Abstract
Background
Legg-Calvé-Perthes disease (LCP) is thought to be associated with ischemic events in the femoral head. However, the types and patterns of reperfusion after these ischemic events are unclear.
Purposes
We therefore determined whether (1) there would be any age-related diffusion changes; (2) diffusion-weighted MR imaging would reveal ischemic damage; and (3) diffusion changes are correlated with prognostic MR findings in patients with LCP.
Methods
We prospectively performed conventional, perfusion, and diffusion-weighted MR imaging studies in 17 children with unilateral LCP. We then measured the apparent diffusion coefficient (ADC) values in the epiphysis and the metaphysis, and compared them with those of the contralateral normal side. Based on perfusion MR imaging, we assessed reperfusion to the epiphysis as either periphyseal or transphyseal. We studied T2-signal intensity changes in the metaphysis and the presence of focal physeal irregularity. We correlated diffusion changes with reperfusion to the epiphysis, T2-signal intensity change, and focal physeal irregularity.
Results
Normal diffusion decreased with age. In LCP hips, epiphyseal diffusion increased early and remained elevated through the healing stage. Six of the 17 patients who had a metaphyseal ADC greater than 50% over the normal side had 13 times greater odds of having an association with transphyseal reperfusion to the epiphysis. The increase of metaphyseal ADC also was associated with an increased T2-signal intensity in the metaphysis and presence of focal physeal irregularity.
Conclusions
Diffusion-weighted MR imaging can be used as a complimentary modality to evaluate ischemic tissue damage with a potential prognostic value in patients with LCP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
LCP is believed to be associated with ischemic events in the capital femoral epiphysis, delayed skeletal maturation, and disturbance of proportionate growth in various regions of the body [30], but its exact pathophysiology is uncertain. Patients with LCP also have various clinical courses: some patients never have any subsequent difficulty with the hip throughout their lifetimes whereas others have collapse of the femoral head leading to deformity and predisposing them to degenerative changes in adulthood [17, 25, 29]. Current treatment strategies are directed toward maintenance of femoral head sphericity during the early revascularization period by maintaining hip motion and containment of the collapsing femoral head in the acetabulum [7, 21, 23, 27]. As the goal of treatment is to maintain a round femoral head, an imaging modality that could predict femoral head deformity would be beneficial in guiding early treatment.
Plain radiography is the primary imaging modality by which LCP is diagnosed and classified. The amount of epiphyseal involvement [3] and height of the lateral pillar [8] observed on plain radiographs are associated with femoral head deformity at maturity. However, these classifications depend on the presence of early collapse of the femoral epiphysis in the fragmentation stage, while the goal of early treatment is to prevent collapse before fragmentation.
Bone scintigraphy and contrast-enhanced MR imaging are used to assess the extent of perfusion abnormality in LCP. Some authors suggest the pattern of epiphyseal reperfusion evaluated with those modalities could be an important determinant of prognosis [5, 12, 24, 26]. However, in a contrast-enhanced subtraction MR imaging study, Sebag et al. [24] suggested metabolically inactive tissue might be enhanced by reperfusion of the necrotic tissue. Perfusion can be influenced by vascular permeability and blood volume. Thus, perfusion-based imaging modalities alone may be limited for evaluating the amount of ischemic tissue damage in LCP. Diffusion-weighted MR imaging has been used to evaluate tissue breakdown following ischemia, especially in acute stroke [4, 13, 14]. This imaging modality can detect ischemic changes in tissue by measuring microscopic alterations in water mobility. Its use in the musculoskeletal system previously was limited because of magnetic susceptibility artifacts [15], but several studies have shown various diffusion MR sequences can be used to evaluate musculoskeletal abnormalities [2, 6, 16, 20, 22]. In an animal model, diffusion-weighted MR imaging appeared sensitive to early ischemic changes and followed a distinct time course compared with perfusion MR imaging [9]. Perfusion varies with time from markedly decreased to abnormally increased, whereas diffusion remains increased shortly after the initial insult [18]. However, diffusion change and its implications in patients with LCP have not been much studied. A recent study [19] showed that increased metaphyseal diffusion was correlated with absent lateral pillar enhancement at dynamic gadolinium-enhanced subtracted MR imaging, although correlation with other prognostic findings of LCP has not been studied.
Reperfusion in LCP is believed to occur by one of two processes: recanalization of preexisting epiphyseal vessels primarily in the lateral pillar and neovascularization through the physis [5]. Recanalization can be detected early in the disease, but neovascularization usually starts in the fragmentation stage. When reperfusion occurs through recanalization in the periphery, the prognosis is better, particularly if the lateral pillar is preserved. MR imaging can distinguish between the two patterns of reperfusion, but this distinction is not possible early.
We therefore asked whether (1) there would be any age-related diffusion changes; (2) diffusion-weighted MR imaging would reveal ischemic damage of the femoral head; and (3) diffusion changes are correlated with prognostic findings in MR imaging in patients with LCP.
Patients and Methods
We prospectively studied the hips of 21 children being evaluated with MRI for probable necrosis of the femoral head showing epiphyseal abnormalities on plain radiography. We excluded patients with conditions resulting in marrow abnormality, such as sickle cell disease or leukemia, and patients receiving medications predisposing to avascular necrosis of the femoral head, such as steroids and chemotherapeutic drugs. Of these, 17 children had a diagnosis of LCP by typical radiographic findings and/or perfusion abnormalities in bone scintigraphy. We excluded the remaining four children, two of whom had epiphyseal dysplasia and two with what we interpreted as a normal radiographic variation. The mean age of the 17 patients with LCP was 6 years (range, 2–12 years). Fifteen patients were boys and only one hip was involved in each child. We established the lack of involvement of the contralateral femoral head using normal serial radiographs and a normal MRI examination, allowing us to use the contralateral hip as a control. The interval between onset of symptoms and imaging ranged from 1 day to 5.8 years (mean, 47 weeks; median, 16.3 weeks).
All MR information was obtained from one study in each patient. We performed MR imaging using a 1.5 Tesla system (Signa, GE Medical Systems, Milwaukee, WI, USA) with a phased array torso or cardiac coil. In all studies, we examined both hips with the following sequences: spin echo T1-weighted (repetition time [TR] = 800 ms and echo time [TE] = 20 ms) and intermediate-weighted (TR/TE = 2500/40) imaging, spin echo fat-suppressed intermediate-weighted (TR/TE = 4000/35) and T2-weighted (TR/TE = 4000/85) imaging, and fat-suppressed spoiled gradient recalled (TR/TE = 140/5) imaging. Imaging parameters included: field of view (FOV), 240 mm × 240 mm; matrix size, 512 × 384; slice thickness, 2 mm with a 0.4-mm intersection gap; and two averages.
We performed diffusion-weighted MR imaging using line-scan diffusion imaging [16] measuring diffusion in six directions with TR/TE = 2100/70, b = 5 and 700 seconds/mm2, 4 mm slice thickness, and 1.25 mm in-plane resolution. Two images were obtained from sagittal sections in each hip. The sequence lasted less than 10 minutes.
We performed contrast-enhanced MR imaging to evaluate femoral head perfusion. After manual injection of 0.2 mmol/kg of gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ, USA), we obtained a spoiled gradient echo sequence (TR/TE = 200/2, flip angle = 60º, 3 mm slice thickness) in the coronal plane. We acquired images in the same five coronal slice locations, every minute for 5 minutes after beginning of the injection. We also obtained fat-suppressed T1-weighted (TR/TE = 500/9) imaging in coronal and sagittal planes.
One observer (YJK) blinded to the MR imaging findings, graded the radiographic stage of the hips at the time of MR imaging. The original Waldenström classification [28] described the evolution period (initial and fragmentation stages), healing period, growing period (healed femoral epiphysis with open physis), and definite period (closed physis). We used a modified Waldenström classification to grade the radiographic stage: initial stage (eight patients), fragmentation stage (six patients), healing stage (two patients), and residual stage (one patient). The grading was confirmed by another independent observer (WJY). One pediatric radiologist (JEC) assessed the signal intensity (SI) of the epiphysis and the metaphysis of the femoral head on T2-weighted images as increased, similar, or decreased when compared with the contralateral normal side (control), and investigated focal physeal irregularities (increased undulation, interruption, and/or cystic changes of the physis) using intermediate-weighted and spoiled gradient echo images. Based on observational findings in perfusion MR imaging [12], we determined the epiphyseal reperfusion pattern to be either transphyseal (enhancing vessels entering the epiphysis across the physis) or periphyseal (enhancing vessels entering the epiphysis lateral or medial to the physis).
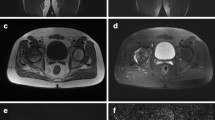
We calculated the ADC values using diffusion-weighted MR imaging [4]. To obtain ADC values, a circular region of interest (ROI) with a diameter of 5 mm was obtained in the center of the epiphyseal ossification center and another identical region was obtained in the subphyseal metaphysis, approximately 1 cm distal from the physis (Fig. 1). Care was taken not to include cortical bone or cartilage. To assess diffusion differences between the affected and control hips, we defined the ADC ratio by the following equation: ADC ratio (%) = (Affected – Control)/Control × 100. We used a metaphyseal ADC ratio greater than 50% as an arbitrary cutoff point as smaller ratios were difficult to perceive on the ADC maps.
In the control hips, we evaluated the normal change of the epiphyseal ADC values with age. Regression analysis using least square regression analysis was performed and Pearson correlation coefficients were calculated. In the affected hips, we compared changes of the epiphyseal ADC values with the modified Waldenström stage. We compared the metaphyseal ADC ratios with a reperfusion pattern to the epiphysis, SI changes in the proximal femur, and focal physeal irregularity. To determine the differences of these associations and odds ratios, we used Fisher’s exact test. To compare ADC value differences between unaffected and affected hips, we used the Mann-Whitney U test. All statistical analyses were conducted using SPSS version 17.0 (SPSS Inc, Chicago, IL, USA).
Results
Normal diffusion decreased with age in the epiphysis and metaphysis (Fig. 2).
Diffusion-weighted MR imaging revealed ischemic damage of the femoral head in all hips with LCP (Table 1). Epiphyseal ADC values were greater (p = 0.005) in the LCP hips than in the control hips. Metaphyseal ADC values also were greater (p = 0.006) in the LCP hips than in the control hips. For the LCP hips, epiphyseal ADC values increased early in the initial stage and remained elevated greater than 35% over the control side in all but one patient in the residual stage (Fig. 3). In all patients, gadolinium-enhanced imaging showed areas of decreased contrast enhancement in the marrow of the secondary ossification center. The metaphyseal ADC values also increased in all hips in the initial and fragmentation stages, with wider variations than in the epiphyseal ADC values (Fig. 4): only six of the 17 patients (35%) had an ADC ratio greater than 50% in the metaphysis.
The graph shows diffusion changes in the epiphysis with radiographic stages of LCP. The dot plot shows that epiphyseal ADC remains increased at least until the healing stage in all cases (ADC - apparent diffusion coefficient; 1 - initial stage; 2 - fragmentation stage; 3 - healing stage; 4 - residual stage).
Diffusion changes in the metaphysis were associated with a reperfusion pattern to the epiphysis, increased SI in the metaphysis, and focal physeal abnormalities. Epiphyseal reperfusion patterns, based on observational findings in perfusion MR imaging, were periphyseal in nine patients (Fig. 5) and transphyseal in eight (Fig. 6). Increased metaphyseal diffusion tended to be associated with (p = 0.05) transphyseal pattern of reperfusion to the epiphysis: metaphyseal ADC values greater than 50% over the control side (ADC ratio > 50%) had 13 times greater odds of being associated with transphyseal reperfusion to the epiphysis. The metaphyseal ADC ratio greater than 50% also was associated with an increased T2-signal intensity of the metaphysis (p < 0.001) and the presence of focal physeal irregularity (p = 0.035) (Table 2).
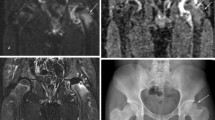
The MR images of a 2-year 11-month-old boy with LCP of the left hip are shown. The (A) ADC map and a (B) gadolinium enhanced fat-suppressed T1-weighted image of the right hip (control side) were compared with a (C) gadolinium enhanced fat-suppressed T1-weighted image of the left hip showing periphyseal reperfusion (arrows) in the epiphysis and the (D) ADC map of the left hip showing increased diffusion to the epiphysis.
The MR images of a 6-year-old boy with LCP of the left hip are shown. The (A) ADC map and a (B) gadolinium enhanced fat-suppressed T1-weighted image of the right hip (control side) were compared with a (C) gadolinium enhanced fat-suppressed T1-weighted image of the left hip showing transphyseal reperfusion (arrows) to the epiphysis and (D) the ADC map of the left hip showing increased diffusion to the epiphysis and the metaphysis.
Discussion
Diffusion-weighted MR imaging, a modality used to assess ischemic tissue damage, is reportedly sensitive to prolonged ischemia of the femoral head in an animal model [9, 18]. However, diffusion change in LCP and its implications have not been much studied [19]. We therefore attempted to determine whether there would be any age-related diffusion changes in the femoral head; whether diffusion-weighted MR imaging would reveal ischemic damage of the femoral head; and whether diffusion changes are correlated with prognostic findings in MR imaging in patients with LCP.
Certain limitations of this study require consideration. First, we lacked serial data in the patients studied. A followup study in which patients will be studied serially to evaluate the course of the disease is being planned. We hope that data obtained from longitudinal followup studies would be used as a reference and therefore we do not need to perform serial MR imaging studies in practice to predict a prognosis. Second, interpretation of increased epiphyseal diffusion could be complicated by the dual nature of the femoral epiphysis that varies with skeletal maturity, and individual disease progress, although we could exclude the influence of skeletal maturity by using the ADC ratio instead of ADC values. Third, our study is based on line-scan diffusion, which is less prone to susceptibility artifacts and allows better sagittal and coronal imaging than standard, more readily available diffusion-weighted sequences based on echo planar imaging. However, we believe that the results can be generalized, regardless of the diffusion technique used [15]. Fourth, we evaluated patients at different stages of the disease, which induces variability in the results. Fifth, we used contralateral hips as controls. We established the lack of involvement of the contralateral femoral head using normal serial radiographs and a normal MRI examination, but this would not exclude a possibility of subtle or subclinical disease that might influence blood flow. Sixth, we had no actual measures of outcome, and rather used surrogate imaging parameters.
Our observations reveal that marrow diffusion in the femoral heads decreases with age. Immature skeletal structures in children, including the proximal femur, contain more water than in adults, making them well suited for evaluation. Diffusion is normally greater in areas of higher cellularity and appears to be greater in hematopoietic than in fatty marrow [1]. As would be expected from the greater diffusion in hematopoietic marrow, it is not surprising that marrow diffusion decreases with age. It decreases faster in the epiphysis, which correlates with the faster epiphyseal marrow conversion. Such normal, age-related changes need to be accounted for in comparing diffusion findings with time, as may occur when monitoring disease progression. The use of the ADC ratio for the contralateral normal side instead of ADC values might offset the influence of normal age-related diffusion changes in patients. We believe that in older patients, as there is less water in the normal epiphyseal marrow, the diffusion-weighted imaging evaluation of the normal epiphysis becomes less reliable. The ischemic epiphysis, however, continues to have very elevated diffusion, and therefore the technique is also useful in older patients for whom early treatment is more important.
Our observations also confirm diffusion MR imaging can be used to clinically evaluate ischemia of the femoral head in skeletally immature patients. Pathologic processes, such as ischemia, result in cellular damage that alters the diffusivity of water in tissues, which is measured with diffusion-weighted MR imaging. Once there is tissue breakdown, as in long-standing bone ischemia, diffusion increases. In two studies in an animal model of epiphyseal ischemia in piglets, diffusion increases above normal limits within hours after the onset of ischemia, and remains elevated for a long period afterward [9, 18]. In our patient population, all abnormal areas had increased diffusion. Therefore, in the evaluation of patients with LCP, ADC maps, which are sensitive to increased diffusion, are better than diffusion-weighted images, which are more sensitive to restrictive diffusion [15].
Metaphyseal diffusion changes had a potential prognostic value in patients with LCP. Diffusion-weighted MR imaging not only showed the increased diffusion, but also differentiated metaphyseal from epiphyseal changes in our patients. We found the epiphyseal ADC increased and remained elevated throughout the course of the disease. There is very little variation in the epiphyseal ADC values, particularly as disease progresses. However, metaphyseal ADC is quite variable. We suspect the variability is related to the presence of patients with varying types of reperfusion: one with transphyseal reperfusion and the other with periphyseal reperfusion, as prior studies using other modalities have shown [5, 12, 26]. Conway et al. [5] suggested that the epiphyseal reperfusion pattern observed in bone scintigraphy has a prognostic value in LCP. Some authors support this concept through observations of serial bone scintigraphy [26] and dynamic gadolinium-enhanced subtraction MR imaging [12]. According to this concept, reperfusion occurs spontaneously and follows one of three patterns: periphyseal reperfusion involving the lateral or medial pillar, transphyseal reperfusion through the physis, or regression from a periphyseal to transphyseal pattern. When reperfusion occurs across the physis, there is an increased risk of premature physeal dysfunction that leads to femoral neck shortening and deformity. Conversely, when reperfusion occurs in the periphery, the prognosis is better, particularly if the lateral pillar is preserved. In our study, metaphyseal diffusion was greater in patients with transphyseal reperfusion than in those with periphyseal reperfusion, suggesting that increased diffusivity of the metaphyseal tissues may be predictive of a bad prognosis of patients with LCP. Unlike gadolinium-enhanced imaging or scintigraphy, diffusion-weighted imaging can provide information regarding transphyseal reperfusion and prognosis without the risks entailed by using contrast materials or radiation. Increased diffusivity of the metaphysis is thought to be caused by extensive ischemic damage that appears to be represented by an increased T2-SI in the metaphysis and a focal irregularity of the physis [10, 11]. Our findings correspond well with those in a recent study [19], in which cut-off ADC values were used to determine whether the femoral heads in patients with LCP are pathologic, and to predict prognosis of the pathologic hips. In that study, increased metaphyseal diffusion was correlated with absent lateral pillar enhancement at dynamic gadolinium-enhanced subtracted MR imaging. In our study, increased metaphyseal diffusion was associated not only with transphyseal reperfusion, but also with focal physeal irregularity, suggesting that the physeal damage and subsequent proximal femoral growth arrest are what ultimately lead to poor prognosis in these cases.
This preliminary study showed there is an age-related diffusion change in normal femoral heads, diffusion-weighted MR imaging is sensitive to epiphyseal changes and can reveal whether there are metaphyseal changes developing that are associated with transphyseal reperfusion to the epiphysis, increased T2-SI in the metaphysis, and focal physeal irregularity in children with LCP. Diffusion-weighted MR imaging provides information regarding epiphyseal and metaphyseal involvement different from that provided by perfusion studies, suggesting it can be used as a complimentary tool to evaluate ischemic tissue damage in patients with LCP. Additional longitudinal studies would be essential to really understand the potential benefit of diffusion-weighted MR imaging in identifying a poor outcome early in the disease, and to determine whether this method can change the treatment plan.
References
Baur A, Dietrich O, Reiser M. Diffusion-weighted imaging of bone marrow: current status. Eur Radiol. 2003;13:1699–1708.
Baur A, Stabler A, Bruning R, Bartl R, Krodel A, Reiser M, Deimling M. Diffusion-weighted MR imaging of bone marrow: differentiation of benign versus pathologic compression fractures. Radiology. 1998;207:349–356.
Catterall A. The natural history of Perthes’ disease. J Bone Joint Surg Br. 1971;53:37–53.
Chien D, Kwong KK, Gress DR, Buonanno FS, Buxton RB, Rosen BR. MR diffusion imaging of cerebral infarction in humans. AJNR Am J Neuroradiol. 1992;13:1097–1102; discussion 1103–1095.
Conway JJ. A scintigraphic classification of Legg-Calve-Perthes disease. Semin Nucl Med. 1993;23:274–295.
Gudbjartsson H, Maier SE, Mulkern RV, Morocz IA, Patz S, Jolesz FA. Line scan diffusion imaging. Magn Reson Med. 1996;36:509–519.
Harrison MH, Menon MP. Legg-Calvé-Perthes disease. The value of roentgenographic measurement in clinical practice with special reference to the broomstick plaster method. J Bone Joint Surg Am. 1966;48:1301–1318.
Herring JA, Neustadt JB, Williams JJ, Early JS, Browne RH. The lateral pillar classification of Legg-Calve-Perthes disease. J Pediatr Orthop. 1992;12:143–150.
Jaramillo D, Connolly SA, Vajapeyam S, Robertson RL, Dunning PS, Mulkern RV, Hayward A, Maier SE, Shapiro F. Normal and ischemic epiphysis of the femur: diffusion MR imaging study in piglets. Radiology. 2003;227:825–832.
Jaramillo D, Kasser JR, Villegas-Medina OL, Gaary E, Zurakowski D. Cartilaginous abnormalities and growth disturbances in Legg-Calvé-Perthes disease: evaluation with MR imaging. Radiology. 1995;197:767–773.
Johnson C, May DA, McCabe KM, Guse R, Resnick D. Non-cartilaginous metaphyseal cysts in Legg-Calvé-Perthes disease: report of a case. Pediatr Radiol. 1997;27:824–826.
Lamer S, Dorgeret S, Khairouni A, Mazda K, Brillet PY, Bacheville E, Bloch J, Pennecot GF, Hassan M, Sebag GH. Femoral head vascularisation in Legg-Calve-Perthes disease: comparison of dynamic gadolinium-enhanced subtraction MRI with bone scintigraphy. Pediatr Radiol. 2002;32:580–585.
Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407.
Lee LJ, Kidwell CS, Alger J, Starkman S, Saver JL. Impact on stroke subtype diagnosis of early diffusion-weighted magnetic resonance imaging and magnetic resonance angiography. Stroke. 2000;31:1081–1089.
MacKenzie JD, Gonzalez L, Hernandez A, Ruppert K, Jaramillo D. Diffusion-weighted and diffusion tensor imaging for pediatric musculoskeletal disorders. Pediatr Radiol. 2007;37:781–788.
Maier SE, Gudbjartsson H, Patz S, Hsu L, Lovblad KO, Edelman RR, Warach S, Jolesz FA. Line scan diffusion imaging: characterization in healthy subjects and stroke patients. AJR Am J Roentgenol. 1998;171:85–93.
McAndrew MP, Weinstein SL. A long-term follow-up of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 1984;66:860–869.
Menezes NM, Connolly SA, Shapiro F, Olear EA, Jimenez RM, Zurakowski D, Jaramillo D. Early ischemia in growing piglet skeleton: MR diffusion and perfusion imaging. Radiology. 2007;242:129–136.
Merlini L, Combescure C, De Rosa V, Anooshiravani M, Hanquinet S. Diffusion-weighted imaging findings in Perthes disease with dynamic gadolinium-enhanced subtracted (DGS) MR correlation: a preliminary study. Pediatr Radiol. 2010;40:318–325.
Oner AY, Aggunlu L, Akpek S, Tali T, Celik A. Diffusion-weighted imaging of the appendicular skeleton with a non-Carr-Purcell-Meiboom-Gill single-shot fast spin-echo sequence. AJR Am J Roentgenol. 2007;189:1494–1501.
Petrie JG, Bitenc I. The abduction weight-bearing treatment in Legg-Perthes’ disease. J Bone Joint Surg Br. 1971;53:54–62.
Raya JG, Dietrich O, Reiser MF, Baur-Melnyk A. Techniques for diffusion-weighted imaging of bone marrow. Eur J Radiol. 2005;55:64–73.
Salter RB. Legg-Perthes disease: the scientific basis for the methods of treatment and their indications. Clin Orthop Relat Res. 1980;150:8–11.
Sebag G, Ducou Le Pointe H, Klein I, Maiza D, Mazda K, Bensahel H, Hassan M. Dynamic gadolinium-enhanced subtraction MR imaging: a simple technique for the early diagnosis of Legg-Calve-Perthes disease: preliminary results. Pediatr Radiol. 1997;27:216–220.
Stulberg SD, Cooperman DR, Wallensten R. The natural history of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 1981;63:1095–1108.
Tsao AK, Dias LS, Conway JJ, Straka P. The prognostic value and significance of serial bone scintigraphy in Legg-Calve-Perthes disease. J Pediatr Orthop. 1997;17:230–239.
Thompson GH, Salter RB. Legg-Calvé-Perthes disease. Current concepts and controversies. Orthop Clin North Am. 1987;18:617–635.
Waldenström H. The definite form of the coxa plana. Acta Radiol. 1922;1:384–394.
Weinstein SL. Legg-Calvé-Perthes disease: results of long-term follow-up. Hip. 1985:28–37.
Weinstein SL. Long-term follow-up of pediatric orthopaedic conditions: natural history and outcomes of treatment. J Bone Joint Surg Am. 2000;82:980–990.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Work performed at Massachusetts General Hospital and Children’s Hospital Boston.
About this article
Cite this article
Yoo, W.J., Kim, YJ., Menezes, N.M. et al. Diffusion-weighted MRI Reveals Epiphyseal and Metaphyseal Abnormalities in Legg-Calvé-Perthes Disease: A Pilot Study. Clin Orthop Relat Res 469, 2881–2888 (2011). https://doi.org/10.1007/s11999-011-1931-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-011-1931-x