Abstract
Background
While some clinical reports suggest minimally invasive surgical (MIS) techniques improve recovery and reduce pain in the first months after TKA, it is unclear whether it improves gait and thigh muscle strength.
Questions/Purposes
We hypothesized TKA performed through a mini-subvastus approach would improve subjective and objective and subjective function compared to a standard medial parapatellar approach 2 months after surgery.
Methods
We randomized 40 patients into two groups using either the mini-subvastus approach or standard medial parapatellar approach. Patients were evaluated preoperatively and 2 months after surgery. We assessed subjective functional outcome and quality of life (QOL) using routine questionnaires (SF-12, Knee Society Score [KSS], Knee Injury and Osteoarthritis Outcome Score [KOOS], UCLA activity, patient milestone diary of activities). We determined isometric strength of the thigh muscles and assessed gait with a three-dimensional (3-D) analysis during level walking and stair climbing.
Results
We observed improvements from preoperatively to 2 months postoperatively in functional scores, QOL, and knee kinematic and kinetic gait parameters during level and stair walking. Isometric quadriceps strength increased in both groups, although remaining lower when compared to sound limbs. We found no differences between the groups in KSS, SF-12, KOOS, UCLA activity, patient milestone diary of activities, isometric quadriceps strength, or 3-D gait parameters, except a marginally higher speed of stair ascent in the MIS group.
Conclusions
Our observations suggest an MIS approach does not confer a substantial advantage in early function after TKA.
Level of Evidence
Level I, therapeutic study. See Instructions to Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, several authors have demonstrated minimally invasive surgical (MIS)-TKA techniques can be reliably performed and have judged them acceptable in routine clinical practice [8, 23, 29]. The main goals of MIS TKA are to minimize surgical trauma, decrease postoperative pain, and improve the rehabilitation and early functional recovery after surgery [8, 29]. While the definition of MIS TKA is the subject of debate, many authors would include a smaller skin incision, minimal disruption of the suprapatellar pouch, no eversion of the patella, no dislocation of the tibiofemoral joint, and minimal disruption of the quadriceps tendon [8, 29, 32, 33]. MIS-TKA procedures have evolved to include the use of surgical instrumentation designed specifically to minimize soft tissue damage and accommodate the smaller exposure [8, 10, 29]. Surgeons have described several surgical approaches, including the mini-parapatellar approach [13], the quad-sparing approach [20], and the mini-subvastus approach [8, 29]. Anatomic and clinical studies show the mini-subvastus approach provides a good exposure through a small incision, preserves all four attachments of the quadriceps to the patella, does not require patella eversion, minimizes disruption in the suprapatellar pouch, and allows a rapid and reliable closure of the knee [10, 32, 33, 39, 48]. Clinical trials suggest patients who underwent a MIS TKA using a mini-subvastus approach benefit in the short term from quicker recovery time, active straight-leg raising, improved early gains in flexion, reduced pain scores and analgesic use, improved quadriceps strength, and shortened length of postoperative stay within the first months after surgery when compared to a standard medial parapatellar approach [6, 17, 33, 34, 40, 48]. Other authors have raised concerns about the potential for perioperative complications, component malposition issues, and lack of convincing data regarding long-term benefit with MIS techniques [5, 6, 9, 11, 45]. Even some advocates of MIS approaches have suggested any short-term improvements in function must be weighed against the downsides of a longer operation and a higher risk of complications [5, 6, 9, 11, 45]. Moreover, beside patient-reported outcome and clinical evaluation based on surgeon examination, the usefulness and the potential benefits of MIS TKA have never been evaluated in a randomized controlled trial using patient’s objective functional outcome parameters with comprehensive gait analysis and strength testing outcomes during the early rehabilitation period 2 months after TKA.
We hypothesized TKA performed through a mini-subvastus approach would result in improved subjective and objective function compared to a standard medial parapatellar approach 2 months after surgery.
Patients and Methods
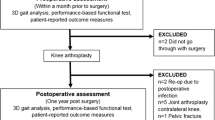
We identified 40 patients scheduled to undergo a primary unilateral TKA between May 2007 to May 2011 who volunteered to participate in the study (Fig. 1) [42]. The initial diagnosis was unilateral knee osteoarthritis (OA) affecting predominantly the medial tibiofemoral compartment (Kellgren-Lawrence grade ≥ 3) in patients between 45 and 80 years old [19]. We excluded patients if they had pain or other symptoms of OA on other lower limb joints (ie, contralateral hip, knee, and ankle and ipsilateral hip and ankle), a preoperative knee flexion of lower than 90°, a valgus or varus knee deformity of greater than 15°, other substantial neurologic or musculoskeletal disorders or diseases (including congenital and developmental etiology) that may affect normal gait or weightbearing ability, history of previous hip and/or knee surgery, a BMI of greater than 40, pregnancy, or presence of infectious diseases. During the study period, we scheduled a total of 525 patients for primary unilateral TKA. Three patients matching the inclusion criteria were excluded as they declined to participate in the study (Fig. 1). Thirty-seven patients were randomized into two groups: a mini-subvastus approach group (MIS group, 19 patients) and a standard medial parapatellar approach group (control group, 18 patients) (Fig. 1). The randomization occurred before surgery to assign patients to a specific treatment group in an unbiased manner. The assigned treatment was generated by a computerized randomization program administered by our department of biostatistics and dynamically balanced the two groups based on age, sex, and BMI. Both the patient and the evaluator physiotherapist were blinded regarding the surgical approach. One patient in the MIS group was lost to followup as he did not return for the postoperative evaluation and did not respond to telephone calls or letters (Fig. 1). Therefore, 18 patients in both groups were analyzed in this study. No differences were found between the patients in the two groups related to the following preoperative parameters: age, sex ratio, BMI, and involved limb side (Table 1). Institutional review board approval and written informed consent were obtained from each patient before their involvement in the study.
A flow diagram illustrates patients’ enrollment, allocation, followup, and analysis [42].
The sample size calculation was based on a difference in walking speed of 0.1 m/second being associated with a clinically important difference in patient’s perception of their walking ability, improved health status, improved physical function, fewer basic disabilities, fewer instrumental disabilities, fewer hospitalization days, and 1-year cost reductions of health care [35, 37]. Assuming the variability in walking speed would be 0.13 m/second, which is similar to other studies evaluating outcomes after TKA [44], a power of 90%, and a significance level of 5%, the required sample size was 18 patients in each group.
TKA was performed at our institution by a single experienced surgeon (MWP). All patients received the same cemented tricompartmental posterior-stabilized prosthesis with a mobile bearing (Sigma® RP knee system; DePuy Orthopaedics, Inc, Warsaw, IN, USA). The patella was systematically resurfaced with a cemented oval-dome component. Only the surgical approach for inserting the components differed between the two groups. All patients had peripheral nerve blockade with an indwelling femoral nerve catheter and a single-shot sciatic nerve block preoperatively. Intraoperative anesthesia was performed with either spinal or general anesthesia at the discretion of the anesthesiologist. All procedures were performed using a tourniquet that was released before closure and with instrumentation specifically dedicated to MIS TKA. In the MIS group, the procedures were performed using an optimized mini-subvastus approach described previously [33]. In this optimized approach, the proximal limb of the arthrotomy was made at the midpole of the patella at a 50° angle relative to the long axis of the femur and stayed parallel to the inferior edge of the vastus medialis obliquus muscle. Therefore, the entire broad and robust tendinous insertion of the vastus medialis obliquus muscle to the patella was spared and consequently provided an ideal location to place the retractors without damaging the quadriceps muscle [33, 39]. In addition, the patella was not everted and the tibiofemoral joint was not dislocated except during the definitive placement of the tibial tray. In the control group, a standard medial parapatellar approach was performed with patellar eversion. Patients were admitted to the hospital after surgery and a 2- or 3-day stay was routine. All patients received appropriate anticoagulation for deep venous thrombosis prophylaxis and routine postoperative antibiotic prophylaxis. No peri- or postoperative surgical complications were reported.
The same postoperative physical rehabilitation protocol was used for each patient regardless of group. Structured physical therapy was begun the day after surgery and continued during the hospitalization period. The patients were instructed to sit up at the bedside the evening of their surgery and to begin ambulating with assistance the day after surgery. Active ROM was encouraged and full weightbearing ambulation was allowed on Postoperative Day 2 when quadriceps inhibition from the femoral nerve block had ceased. Discharge was allowed when patients could ambulate 30 m, ascend and descend three steps, and had pain well controlled with oral medications. Patients were sent home with three specific knee ROM exercises and encouraged to seek formal physical therapy on an outpatient basis two or three times per week for the first month.
Each patient was evaluated preoperatively and 2 months postoperatively in the Biomechanics and Motion Analysis Laboratory. Evaluation was performed by a physiotherapist blinded to the surgical approach and not involved in any other aspect of the patient’s management, care, or rehabilitation. The preoperative and postoperative evaluations entailed a physical examination with specific evaluation of pain, patient’s function, knee motion, and gait. The following parameters were assessed: (1) patient’s subjective function and perceived quality of life (QOL) survey evaluated by the Knee Society Score (KSS) [16], Knee Injury and Osteoarthritis Outcome Score (KOOS) [38], SF-12 [49], and UCLA activity score [3, 49]; (2) return to normal activities of daily living (ADL) recorded by the attainment of early functional milestones; (3) thigh muscle strength; and (4) patient’s objective functional outcome using three-dimensional (3-D) gait analysis methods to study level walking, stair ascent, and stair descent.
In addition, each patient received a milestone diary to record the specific time when he/she discontinued using walker, crutches, or cane or taking narcotic pain medication or when he/she returned to carrying out normal ADL (defined as the time when the patient felt safe to be left at home all day with no additional help), driving a car, negotiating stairs independently without a walker or cane, or walking a six-block distance.
The thigh muscle strength was measured isometrically (Biodex® System 3 Pro; Biodex Medical Systems, Inc, Shirley, NY, USA) [4, 25, 46]. The patients were seated on the testing bench with 100° of hip flexion, the back supported in a comfortable position, and the pelvis and tested thigh stabilized with a belt or a strap to minimize extraneous body movements. The testing was completed at 60° of knee flexion, which represents the angle for maximal isometric quadriceps force generation [25]. The patients warmed up by performing a series of submaximal isometric contractions of the knee extensor and flexor muscles. The patients were then asked to produce their maximal effort as fast and forcefully as possible and to maintain the contraction for 5 seconds. Three 5-second trials of maximal strength effort were collected with a 30-second rest period between contractions. The maximum value of peak torque obtained across the three trials was used.
Gait analysis was performed in a motion analysis laboratory environment equipped with a 10-infrared camera motion capture system sampling at 60 Hz (EvaRT 5.04®; Motion Analysis Corp, Santa Rosa, CA, USA) [18]. Retroreflective markers were placed at bony prominences of the iliac crest, lateral thigh, lateral and medial aspects of the knee, fibular head, lateral malleolus, posterosuperior part of the calcaneal tubercle, and fifth metatarsal head to define the anatomic coordinate system for the pelvis, thigh, shank, and foot. The ground reaction forces were collected at 600 Hz using two AMTI BP2416® force platforms (Advanced Mechanical Technology Inc, Watertown, MA, USA) and two Kistler 9281B® force platforms (Kistler Instrument Corp, Amherst, NY, USA) embedded in the floor in the center of the calibration volume. During stair negotiation, motion was tracked as the patients ascended and descended a seven-step flight of stairs of standard dimension (rise = 16.5 cm; tread = 27.5 cm). Two AMTI ZBP245600® force platforms (Advanced Mechanical Technology) were mounted on the third and fourth step for kinetic data acquisition at 600 Hz. One set of data corresponding to the standing position (static data) was acquired to define the location of the joint centers. After patient instruction and orientation, gait analysis was performed while the patients walked barefoot at a self-selected comfortable speed over a 15-m level walking or during stair ascent and descent. Three successful trials (full contact on the force plate and all camera views) were collected for each condition. The 3-D marker trajectories were smoothed using a two-pole, low-pass Butterworth filter implemented with time reversal to induce zero phase lag and a cutoff frequency of 7.4 Hz given the 60-Hz sampling frequency. The 3-D marker coordinate data and force plate data were used as input to a commercial software program (Visual3D® 4.0; C-Motion, Germantown, MD, USA) to calculate the joint kinematics and kinetics. The gait cycle periods were selected by heel strike to heel strike events. All gait events were expressed as a percentage of the gait cycle, irrespective of the actual time for a stride, to yield a normalized gait cycle. The joint moments were reported as internal moments normalized by body mass (kilograms) to minimize discrepancies between patients due to body size and sex. The peak and SD of the joint angles during the gait cycle and joint kinetics during the stance phase were extracted as variables of interest in all three planes for the hips, knees, and ankles. For each gait condition, the variables acquired from the three trials were averaged for data analysis. Furthermore, spatiotemporal parameters were extracted and analyzed.
Data descriptive statistics are presented as mean ± SD. We compared qualitative variables using the chi-square test. We analyzed quantitative and continuous variables using the following parametric tests: (1) Student’s paired t-tests for intragroup comparison of two variables and (2) two-sample t-tests for intergroup comparison of two variables. We performed statistical analyses using SPSS® 19.0 (IBM Corp, Somers, NY, USA).
Results
At 2 months postoperatively, we found improvement (p < 0.001–0.003) in all scores in both groups compared to preoperatively, except for the SF-12 mental component subscale in both groups and the KOOS sports and recreation function dimension in the standard-TKA group (Table 2). There were no differences between groups as assessed by clinical scores, patients’ perceived functional outcome, and QOL surveys at 2 months postoperatively. We found no differences in pain relief between groups in the KSS and KOOS pain subscales (p = 0.24 and 0.13, respectively). There were no differences in ADL and function between groups in the KSS function, KOOS ADL, and SF-12 physical subscales and UCLA activity scale (p = 0.09–0.91). We observed no differences in the patients’ self-assessed QOL between groups in the KOOS QOL and SF-12 mental subscales (p = 0.48 and 0.79, respectively). We also found no differences between groups (p = 0.14–1.00) in patients’ attainment of functional milestones early after surgery as assessed by the patient milestone diary (Table 3).
At 2 months postoperatively compared to preoperatively, isometric quadriceps strength increased in both groups (p = 0.022 and 0.038, respectively), but both groups remained weaker compared to the nonoperated knee (p = 0.007 and 0.002, respectively) (Table 2). The flexion strength of the involved knee was also consistently weaker than that of the nonoperated knee (p = 0.005–0.010). There was no difference between groups in either the isometric quadriceps strength as assessed by the isometric knee extension peak (p = 0.79) or the knee flexion strength of the operated knee at 2 months postoperative.
At 2 months postoperatively compared to preoperatively, we observed improvements in each of the spatiotemporal parameters of gait in both the MIS group (Table 4) and the control group (Table 5). The involved single-limb support time increased (p = 0.022 and p = 0.004, respectively); double-limb support time decreased (p = 0.003 and p = 0.002, respectively); and walking speed and stride length increased (p < 0.0001–0.005). We found no differences between groups in the spatiotemporal gait parameters during level walking or stair climbing at 2 months postoperative (p = 0.068–0.94), except that stair ascent was marginally faster (p = 0.018) in the MIS group (0.44 ± 0.02 versus 0.41 ± 0.04 m/second, respectively). We also found improvements in each of the kinematic and kinetic parameters of gait for all walking conditions for both the MIS group (Table 4) and the control group (Table 5). Along with an increase in knee valgus angle, knee varus angle and moment decreased in the MIS group (p < 0.0001–0.005) and knee varus moment decreased in the standard-TKA group (p = 0.044). Knee power generation increased during level walking in both groups, as well as during stair ascent in the control group (p < 0.0001–0.048). Knee extension moment and knee power absorption were improved in both groups during stair descent (p = 0.007–0.038). In addition, ankle plantarflexion moment and power generation of the involved lower limb in both groups, as well as ankle power absorption in the control group, increased during level walking (p = 0.014–0.048). No differences in the kinematic and kinetic gait parameters during level walking or stair climbing were detected between groups at 2 months postoperatively (p = 0.65–0.98).
Discussion
Patient expectations and demands after TKA have increased in recent years [28, 31]. Prolonged postoperative pain and delayed return of function remain the two greatest concerns of today’s patient and may contribute to dissatisfaction after TKA [28, 31, 47]. Some surgeons have suggested MIS TKA might result in earlier hospital discharge, quicker functional recovery, and better patient satisfaction [7, 23, 29]. However, at least three recent meta-analyses raise questions as to whether quicker recovery and better satisfaction are consistently associated with MIS TKA [9, 11, 45]. During the evolution of MIS TKA, there has also been the introduction of advanced pain management protocols, rapid rehabilitation protocols, early hospital discharge protocols, and comprehensive patient education initiatives. Objective evidence of a clear and convincing benefit of MIS-TKA techniques over standard-TKA techniques when both are coupled with advanced anesthetic, pain management, and rapid rehabilitation protocols has been lacking. We specifically determined whether TKA performed through a mini-subvastus approach would result in an improved functional outcome when compared to a standard medial parapatellar approach 2 months after surgery as assessed by comprehensive gait analysis, strength testing, a patient milestone diary of activities, and multiple questionnaire-based outcome tools.
In addition to intrinsic limitations common to all motion analysis studies (ie, variability in gait measurements due to body anthropometrics and independent skin motion, definition of the neutral position, and time and expense of gait studies), our study presented with some limitations [12, 43, 50]. The first limitation is due to our limited sample size of 18 patients/group. Although the two populations were homogeneous in both the pre- and postoperative periods, a larger sample size might reveal differences in the objective functional outcome parameters not found in our study. However, the meaningfulness of such differences remained of unclear clinical importance to the patients. Second, despite the fact that the study covered a short but critical term when the benefits of the MIS approach to TKA are maximal, this 2-month followup does not allow one to determine any additional improvement in an intermediate-term followup period of 6 months to 1 year postoperatively or any potential adverse long-term effects such as higher failure rates. However, our study was focused on the potential benefits of MIS TKA during the early recovery period.
Despite a strict surgical protocol to ensure no extensor mechanism disruption, no patella eversion, and no knee dislocation during the procedure in the MIS group [32, 33], our results did not demonstrate any differences in objective functional outcome between the MIS- and standard-TKA groups. In our study, there was no difference of the mini-subvastus approach when compared to medial parapatellar approach in terms of postoperative pain reduction, patient’s perceived functional outcome and QOL, quadriceps strength, or gait performance 2 months after TKA. In addition, patients did not recover faster after MIS TKA than after conventional TKA as measured by the attainment of functional milestones in the early postoperative period.
Our finding of no substantial objective functional benefit of MIS TKA as compared to standard TKA stands in distinction to previous randomized controlled trials in which patients achieved and sustained better perceived functional outcomes, higher knee flexion, earlier straight-leg rise, earlier ambulation, or less pain after surgery [6, 17, 34, 40, 41, 48]. However, in our study, the same patient education, rapid rehabilitation, and optimized multimodal pain regimen with femoral nerve block and local injection were systematically applied in both groups regardless of surgical approach [21, 24]. Differences in the pain management, rehabilitation, and patient education protocols in the published cohort studies could be an important confounding factor and strongly influence the early postoperative rehabilitation and recovery. Rapid rehabilitation protocols reportedly accelerate recovery after TKA, including those performed using a standard surgical approach, and are useful in reducing the length of hospital stay [15, 24]. Reduced postoperative pain, higher knee flexion, and early walking day and straight-leg rise can be objectively compared only when the patient education, pain management, and rehabilitation protocols are the same between the surgical groups.
While we found no differences in perioperative complications between the MIS- and standard-TKA groups, other authors have encountered surgical technical difficulties with MIS techniques [5, 6, 11, 34]. In our study, the low rate of complications, particularly in the MIS group, might be attributable to the high-volume experience in MIS TKA performed at our institution or simply reflect the relatively small number of knees included in this trial [1, 8]. A meta-analysis reviewing complications related to MIS TKA suggested MIS approaches offered early but short-lived benefits for patients at the expense of a longer operation and a higher risk of complications, such as patellar ligament injury or rupture, lateral femoral condylar fracture, femoral notching, peroneal nerve palsy, gross component malposition, delayed wound healing due to excessive retractor tension, and skin necrosis or infections as a consequence of a reduced access and overall visibility [11]. In addition, Barrack et al. [5] showed, in a consecutive series of TKA revisions performed at three referral centers, the limited exposure in MIS TKAs predisposed to malrotation and/or malalignment and ligament imbalance or instability that may lead to early failure within 24 months in younger patients when compared with the standard procedure.
To our knowledge, no published study is dedicated to gait performance analysis after MIS TKA with direct comparison to conventional TKA. Gait analysis can objectively document comprehensive joint mechanics after TKA particularly on knee loading and function with pathologic conditions [18, 22, 26]. In our study, the sagittal plane kinematics and kinetics were improved in the two groups during stair negotiation. Importantly, the knee extension moment and power absorption were enhanced in both groups during stair descent and corresponded to an improved function and loading condition for stabilizing and damping the body weight excursion during a challenging locomotor task. This is the first study to report knee mechanics during stair descent after TKA, and thus, no comparison for this task could be made with the literature. In addition, the kinematics and kinetics in the frontal plane were also improved in the two groups during level walking. Importantly, the knee varus angle and moment were decreased, highlighting the frontal plane knee deformity correction after TKA. Our results were in accordance with previous studies showing a normalization of the knee varus angle and moment as a result of surgery when compared with normal subjects and underlined the tibiofemoral joint realignment effect of TKA [2, 14, 27, 30, 36]. Therefore, all these improvements in knee kinematics and kinetics parameters found in our study corresponded to improvements in gait after TKA and should be considered as higher impact attenuation ability and functional changes toward a more asymptomatic pattern regardless of the surgical approach used.
In conclusion, we found no substantial differences between TKA using the MIS approach and the standard medial parapatellar approach with regard to function at 2 months after surgery as assessed by comprehensive gait analysis, strength testing, patient milestone diary of daily activities, and multiple questionnaire-based outcome tools. In the contemporary setting where advanced anesthetic, pain management, rapid rehabilitation, and patient education protocols are employed routinely, an MIS approach does not appear to confer a substantial advantage in regard to early functional outcome after TKA.
References
Aglietti P, Baldini A, Giron F, Sensi L. Minimally invasive total knee arthroplasty: is it for everybody? HSS J. 2006;2:22–26.
Alnahdi AH, Zeni JA, Snyder-Mackler L. Gait after unilateral total knee arthroplasty: frontal plane analysis. J Orthop Res. 2011;29:647–652.
Amstutz HC, Thomas BJ, Jinnah R, Kim W, Grogan T, Yale C. Treatment of primary osteoarthritis of the hip: a comparison of total joint and surface replacement arthroplasty. J Bone Joint Surg Am. 1984;66:228–241.
Bade MJ, Kohrt WM, Stevens-Lapsley JE. Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther. 2010;40:559–566.
Barrack RL, Barnes CL, Burnett RS, Miller D, Clohisy JC, Maloney WJ. Minimal incision surgery as a risk factor for early failure of total knee arthroplasty. J Arthroplasty. 2009;24:489–498.
Boerger TO, Aglietti P, Mondanelli N, Sensi L. Mini-subvastus versus medial parapatellar approach in total knee arthroplasty. Clin Orthop Relat Res. 2005;440:82–87.
Bonutti PM, Zywiel MG, McGrath MS, Mont MA. Surgical techniques for minimally invasive exposures for total knee arthroplasty. Instr Course Lect. 2010;59:83–91.
Bonutti PM, Zywiel MG, Ulrich SD, McGrath MS, Mont MA. Minimally invasive total knee arthroplasty: pitfalls and complications. Am J Orthop. 2010;39:480–484.
Cheng T, Liu T, Zhang G, Peng X, Zhang X. Does minimally invasive surgery improve short-term recovery in total knee arthroplasty? Clin Orthop Relat Res. 2010;468:1635–1648.
Coon TM. Specialized instruments and modular implants for minimally invasive total knee arthroplasty. Am J Orthop. 2006;35:12–17.
Gandhi R, Smith H, Lefaivre KA, Davey JR, Mahomed NN. Complications after minimally invasive total knee arthroplasty as compared with traditional incision techniques: a meta-analysis. J Arthroplasty. 2011;26:29–35.
Growney E, Meglan D, Johnson M, Cahalan T, An KN. Repeated measures of adult normal walking using a video tracking system. Gait Posture. 1997;6:147–162.
Han I, Seong SC, Lee S, Yoo JH, Lee MC. Simultaneous bilateral MIS-TKA results in faster functional recovery. Clin Orthop Relat Res. 2008;466:1449–1453.
Hatfield GL, Hubley-Kozey CL, Astephen Wilson JL, Dunbar MJ. The effect of total knee arthroplasty on knee joint kinematics and kinetics during gait. J Arthroplasty. 2011;26:309–318.
Healy WL, Iorio R, Ko J, Appleby S, Lemos SW. Impact of cost reduction programs on short-term patient outcome and hospital cost of total knee arthroplasty. J Bone Joint Surg Am. 2002;84:348–353.
Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14.
Kashyap SN, van Ommeren JW. Clinical experience with less invasive surgery techniques in total knee arthroplasty: a comparative study. Knee Surg Sports Traumatol Arthrosc. 2008;16:544–548.
Kaufman K, Hughes C, Morrey B, Morrey M, An K. Gait characteristics of patients with knee osteoarthritis. J Biomech. 2001;34:907–915.
Kellgren J, Lawrence J. Radiological assessment of osteo-arthritis. Ann Rheumatic Dis. 1957;16:494–502.
Kim YH, Kim JS, Kim DY. Clinical outcome and rate of complications after primary total knee replacement performed with quadriceps-sparing or standard arthrotomy. J Bone Joint Surg Br. 2007;89:467–470.
Krych AJ, Horlocker TT, Hebl JR, Pagnano MW. Contemporary pain management strategies for minimally invasive total knee arthroplasty. Instr Course Lect. 2010;59:99–109.
Kuster MS, Wood GA, Stachowial GW, Gachter A. Joint load considerations in total knee replacements. J Bone Joint Surg Br. 1997;79:109–113.
Leopold SS. Minimally invasive total knee arthroplasty for osteoarthritis. New Eng J Med. 2009;360:1749–1758.
Lombardi AV Jr, Viacava AJ, Berend KR. Rapid recovery protocols and minimally invasive surgery help achieve high knee flexion. Clin Orthop Relat Res. 2006;452:117–122.
Maffiuletti NA, Bizzini M, Widler K, Munzinger U. Asymmetry in quadriceps rate of force development as a functional outcome measure in TKA. Clin Orthop Relat Res. 2010;468:191–198.
Mandeville D, Osternig LR, Chou LS. The effect of total knee replacement on dynamic support of the body during walking and stair ascent. Clin Biomech (Bristol, Avon). 2007;22:787–794.
Mandeville D, Osternig LR, Lantz BA, Mohler CG, Chou LS. The effect of total knee replacement on the knee varus angle and moment during walking and stair ascent. Clin Biomech (Bristol, Avon). 2008;23:1053–1058.
Masson JB. The new demands by patients in the modern era of total joint arthroplasty. Clin Orthop Relat Res. 2008;466:146–152.
Mont MA, Zywiel MG, McGrath MS, Bonutti PM. Scientific evidence for minimally invasive total knee arthroplasty. Instr Course Lect. 2010;59:73–82.
Mundermann A, Dyrby CO, Andriacchi TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum. 2005;52:2835–2844.
Noble PC, Conditt MA, Cook KF, Mathis KB. The John Insall Award. Patient expectations affect satisfaction with total knee arthroplasty. Clin Orthop Relat Res. 2006;452:35–43.
Pagnano MW, Meneghini RM. Minimally invasive total knee arthroplasty with an optimized subvastus approach. J Arthroplasty. 2006;21:22–26.
Pagnano MW, Meneghini RM, Trousdale RT. Anatomy of the extensor mechanism in reference to quadriceps-sparing TKA. Clin Orthop Relat Res. 2006;452:102–105.
Pan WM, Li XG, Tang TS, Qian ZL, Zhang Q, Zhang CM. Mini-subvastus versus a standard approach in total knee arthroplasty: a prospective, randomized, controlled study. J Int Med Res. 2010;38:890–900.
Purser JL, Weinberger M, Cohen HJ, Pieper CF, Morey MC, Li T, Williams GR, Lapuerta P. Walking speed predicts health status and hospital costs for frail elderly male veterans. J Rehabil Res Dev. 2005;42:535–546.
Ramsey DK, Snyder-Mackler L, Lewek M, Newcomb W, Rudolph KS. Effect of anatomic realignment on muscle function during gait in patients with medial compartment knee osteoarthritis. Arthritis Rheum. 2007;57:389–397.
Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the six minute walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155:1278–1282.
Roos EM, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS) —validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17.
Rossi R, Maiello A, Bruzzone M, Bonasia DE, Blonna D, Castoldi F. Muscle damage during minimally invasive surgical total knee arthroplasty traditional versus optimized subvastus approach. Knee. 2011;18:254–258.
Schroer WC, Diesfeld PJ, Reedy ME, LeMarr AR. Mini-subvastus approach for total knee arthroplasty. J Arthroplasty. 2008;23:19–25.
Schroer WC, Diesfeld PJ, Reedy ME, LeMarr AR. Isokinetic strength testing of minimally invasive total knee arthroplasty recovery. J Arthroplasty. 2010;25:274–279.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–732.
Simon SR. Quantification of human motion: gait analysis—benefits and limitations to its application to clinical problems. J Biomech. 2004;37:1869–1880.
Smith AJ, Lloyd DG, Wood DJ. A kinematic and kinetic analysis of walking after total knee arthroplasty with and without patellar resurfacing. Clin Biomech (Bristol, Avon). 2006;21:379–386.
Smith TO, King JJ, Hing CB. A meta-analysis of randomized controlled trials comparing the clinical and radiological outcomes following minimally invasive to conventional exposure for total knee arthroplasty. Knee. 2012;19:1–7.
Staehli S, Glatthorn JF, Casartelli N, Maffiuletti NA. Test-retest reliability of quadriceps muscle function outcomes in patients with knee osteoarthritis. J Electromyogr Kinesiol. 2010;20:1058–1065.
Trousdale RT, McGrory BJ, Berry DJ, Becker MW, Harmsen WS. Patients’ concerns prior to undergoing total hip and total knee arthroplasty. Mayo Clin Proc. 1999;74:978–982.
Varela-Engocheaga JR, Suarez-Suarez MA, Fernandez-Villan M, Gonzales-Sastre V, Varela-Gomez JR, Rodriguez-Merchan C. Minimally invasive subvastus approach: improving the results of total arthroplasty: a prospective, randomized trial. Clin Orthop Relat Res. 2010;468:1200–1208.
Ware JE, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233.
Yu B, Kienbacher T, Growney ES, Johnson ME, An KN. Reproducibility of the kinematics and kinetics of the lower extremity during normal stair-climbing. J Orthop Res. 1997;15:348–352.
Acknowledgments
We thank Mrs. Barbara Iverson for her assistance in manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his/her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Wegrzyn, J., Parratte, S., Coleman-Wood, K. et al. The John Insall Award: No Benefit of Minimally Invasive TKA on Gait and Strength Outcomes: A Randomized Controlled Trial. Clin Orthop Relat Res 471, 46–55 (2013). https://doi.org/10.1007/s11999-012-2486-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-012-2486-1