive results. Although the results of the enzymatic interesterification process using methyl and ethyl acetates have been published, the use of enzymes for interesterification with methyl formate has not been studied so far. Therefore, the aim of our work was to investigate the feasibility of interesterification of triglycerides with methyl formate by enzymatic catalysis and to determine the conditions under which the highest yield of rapeseed oil methyl esters is obtained.
2 Materials and methods
Refined rapeseed oil purchased from a trade network was used for the interesterification studies. Analytical grade methyl formate (JSC Labochema, Lithuania) was used as the acyl receptor. The following lipases were used to determine the efficiency of lipases as catalysts in the interesterification process and to select the most efficient ones: Lipase F-EC from Rhizopus oryzae (1); Lipase G “Amano” 50 from Penicillium camemberti (2); Lipase with perlite from Enterobacter aerogenes E13 (3); Lipozyme RM IM from Rhizomucor miehei (4); Lipozyme TL IM from Thermomyces lanuginosus (5); Lipozyme 435 from Candida antarctica (6); Novozym 435 from Candida antarctica (7); Lipolase 100L (from Thermomyces lanuginosus (8); Lipex 100L from Thermomyces lanuginosus (9); Palatase 20000L (10); Lecitase Ultra from Fusarium oxysporum (11); Resinase 2X from Aspergillus oryzae (12); Lipozyme CALB from Candida antarctica B (13); Lipozyme TL 100L from Thermomyces lanuginosus (14); Lipex 100L from Thermomyces lanuginosus (15). Lipases were donated from the global biotechnology company Novozymes A/S (Denmark) and the Institute of Biochemistry (Lithuania). Rapeseed oil (RA), glycerol (KG), and rapeseed oil methyl esters (RME) were used for control.
Interesterification studies were performed in a chemical reactor equipped with a stirrer, a temperature controller, and a reflux condenser. The required amount of rapeseed oil was charged to the reactor; the calculated amount of enzyme preparation and the required amount of methyl formate were added. After refluxing, the mixture was stirred at 20 °C for an appropriate time. In the end, the biocatalyst was separated from the reaction product by filtration and the excess methyl formate was evaporated in a vacuum evaporator. Lipase screening was performed under the same conditions: taking 10% of the lipase from the oil content, at a temperature of 20 °C using a molar ratio of methyl formate to oil of 14:1. The process duration was 5 h. The reaction product was analyzed by thin-layer chromatography as described previously [32]. After selecting the more efficient lipases by thin-layer chromatography, their efficiency was examined by gas chromatography. All experiments were performed in triplicate, and the final result was the average of three measurements.
Optimal interesterification conditions were determined using the most efficient lipase in the same laboratory reactor. The tests were performed at 20 °C; using from 5 to 17% of the biocatalyst from the amount of rapeseed oil; at a molar ratio of 10:1 to 40:1 of methyl formate to oil; the duration of the process ranged from 8 to 48 h. Ester content was assessed by PerkinElmer Clarus 500 gas chromatograph (detector—FID, column—Restek MXT-Biodiesel TG (0, 15 m, − 0.32 mm, − 0.10 µm) as described previously [32] according to LST EN 14,105. The yield of rapeseed oil methyl esters (RME) in the reaction product was calculated as the ratio of the determined content of (RME) to the theoretical content of RME could be obtained after the complete reaction of triglycerides with methyl formate. The theoretical content of RME in the reaction product is 84%, and the triformylglycerol content is 16% [31].
3 Results and discussions
3.1 Influence of catalyst type and amount on interesterification efficiency
The process of synthesis of conventional biodiesel by oil transesterification with alcohols is catalytic and can use a variety of heterogeneous catalysts, including metal oxides and hydroxides [33, 34], ion exchange resins [35], and biocatalysts–enzymatic preparations [36]. Heterogeneous catalysts, lipases, which catalyze the transesterification of oil, can also be used to catalyze the process of oil interesterification with carboxylate esters. The activity of lipases and catalytic efficiency are different and depend on the acyl receptor used. Although some researchers have tried to use other lipases (lipases from porcine pancreas, LPP; Pseudomonas cepacea, LPsC; Candida cylindracea, LCC; Pseudomonas sp., LPs; Mucor javanicus, LMJ; Candida rugosa OF, LOF; and Candida antarctica, LCA), the highest ester yield was obtained by catalyzing the process on immobilized Novozyme 435 lipase [2]. However, the enzyme preparation Novozyme 435 is active in the oil transesterification and interesterification processes and is relatively expensive compared to other lipases that are sometimes used in the synthesis of biodiesel. One of the tasks of our work is to compare the activity of enzymatic preparations for the interesterification of oil using methyl formate and to select a more active lipase. It should be noted that lipases are sensitive to alcohols, and it has been observed that the high amount of methanol used in transesterification inactivates the lipases, so it is advisable to introduce it into the reaction medium in stages. Given that the enzymatic process of interesterification of oil using carboxylate esters has not been studied, 15 lipase efficacy studies were performed using them under the same conditions and analyzing the resulting interesterification product by thin-layer chromatography. The chromatogram is presented in Fig. 2.
The data shows that lipases Novozyme 435, Lipozyme RM IM, and Lipozyme TL IM (commercial lipases produced by company Novozymes A/S) were more effective in the process of interesterification of rapeseed oil with methyl formate. The resulting reaction product was further analyzed by gas chromatography. The results showed that the highest yield of esters was obtained using the immobilized lipase Lipozyme RM IM, which was 26.5%. Lipozyme RM IM (14.8% RME yield) and Novozyme 435 (21.9% RME yield) were less effective. Although Novozym 435 has been shown to be effective in the interesterification of vegetable oil with ethyl acetate [37, 38], the results of our studies using methyl formate demonstrated that Novozyme 435 was less active than Lipozyme RM IM. It should be noted that most interesterification studies were performed using the enzyme preparation Novozyme 435 and methyl or ethyl acetate as acyl receptors, the enzyme preparation Lipozyme RM IM was not used for studies, and Lipozyme TL IM demonstrated the catalytic activity in interestification waste cooking oil with methyl acetate [39]. Based on the results of the screening of enzyme preparations, the lipase Lipozyme RM IM (> 30 U/g) was selected for further research of the interesterification process and determination of optimal conditions.
The efficiency of the interesterification process is greatly influenced not only by the type of catalyst but also by the amount; therefore, the influence of catalyst amount on the yield of esters was investigated in the initial stage. Experiments were performed under the same conditions: at a temperature of 20 °C and a molar ratio of 14:1 of methyl formate to rapeseed oil. The process duration was 7, 9, 12, and 15 h, varying the amount of catalyst from 5 to 17% by weight of the oil. The dependence of the RME yield on the amount of catalyst determined by gas chromatography is shown in Fig. 3.
The data presented show that increasing both the enzyme concentration and the process time increases the ester yield. At the lowest enzyme concentration within 5 h, 30% RME yield was obtained. Increasing the process time to 15 h positively affected the interesterification process; the yield reached 37.8%. Increasing the catalyst content from 5 to 17% RME yield in all cases was higher than 35%. The highest yield of 59.6% was obtained at 15% catalyst concentration and 15-h duration. It can be stated from the obtained data that the optimal catalyst concentration in the process of interesterification of rapeseed oil with methyl formate is 15%. When the highest catalyst amount was used and the duration of the process was 12 h and 15 h, the RME yield differed only slightly.
There are no data on the efficiency of interesterification and its dependence on the amount of biocatalyst using methyl formate as an acyl receptor, but some researchers have investigated the use of enzymes in the process of interesterification with methyl and ethyl acetate. Modi et al. [38] stated that the optimal content of Novozyme 435 in the interesterification with ethyl acetate is 10%. Du et al. [28] indicate the optimal concentration of this enzyme in the interesterification of vegetable oil with methyl acetate is 30%. The results showed that a high yield of interesterification products is obtained using a higher content of enzymatic catalysts comparing with the application of chemical catalysts. The yield of 73.4% rapeseed oil ethyl esters was obtained using 0.4% sodium methanoate and sodium metal as a catalyst, and 60% ester yield was obtained using 5% p-toluenesulfonic acid [40]. Maddikeri et al. [41] obtained 90% yield of ethyl esters using 1% potassium methoxide. Sustere and Kampars [31] investigated the interesterification of rapeseed oil with methyl and ethyl acetates and found that high ester yields of 84.7% and 80.7%, respectively, could be obtained using 0.12 and 0.16 potassium t-butoxide/t-butanol catalyst molar ratio to oil. Using the same amount of alcohol alcoxides (methoxide, ethoxide, isopropoxide, tert-butoxide) as a catalyst for interesterification of rapeseed oil with methyl formate, the authors obtained 77–81% yield of RME in the reaction product [42]. Moisture has also been observed to negatively affect the chemical interesterification process [1]. The RME content of more than 77.6% was obtained only at a moisture content of not more than 0.005% in the oil.
Heterogeneous chemical catalysts were also used for interesterification with methyl and ethyl acetate, but their activity was lower than that of homogeneous catalysts. Yields greater than 90% of methyl and ethyl esters for interesterification using methyl and ethyl acetates were obtained using 10% of tin(II) octanoate catalyst [43], 61.5% yield of methyl esters was obtained with 5% niobium phosphate, and 60.5% yield of methyl esters was obtained with 2% ɣ-aluminum catalysts [44].
3.2 Influence of molar ratio of methyl formate to oil on the efficiency of interesterification
The amount of reactants in the reaction medium also has a significant effect on the interesterification efficiency of triglycerides. In order for a triglyceride molecule to react completely, three molecules of carboxylate esters are required. In the case of methyl formate, three methyl radicals react with the fatty acid chains to form 3 molecules of fatty acid methyl esters (conventional biodiesel), and three formic acid groups with the glycerol residue give triformylglycerol. The interesterification reaction takes place in stages to form intermediates (MFG and DFG). The reaction is equilibrium; in order to shift the equilibrium towards the reaction products and increase the yield of the reaction products, an excess of reactants is used. The efficiency of the interesterification can be increased by taking a ratio of methyl formate to oil greater than 3:1. In order to determine the optimal amount of methyl formate, the interesterification process was studied by varying the molar ratio of methyl formate to rapeseed oil from 10:1 to 40:1. The process was performed using 15% lipase Lipozyme RM IM at 20 °C and 7-h process duration. The content of formyl glycerides in the reaction product, the yield of RME, and the amount of unreacted triglycerides were analyzed. The obtained data are presented in Fig. 4 and Fig. 5.
It is clear that the yield of RME increases with increasing molar ratio of methyl formate to oil. With an increase in the molar ratio to 20:1, the increase in RME yield is negligible. A more significant increase is observed when the molar ratio is increased above 20:1, but at molar ratios greater than 32:1, the yield of RME differs slightly and is higher than 46%. It should be noted that the reaction mixture contains a high amount of unreacted oil. The lowest triglyceride content is about 52.33% at the highest molar ratio of 40:1 of methyl formate to oil. Even at high methyl formate ratios, no more than 50% of triglycerides react under the test conditions. The content of MFG, DFG, and TFG in the reaction product also increases slightly with increasing molar ratio of methyl formate to oil. Diformylglycerides account for the largest share of glycerides. The presence of mono- and DFG in the reaction product indicates that some of the triglycerides did not react completely with the methyl formate, i.e., the process stopped at the first or second interesterification stage and only one or two formic acid radicals replaced fatty acid radical to form MFG or DFG. The content of MFG in the reaction product ranged from 1.56 to 2.39%, the content of DFG was almost twice as high and ranged from 3.38 to 4.39%, while the content of TFG, the fully reacted triglyceride products, ranged from 1.35 to 1.91%. The higher content of DFG indicates that the first and second stages of the interesterification reaction were more efficient, while the attachment of the third formic acid group to the DFG was more difficult. The increase in the amount of methyl formate did not significantly affect the rate of the third interesterification stage. At a molar ratio of methyl formate to oil greater than 32:1, no significant changes in formylglyceride and triglyceride concentrations as in the ester yield were observed, so this molar ratio was chosen as optimal and used in further studies.
Unlike conventional biodiesel production by transesterification with methanol, the interesterification process requires a significantly higher excess of methyl formate. This indicates that the interesterification process is more complex and slower than the transesterification process. The results obtained confirm the results reported by other researchers who studied the interesterification of oil using methyl and ethyl acetates using chemical catalysts. The use of 0.4% sodium methanolate and metal sodium dispersion catalyst for the interesterification of rapeseed oil gave a high yield of ethyl esters (89.9%) only at a molar ratio of 11:1 methyl acetate to oil, and using 5% of p-toluenesulfonic acid as a catalyst at a molar ratio of 22:1 the yield of esters of 73.5% was obtained [40]. Casas et al. [5, 45] studied the process of interesterification of sunflower oil with methyl acetate and found that the yield of esters of 94.8% and 95.1% was obtained using only a very high ratio of methyl acetate to oil, which was 50:1 and 48:1, respectively. Sustere and Kampars [31] used rapeseed oil interesterification with various carboxylate esters of low molecular weight to study the interesterification process and an 87.8% of RME yied obtained at molar ratio of 18:1. Interesterification with methyl formate in an 18:1 molar ratio with oil and potassium alkoxide catalyst yielded more than 70% of RME in the upper layer of reaction product [42]. An even lower molar ratio was used by Maddikerti et al. [41] for waste cooking oil for interesterification with methyl acetate. At a molar ratio of methyl acetate to oil of 12:1 and additional sonication, a yield of 90% fatty acid methyl esters was obtained. High efficiency of interesterification was achieved only by using large excesses of methyl or ethyl acetate to catalyze interesterification with heterogeneous catalysts. Galia et al. [43] and Interrante et al. [46] obtained 90% yield of esters using a molar ratio of 40:1 methyl acetate to rapeseed oil and tin(II) octanoate as a catalyst, while Galia et al. [43] found that interesterification with ethyl acetate was not as efficient as methyl acetate. Using the same 40:1 ratio of ethyl acetate to rapeseed oil and tin(II) octanoate catalyst, only 68% yield of rapeseed ethyl esters was obtained. At lower molar ratios of methyl acetate and oil of 30:1 and 20:1, the yields of RME were 61.5% and 60.5%, respectively [47].
3.3 Influence of process duration on interesterification efficiency
The duration of the process is one of the factors influencing the yield of biotechnological processes. In order to evaluate the influence of process time on the effectiveness of rapeseed oil interesterification with methyl formate, the experiments were performed by varying the process time from 4 to 48 h at a molar ratio of methyl formate to oil of 32:1, enzyme content of 15%, and temperature of 20 °C (Fig. 6 and Fig. 7).
The presented results show that the duration of the interesterification process has a significant effect on the yield of RME. As the reaction time increases, the yield of esters increases. When the process time is 8 h, the RME yield is 41.65%; increasing the time to 48 h, the yield of RME increases to 68.13%.
With a further increase in process time, the increase in RME yield is slower and with a process duration of 40 to 48 h, and the yield varies slightly: 67.9% RME yield was obtained in 40 h and 68.13% in 48 h of process. Therefore, it can be concluded that increasing the duration of the interesterification process beyond 40 h not appropriate. The same is confirmed by the results of the unreacted oil tests. As the RME yield increased, the triglyceride concentration gradually decreased increasing not only the ester yield but also the formyl glyceride concentrations in the reaction product. The distribution of formyl glycerides in the reaction product shows that more MFG and glycerides are formed as the process time increases, while the amount of DFG is lower. The TFG content of the final interesterification reaction product is 2.32% at 8 h, compared to 4.86% at 40 h. This is explained because, over time, the new formyl groups are able to bind to the MFG and DFG formed by replacing the radicals of the fatty acids at the second and third positions of the glycerol group. The amount of MFG in the reaction product increases with the duration of the process and remains relatively high compared to the amount of TFG, indicating that the third step of the interesterification reaction is more complex than the first, with only part of the triglycerides being fully interesterified. 4.35% of TFG are found after 48 h, and 2.94% of DFG and 5.46% of MFG are present in the reaction mixture, which make up the majority of the formic acid and glycerol esters. Other researchers who studied the interesterification process using another acyl receptor and running the process from 20 min up to 40 h also emphasize the significant influence of duration on the efficiency of the process. It was found that the rate of interesterification is higher when chemical alkaline catalysts are used [48]. Interesterification with ethyl acetate using sodium methanolate and metal sodium dispersion as catalyst gave 89.9% fatty acid ethyl esters yield in 20 min. [40]. However, it has been observed that intermediate products are formed during the process, which must be separated by distillation. When using acid catalysts (p-toluensulphonic acid), the process time is longer, and only 73.5% fatty acid ethyl esters yield reached 40 h. The duration of heterogeneous interesterification also varies depending on the catalysts and acyl receptors used. However, Galia et al. [43] indicate that using tin(II) octanoate within 20 h 90% yield of methyl esters and 68% yield of ethyl esters is obtained for interesterification using methyl and ethyl acetate. Shorter process times are reported by Interrante et al. [46], in 4 h yielding 90% of methyl esters. About 60% yield was also obtained by Ribeiro et al. [44, 47] using methyl acetate and ɣ-aluminum (ɣAl2O3) and niobium phosphate catalyst for 1 h of interesterification.
Summarizing the obtained research results, it can be stated that the interesterification process is more complicated than the process of oil transesterification with methanol using alkaline catalysts applied in the synthesis of conventional biodiesel. Although enzymatic preparations can be used as catalysts for the interesterification of triglycerides with carboxylate esters of low molecular weight, the lipase-catalyzed process is slow, requiring a longer time to achieve high ester yields. Interesterification with methyl formate can be used in the synthesis of biodiesel but requires not only a high amount of enzyme and long duration but also a large excess of methyl formate. Increasing the process temperature and at the same time the process speed is not appropriate because methyl formate is very volatile and has a low boiling and evaporation temperature.
4 Conclusions
To prevent the formation of glycerol, the oil transesterification using short-chain alcohols applied in the synthesis of biodiesel can be replaced by the oil interesterification with carboxylate esters.
The interesterification of rapeseed oil with methyl formate can be carried out by a biotechnological method using a catalyst using lipase preparations. In this way, conventional biodiesel and esters of glycerol and formic acid, which are soluble in biodiesel, are obtained. Of the enzyme preparations tested, the industrial enzyme preparation Lipozyme RM IM has a higher catalytic efficiency. The optimum content in the reaction medium is 15% by weight of the oil.
The molar ratio of metiformate to oil has a significant effect on the yield of reaction products. The highest yield of rapeseed oil methyl esters and the lowest concentration of unreacted triglycerides were obtained by carrying out the interesterification process at 20 °C in a molar ratio of methylformate to oil of 32:1 At this molar ratio, a 46.03% yield of RME was obtained, difromylglycerides predominate in the reaction product, and the concentrations of monoformylglicerides and triformylglicerol are low, but increase gradually with increasing molar ratio of methyl formate to oil.
The duration of the process also has a significant impact on the efficiency of the interesterification process. The yield of rapeseed oil methyl esters increases with increasing process time. The yield of esters reaches 67.9% with a process duration of 40 h. Changes in RME yield over an even longer period are not significant. The amount of triformylglycerol in the reaction product also increases with increasing process time. At 40 h, the triformylglycerol concentration is 4.86% and higher than the diformylglyceride concentration.
Data availability
Data sharing is not applicable to this article.
References
Lazdovica K, Kampars V (2020) Influence of moisture and acids on the chemical interesterification of rapeseed oil and ability of the catalyst to promote the glycerol as an undesirable by-product. J Taiwan Inst Chem Eng 111:110–118. https://doi.org/10.1016/j.jtice.2020.04.016
Usai EM, Gualdi E, Solinas V, Battistel E (2010) Simultaneous enzymatic synthesis of FAME and triacetyl glycerol from triglycerides and methyl acetate. Bioresour Technol 101:7707–7712. https://doi.org/10.1016/j.biortech.2010.05.044
Goembira F, Matsuura K, Saka S (2012) Biodiesel production from rapeseed oil by various supercritical carboxylate esters. Fuel 97:373–378. https://doi.org/10.1016/j.fuel.2012.02.051
Goembira F, Saka S (2012) Factors affecting biodiesel yield in interesterification of rapeseed oil by supercritical methyl acetate. Zero-Carbon Energy Kyoto 2011. Japan: Springer 147–152 https://doi.org/10.1007/978-4-431-54067-0_16
Casas A, Ramos AMJ, Pérez A (2011) New trends in biodiesel production: chemical interesterification of sunflower oil with methyl acetate. Biomass Bioenergy 35:1702–1709. https://doi.org/10.1016/J.BIOMBIOE.2011.01.003
Huang X, Bi J, Wang J, Ouyang J, Xiao Y, Hao H, Wang Y, Yin Q (2015) Liquid–liquid equilibrium of binary and ternary systems composed by palm oil or palm oil fractions with methanol/ ethanol and water. Fluid Phase Equilib 404:17–25. https://doi.org/10.1016/j.fluid.2015.06.028
Alavianmehr MM, El-Shaikh M, Akbari F, Behjatmanesh-Ardakani R (2017) A new equation of state for modeling thermodynamic properties of some fatty acids alkyl esters, methyl ester-based biodiesels and their blends. Fluid Phase Equilib 442:53–61. https://doi.org/10.1016/j.fluid.2017.03.004
Moreira K, de Oliveira ALB, de M Júnior LS, Monteiro RRC, da Rocha TN, Menezes FL, Fechine LMUD, Denardin JC, Michea S, Freire RM, Fechine PBA, Souza MCM, dos Santos JCS (2020) Lipase from Rhizomucor miehei immobilized on magnetic nanoparticles: performance in fatty acid ethyl ester (FAEE) optimized production by the Taguchi method. Front Bioeng Biotechnol 8:693. https://doi.org/10.3389/fbioe.2020.00693
Fernandez-Lopez L, Bartolome-Cabrero R, Maria Daniela Rodriguez MD,Cleiton S. Dos Santos C, Rueda N, Roberto Fernandez-Lafuente R (2015) Stabilizing effects of cations on lipases depend on the immobilization protocol. RSC Advances 102 https://doi.org/10.1039/c5ra18344h
Virgen-Ortíz JJ, dos Santos JCS, Ortiz C, Berenguer-Murcia A, Barbosa O, Rodrigues RC, Fernandez-Lafuente R (2019) Lecitase ultra: a phospholipase with great potential in biocatalysis. Mol Catal 473:110405. https://doi.org/10.1016/j.mcat.2019.110405
Melo ADQ, Silva FFM, Santos JCS, Fernández-Lafuente R, Lemos TLG, Filho FAD (2017) Synthesis of benzyl acetate catalyzed by lipase immobilized in nontoxic chitosan-polyphosphate beads. Molecules 22(12):2165. https://doi.org/10.3390/molecules22122165
de Oliveira UMF, de Matos LJBL, de Souza MCM, Pinheiro BB, dos Santos JCS, Gonçalves LRB (2019) Efficient biotechnological synthesis of flavor esters using a low-cost biocatalyst with immobilized Rhizomucor miehei lipase. Mol Biol Rep 46:597–608. https://doi.org/10.1007/s11033-018-4514-z
Rios NS, Neto DMA, Santos JCS, Fechine PBA, Fernández-Lafuente R, Gonçalves LRB (2019) Comparison of the immobilization of lipase from Pseudomonas fluorescens on divinylsulfone or p-benzoquinone activated support. Int J Biol Macromol 134:936–945. https://doi.org/10.1016/j.ijbiomac.2019.05.106
Zahirinejad S, Hemmati R, Homaei A, Dinari A, Hosseinkhani S, Mohammadi S, Vianello F (2021) Nano-organic supports for enzyme immobilization: scopes and perspectives. Colloids Surf B 204:111774. https://doi.org/10.1016/j.colsurfb.2021.111774
Galvão WS, Pinheiro BB, Golçalves LRB, de Mattos MC, Fonseca TS, Regis T, Zampieri D, dos Santos JCS, Costa LS, Correa MA, Bohn F, Fechine PBA (2018) Novel nanohybrid biocatalyst: application in the kinetic resolution of secondary alcohols. J Mater Sci 53:14121–14137. https://doi.org/10.1007/s10853-018-2641-5
Manoel EA, Pinto M, dos Santos JCS, Tacias-Pascacio VG, Freire DMG, Pinto JC, Fernandez-Lafuente R (2016) Design of a core–shell support to improve lipase features by immobilization. RSC Advances 67:62814. https://doi.org/10.1039/C6RA13350A
Nunes YL, de Menezes FL, de Sousa IG, Cavalcante ALG, Cavalcante FTT, da Silva MK, de Oliveira ALB, Mota GFM, da Silva Souza JE, de Aguiar Falcão IR, Rocha TG, Valério RBR, Fechine PBA, de Souza MCM, dos Santos JCS (2021) Chemical and physical chitosan modification for designing enzymatic industrial biocatalysts: how to choose the best strategy? Int J Biol Macromol 181:1124–1170. https://doi.org/10.1016/j.ijbiomac.2021.04.004
Monteiro RRC, Lima PJM, Pinheiro BB, Freire TM, Dutra LMU, Fechine PBA, Gonçalves LRB, De Souza MCM, dos Santos JCS, Fernández-Lafuente R (2019) Immobilization of lipase a from Candida antarctica onto chitosan-coated magnetic nanoparticles. Int J Mol Sci 20(16):E4018. https://doi.org/10.3390/ijms20164018
Pinheiro MP, Rios NS, de S Fonseca T, de Aquino Bezerra F, Rodríguez-Castellón E, Fernandez-Lafuente R, de Mattos MC, dos Santos JCS, Gonçalves LRB (2018) Kinetic resolution of drug intermediates catalyzed by lipase B from Candida antarctica immobilized on immobead-350. Biotechnol Prog 34(4):878–889. https://doi.org/10.1002/btpr.2630
Bonazza HL, Manzo RM, dos Santos JSC, Mammarella EJ (2018) Operational and thermal stability analysis of thermomyces lanuginosus lipase covalently immobilized onto modified chitosan supports. Appl Biochem Biotechnol 84(1):182–196. https://doi.org/10.1007/s12010-017-2546-9
Bezerra RM, Monteiro RRC, Neto DMA, da Silva FFM, de Paula RCM, de Lemos TLG, Fechine PBA, Correa MA, Bohn F, Gonçalves LRB, dos Santos JCS (2020) A new heterofunctional support for enzyme immobilization: PEI functionalized Fe3O4 MNPs activated with divinyl sulfone. Application in the immobilization of lipase from Thermomyces lanuginosus. Enzyme Microb Technol 138:109560. https://doi.org/10.1016/j.enzmictec.2020.109560
Liu DM, Dong Ch (2020) Recent advances in nano-carrier immobilized enzymes and their applications. Process Biochem 92:464–475. https://doi.org/10.1016/j.procbio.2020.02.005
Cavalante FTT, Cavalante ALG, de Sousa IG, Neto FS, dos Santos JCS (2021) Current status and future perspectives of supports and protocols for enzyme immobilization. Catalysts 11(10):1222. https://doi.org/10.3390/catal11101222
Sustere Z, Murnieks R, Kampars V (2016) Chemical interesterification of rapeseed oil with methyl, ethyl, propyl and isopropyl acetates and fuel properties of obtained mixtures. Fuel Process Technol 149:320–325. https://doi.org/10.1016/j.fuproc.2016.04.033
Fabbri D, Bevoni V, Notari M, Rivetti F (2007) Properties of a potential biofuel obtained from soybean oil by transmethylation with dimethyl carbonate. Fuel 86:690–697. https://doi.org/10.1016/j.fuel.2006.09.003
Ognjanovic N, Bezbradica D, Knezevic-Jugovic Z (2009) Enzymatic conversion of sunflower oil to biodiesel in a solvent-free system: process optimization and the immobilized system stability. Bioresour Technol 100:5146–5154. https://doi.org/10.1016/j.biortech.2009.05.068
Ruzich NI, Bassi AS (2010) Investigation of lipase-catalyzed biodiesel production using ionic liquid [BMIM][PF6] as a co-solvent in 500 ml jacketed conical and shake flask reactors using triolein or waste canola oil as substrates. Energy Fuels 24:3214–3222. https://doi.org/10.1021/ef901428k
Du W, Xu Y, Liu D, Zeng J (2004) Comparative study on lipase-catalyzed transformation of soybean oil for biodiesel production with different acyl acceptors. J Mol Catal B Enzym 30:125–129. https://doi.org/10.1016/j.molcatb.2004.04.004
Razack SA, Duraiarasan S (2016) Response surface methodology assisted biodiesel production from waste cooking oil using encapsulated mixed enzyme. Waste Manag 47:98–104. https://doi.org/10.1016/j.wasman.2015.07.036
Patil PD, Reddy H, Muppaneni T, Deng S (2017) Biodiesel fuel production from algal lipids using supercritical methyl acetate (glycerin-free) technology. Fuel 195:201–207. https://doi.org/10.1016/j.fuel.2016.12.060
Sustere Z, Kampars V (2017) The influence of acyl and alcohol moieties of carboxylate esters on rapeseed oil chemical interesterification. Mater Method Technol 11:1–7
Sendzikiene E, Makareviciene V, Gumbyte M (2015) Reactive extraction and fermental transesterification of rapeseed oil with butanol in diesel fuel media. Fuel Process Technol 138:758–776. https://doi.org/10.1016/j.fuproc.2015.07.020
Yoo SJ, Lee HS, Veriansyah B, Kim J, Kim JD, Lee YW (2010) Synthesis of biodiesel from rapeseed oil using supercritical methanol with metal oxide catalysts. Bioresour Technol 101:8686–8689. https://doi.org/10.1016/j.biortech.2010.06.073
Faungnawakij K, Yoosuk B, Supawadee N, Krasae B, Viriya-empikul N (2012) Sr–Mg mixed oxides as biodiesel production catalysts. ChemCatChem 4:209–216. https://doi.org/10.1002/cctc.201100346
Shibasaki-Kitakawa N, Honda H, Kuribayashi H, Toda T, Fukumura T, Yonemoto Y (2007) Biodiesel production usinanionic ion-exchange resin as heterogeneous catalyst. Bioresour Technol 98:416–421. https://doi.org/10.1016/j.biortech.2005.12.010
Gog A, Roman M, Tosa M, Paizs C, Irimie FD (2012) Biodiesel production using enzymatic transesterification-current state and perspectives. Renew Energy 39:10–16. https://doi.org/10.1016/j.renene.2011.08.007
Nguyen HC, Liang SH, Chen SS, Suc CH, Lin JH, Chienc CC (2018) Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: optimization by using response surface methodology. Energy Convers Manag 158:168–175. https://doi.org/10.1016/j.enconman.2017.12.068
Modi MK, Reddy JRC, Rao B, Prasa RBN (2007) Lipase-mediated conversion of vegetable oils into biodiesel using ethyl acetate as acyl acceptor. Bioresour Technol 98:1260–1264. https://doi.org/10.1016/j.biortech.2006.05.006
Subhedar PB, Gogate PER (2016) Ultrasound assisted intensification of biodiesel production using enzymatic interesterification. Ultrason Sonochem 29:67–75. https://doi.org/10.1016/j.ultsonch.2015.09.006
Miesiac I, Rogalinski A, Jozwiak P (2013) Transesterification of triglycerides with ethyl acetate. Fuel 105:169–175. https://doi.org/10.1016/j.fuel.2012.06.086
Maddikeri GL, Pandit AB, Gogate PER (2013) Ultrasound assisted interesterification of waste cooking oil and methyl acetate for biodiesel and triacetin production. Fuel Process Technol 116:241–249. https://doi.org/10.1016/j.fuproc.2013.07.004
Abelniece Z, Laipniece L, Kampars V (2020) Biodiesel production by interesterification of rapeseed oil with methyl formate in presence of potassium alkoxides. Biomass Conv Bioref 12:2881–2889. https://doi.org/10.1007/s13399-020-00874-z
Galia A, Centineo A, Saracco G, Schiavo B, Scialdone O (2014) Interesterification of rapeseed oil catalyzed by tin octoate. Biomass Bioenergy 67:193–200. https://doi.org/10.1016/j.biombioe.2014.04.025
Ribeiro JS, Celante D, Simões SS, Bassaco MM, Silva C, Castilhos F (2017) Efficiency of heterogeneous catalysts in interesterification reaction from macaw oil (Acrocomia aculeata) and methyl acetate. Fuel 200:499–505. https://doi.org/10.1016/j.fuel.2017.04.003
Casas A, Ramos MJ, Pérez A (2013) Methanol-enhanced chemical interesterification of sunflower oil with methyl acetate. Fuel 106:869–872. https://doi.org/10.1016/j.fuel.2012.11.037
Interrante L, Bensaid S, Galletti C, Pironeb R, Schiavo B, Scialdone O, Galia A (2018) Interesterification of rapeseed oil catalysed by a low surface area tin (II) oxide heterogeneous catalyst. Fuel Process Technol 177:336–344. https://doi.org/10.1016/j.fuproc.2018.05.017
Ribeiro JS, Celante D, Brondani LN, Trojahn DO, Silva C, Castilhos F (2018) Synthesis of methyl esters and triacetin from macaw oil (Acrocomia aculeata) and methyl acetate over γ-alumina. Ind Crop Prod 124:84–90. https://doi.org/10.1016/j.indcrop.2018.07.062
Freedman B, Pryde EH, Mounts TL (1984) Transesterification kinetics of soybean oil. J Am Oil Chem Soc 61:1638–1643. https://doi.org/10.1007/BF02679606
Acknowledgements
The authors are thankful for samples of biocatalyst presented by JSC “Biopolis.”
Funding
The research was funded by a project MIP-22/59 Synthesis of innovative biodiesel from the Research Council of Lithuania.
Author information
Authors and Affiliations
Contributions
Conceptualization: Violeta Makareviciene, Egle Sendzikiene. Methodology: Kiril Kazancev, Egle Sendzikiene. Formal analysis and investigation: Ieva gaide, Kiril Kazancev. Writing—Ieva Gaide, Violeta Makareviciene. Writing—review and editing: Ieva Gaide, Violeta Makareviciene. Funding acquisition: Violeta Makareviciene. Resources: Violeta Makareviciene, Egle Ssendzikiene. Supervision: Violeta Makareviciene.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Makareviciene, V., Sendzikiene, E., Gaide, I. et al. Application of methyl formate in the process of biotechnological interesterification of triglycerides for the production of biodiesel. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03390-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03390-4