Abstract
Background
In children older than 5 years with a mild form of Legg–Calvé–Perthes disease, the outcome is difficult to predict. In this study, we retrospectively correlated gadolinium-enhanced subtracted (DGS) and diffusion (DWI) MRI findings to the radiographic assessment according to the Catterall and Herring et al. classifications and to the final score according to Stulberg et al.: the aim was to identify a precocious, simple, and objective criterion to differentiate between forms evolving favourably and forms requiring an early surgical treatment in order to avoid femoral head deformity and subsequent osteoarthritis.
Methods
Twelve boys with unilateral mild femoral head involvement (Catterall grade 2 or grade 3) underwent DSG and DWI MR during the early phase of the disease. The absence of enhancement of the external pillar on DSG MRI and the presence of metaphyseal hyperintensity on DWI were considered to be the signs of poor outcome. These findings were correlated with the Catterall and Herring et al. classifications at the initial sclerotic stage and early fragmentation phase and with the Stulberg et al. classifications at least 5 years after the onset of the disease.
Results
DSG MRI findings correctly discriminated three out of four patients with a good outcome but underestimated two out of eight patients with a poor outcome. DWI findings correlated with the Catterall and Herring et al. classifications in 12 out of 12 cases. In only one case, DWI findings did not correlate with the Stulberg et al. classification.
Conclusion
DWI MR provides an objective and accurate prognostic criterion that is relatively easy to recognise. DGS MR findings are less accurate, thus underestimating the gravity of the disease in one-fourth of the patients with a poor outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Legg–Calvé–Perthes (LCP) disease is a juvenile necrosis of the proximal femoral epiphysis of vascular origin [1].
The clinical course and outcome in LCP disease vary considerably between different patients. Earlier prognostic criteria than those offered by conventional radiography are necessary to identify children who require prompt surgical treatment [2]. The Catterall and Herring et al. radiological classifications have shown good-to-excellent correlation with clinical and radiological outcome [3, 4]. However, the Herring et al. classification can only be applied correctly at the mid-fragmentation stage of LCP disease, and the Catterall classification can only be applied at the end of the fragmentation stage [4, 5].
Ideally, a prognosticator of outcome of the disease should be done at the very early stage of the disease and before the development of femoral head deformity in order to set a good treatment decision to prevent the deformity of the femoral head. This ability to distinguish hips, at the early stages of the disease, that will ultimately have a benign or a severe form of disease, may help to avoid operating on patients too late, or avoid operating on patients who really don’t need surgical treatment.
Bone scintigraphy and dynamic gadolinium-enhanced subtracted MR imaging (DGS MR) are used for early detection of the extent of perfusion abnormality in LCP [4, 6]. When reperfusion occurs through recanalisation in the periphery, the prognosis is better, particularly if the lateral pillar is preserved. MR perfusion imaging can distinguish between the two patterns of reperfusion, but this distinction is not possible early [6,7,8,9,10]. However, it was pointed out that even metabolically inactive or necrotic tissue might be enhanced by reperfusion [10, 11]. Moreover, perfusion can be influenced by vascular permeability and blood volume [11].
Thus, the early appearance of lateral pillar enhancement on DGS MR alone has been questioned as indicative of good prognosis [12].
Diffusion-weighted MR imaging (DWI) can detect ischaemic changes in tissue by measuring microscopic alterations in water mobility [13]. Preliminary experimental studies on surgically induced hip ischaemia in piglets have shown that diffusion-weighted MR (DWI) changes are a good marker of epiphyseal destruction [14]. Thus, in 2010 we hypothesised that increased metaphyseal diffusion in LCP disease could be correlated with absent lateral pillar enhancement at dynamic gadolinium-enhanced subtracted MR imaging [15]. Furthermore, Yoo et al. suggested that DWI could be used as a complimentary tool to evaluate ischaemic tissue damage in patients with LCP [16, 17], and the prognostic value of DWI in LCP was also confirmed [17,18,19,20].
Further studies on the subject added interesting data to the mounting volume of knowledge on LCP using more sophisticated techniques such as the Serial Perfusion MRI [20] or apparent diffusion coefficient (ADC) measurement on DWI sequences in the function of age of patients [16] and different timing [19]. ADC ratios are comparing the diffusivity of the pathological hip to that of the contralateral normal hip [17, 21]. However, these techniques are relatively complicated to achieve, and all these authors admit that they are not a part of their clinical routine practice and that they cannot make recommendations on treatment decisions [22].
The aim of this study was to investigate whether there is a sign that is easy to obtain in everyday routine practice that can be used as an early prognostic criterion to help choose the correct treatment of LCP. We thus retrospectively reviewed findings of early performed MRI with DGS and DWI in children with LCP disease with mild femoral head involvement: we correlated MRI findings to the Catterall and Herring et al. classifications evaluated on the plain radiograph under the fragmentation stage and, in addition, to the final radiographic outcome evaluated with the Stulberg et al. classification.
Materials and methods
We retrospectively reviewed the clinical charts of patients with a diagnosis of LCP disease made on plain radiographs and/or on bone scintigraphy. Patients were recruited during the period ranging from January 2006 to May 2008 at a single Institution (Table 1).
Inclusion criteria were: Catterall classification on plain radiography grade 2 (n = 4) or grade 3 (n = 8); unilateral LCP in order to allow comparison with the unaffected contralateral side that formed the control group; and the age at presentation more than 5 years of age.
A senior paediatric orthopaedic surgeon initially saw all patients (VDR). MR imaging was performed 1–4 months after the first onset of symptoms of LCP disease. The Catterall and Herring et al. classifications were compared with the early appearance of lateral pillar enhancement on DGS MR and the presence of the absence of hyperintensity in the metaphysis adjacent to the physis compared to the same region of the control hip on DWI.
Radiographic imaging and analysis
During the study period, each patient underwent standard antero-posterior and frog-lateral radiographs of the pelvis at different stages of the disease. Radiographically, all hips were rated by consensus of three paediatric orthopaedic surgeons at the initial sclerotic stage according to the Catterall classification [23], and the early fragmentation phase according to the Herring et al. classification [4]. The Stulberg et al. classification was used to rate hips at the last follow-up visit [3] at least 5 years after the onset of symptoms (Figs. 1d, 2d, 3d, 4d).
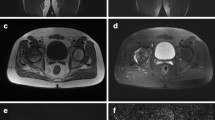
8-year-old boy: MRI performed 3 months after the onset of symptoms. a DWI and b ADC map, showing left metaphyseal hyper intensity (arrows), considered as a sign of poor prognosis. c DGS showing lack of enhancement of the lateral pillar on the left side (arrowheads) considered as a sign of poor prognosis. d X-ray performed 8 year later: he was treated conservatively and the final results were poor (Stulberg 4)
6-year-old boy: MRI performed 2 months after onset of the LCP disease on the left side. a DWI and b ADC map, showing symmetrical metaphyseal intensity (arrows), considered as a sign of good prognosis; c however, DGS showed lack of enhancement of the lateral pillar on the left side (arrowheads) considered as a sign of poor prognosis. d X-ray performed 8 year later: the final results were good (Stulberg 2), according to DWI
6-year-old boy: MRI performed 2 months after onset of the LCP disease. a DWI and b ADC map, showing left metaphyseal hyper intensity (arrows), considered as a sign of poor prognosis. c DGS showing lack of enhancement of the lateral pillar on the left side (arrowheads) considered as a sign of poor prognosis. d X-ray performed 8 year later: surgical Salter procedure was performed (arrow) and the final results were good (Stulberg 2)
9-year-old boy: MRI performed 2 months after the onset of symptoms. a DWI showing left metaphyseal hyper intensity (arrows), considered as a sign of poor prognosis. b DGS showing early enhancement of the lateral pillar on the left side (arrowheads) considered as a sign of good prognosis. c X-ray performed 3 months later: according to Catteral he was classified as 3 and Herrings et al as B. d X-ray performed 8 years later, in spite of the fact that surgical Salter procedure was performed, the final results were poor (Stulberg 3)
MR imaging
MR was performed on a 1.5-T unit (Avanto, Siemens, Germany) using a six-element body-array coil positioned on the anterior ventral part of the child, combined with six elements of the spine coil. Geometric imaging parameters common to all morphological sequences were: FOV 270°—270 mm; slice thickness 5 mm with a 1-mm gap; 367°—448 matrix; 1–3 averages; and voxel size 0.8°—0.7°—5 mm. The following sequences were obtained: T1-weighted (T1-W) coronal spin-echo (SE) (TR 500 ms; TE 20 ms) and both proton-density (PD) and T2-weighted (T2-W) coronal turbo SE (TSE), (TR 2000 ms; TE 80 ms) (T2-W); TE 20 ms (PD). The dynamic T1-W SE sequence (TR 200 ms; TE 20 ms; acquisition time 1 min) was first obtained at five levels, all including the femoral head, in the coronal plane. A bolus of 0.1 mmol/kg gadolinium tetra-azacyclo-dodecane-tetraacetic acid (DOTA; Dotarem, Guerbet, Aulnay, France) was injected manually in < 15 s, followed by a 10-ml saline flush. Images were acquired at the same five levels as before, every minute for 5 min after the beginning of the injection. The 1-, 2-, 3-, 4-, and 5-min contrast-enhanced images were then subtracted from the precontrast images, using the subtraction function available as standard software on our console.
DWI sequences were performed before contrast medium injection and consisted of 20 coronal slices (at least 10 through the femoral head), acquired using an SE-based EPI sequence. Diffusion weighting was obtained using bipolar gradient preparation and three b values: 50, 400, and 800 s/mm2. Diffusion gradients were applied concurrently in read, phase, and slice directions in order to offer three orthogonal direction diffusion weightings sequentially. The MR parameter utilised included: slice thickness 4 mm; phase encoding direction right to left; EPI factor 156; matrix size 156°—192; FOV 267°—328 mm; voxel size 1.7° 1.7°—4 mm; TR 500 ms; TE 76 ms; and bandwidth 1736 Hz/pixel. Also, the sequence was implemented with parallel imaging (grappa factor = 2) and four averages.
No automatic co-registration between DGS and DWI was performed; visual image matching was used.
MRI analysis
On DGS MR, enhancement in the lateral pillar was used as a sign of good prognosis. Enhancement intensity of the ischaemic femoral head lateral pillar was qualitatively compared to the contralateral side on subtraction images, using slices with the most intense enhancement. We did not measure enhancement ratios. Two senior paediatric radiologists with 6 and 15 years of experience (L.M. and M.L., respectively) and a senior orthopaedist (V.D.) separately scored the images blindly. For all investigators, training consisted only of the previous routine use in the paediatric orthopaedic and radiology departments, and no other advice or training was given. All three observers worked independently and no time limit was imposed on the review.
The enhancement intensity of the epiphysis was compared with that of the contralateral hip and classified as not increased (−) with a poor prognosis (Figs. 1c, 2c), or increased (+) with a good prognosis (Fig. 4c). The readers were unaware of the final results according to the Stulberg et al. classification.
On DWI, the same two radiologists assessed the DW images and ADC maps blindly and reached a consensus for image quality with a two-point scale (1 = background noise and distortion artefacts due to susceptibility or eddy currents; 2 = minor background noise and no distortion of the image). Intensity on the femoral metaphysis was blindly qualitatively compared to the contralateral side on DW images and ADC maps for the presence (+) of hyperintensity with a poor prognosis (Fig. 1a, b) or the absence (−) of hyperintensity with a good prognosis (Fig. 2a, b), using all slices (Table 2). ADC values were not measured.
Statistical analysis
Pearson correlation coefficients were calculated for all patients. In particular, we evaluated the degree of correlation between the three observers by calculating interclass correlation coefficients. Intraclass correlation coefficients were also calculated in order to evaluate the reproducibility of the assessment method. The tests were two-sided, with a type I error set at α = 0.05.
Results
Between January 2006 and May 2008, 12 boys aged between 5 and 8 years of age, with a diagnosis of LCP disease made on plain films and/or on bone scintigraphy, met the inclusion criteria (Table 1).
Radiographs
Four children were classified as grade 2 and eight were classified as grade 3 according to the Catterall classification (Table 1).
Concerning the further evolution, all four patients that scored Catterall 2 evolved towards grade A according to the Herring et al. classification. In the eight patients rated Catterall grade 3, four cases evolved towards Herring et al. grade B and four cases towards Herring et al. grade C (Table 1).
Overall, eight hips out of 12 (66.6%) were at risk of poor outcome according to Herring et al. classification.
MRI
When analysing the perfusion pattern of the lateral pillar on DGS MRI, the intra- and interclass correlation between readers showed a very good reliability with an r score of 0.95. The results are shown in Table 1.
We matched the Catterall classification with the results of the early revascularisation of the external pillar on DGS MR. Six out of eight cases of Catterall grade 3 showed no enhancement of the external pillar on DGS MR correctly indicating a poor prognosis (Fig. 1c). In two cases of Catterall grade 3, we had a revascularisation of the external pillar on DGS MR, which incorrectly predicted a good outcome (Fig. 4c). The two patients were subsequently classified as Herring et al. grade B, and a Salter osteotomy of the affected hip was performed.
Three out of four patients with Catterall grade 2 showed early revascularisation of the lateral pillar on DGS MR correctly indicating a good prognosis. One patient with Catterall grade 2 showed the absence of this sign, and it was incorrectly prognosticated as having a poor prognosis. The patient was subsequently classified as Herring et al. grade A and was treated conservatively.
In conclusion, the DGS MR sign of early revascularisation of the external pillar did not correlate with the clinical outcome in three out of 12 cases; in two cases, the DGS MR findings underestimate the clinical outcome, and, in one case, it overestimated the severity of the disease (Table 1).
The second parameter we compared was the presence of hyperintensity in the metaphysis adjacent to physis on DWI and ADC maps, which was supposed to predict a severe form of LCP disease with potential poor outcome. Even for this sign, the intra- and interclass correlation between readers showed a very good reliability with an r score of 0.90.
All eight cases of Catterall grade 3/Herring et al. grade B or C showed the hyperintensity of the metaphysis, while the 4 cases of Catterall grade 2/Herring et al. grade A had no hyperintensity in the metaphysis on DWI (Table 2).
Considering the final outcome evaluated according to Stulberg et al., the DWI sign correlated well with the clinical outcome in 11 out of 12 cases, and it was thus accurate in predicting the clinical outcome (Figs. 1, 2, 4).
Discussion
This study assessed children older than 5 years of age with a mild form of unilateral LCP disease, in whom the prognosis is often unpredictable. Children younger than 5 years of age were excluded because this age group has a good prognosis, and the early prognostic signs are, therefore, of little value [24]. Furthermore, the inhomogeneity of the epiphyseal femoral marrow in early childhood may cause difficulty in interpretation [18, 19].
It is known that hips rated Catterall grade 1 have a good clinical and radiological outcome and, most importantly, do not require surgical treatment. Similarly, hips rated Catterall grade 4 are mostly treated surgically due to the high frequency of a poor clinical and radiological outcome. On the other hand, surgical decision-making can be challenging in hips with mild femoral head involvement (i.e. Catterall grade 2 and Catterall grade 3). In these forms, early detection of patients at risk for a poor outcome would be of help to plan early surgical management. For this reason, only Catterall grade 2 and Catterall grade 3 patients older than 5 years of age were included in our study. In this homogeneous population, we reviewed MRI imaging performed early in order to investigate which sign on DGS or DWI better predicted the outcome of the disease (i.e. Catterall grade 2/Herring et al. grade A not requiring surgical treatment or Catterall grade 3/Herring et al. grade B and C needing a surgical intervention).
To be useful in routine clinical practice, MRI prognostic markers should be technically simple to obtain, not time-consuming, and easy to assess by a busy paediatric radiologist: based on the literature data, we chose the early enhancement of the lateral pillar on DGS as a sign of good prognosis and the presence of metaphyseal hyperintensity on DWI as a sign of poor prognosis [19, 21].
The first finding of our study was that, after a blind assessment performed separately by the two readers, there was no need for a consensus because the agreement was perfect about the presence or the absence of the two MRI signs. Moreover, the method showed good-to-excellent inter- (r = 0.95) and intraclass (r = 0.90) coefficient when raters were asked to predict an outcome according to the MRI signs. It thus seems that the visual assessment of the two signs is not subjective and that the comparison between the affected and healthy side is sufficient to discriminate the presence or the absence of abnormalities.
The match from the Catterall classification score and the early revascularisation of the external pillar on DGS MR shows that this sign was always present in cases with a good outcome (Catterall grade 2), but it was also present in two out of eight patients with a poor outcome (Catterall grade 3) (Fig. 4). We can thus conclude that this sign seems to be accurate in discriminating patients not requiring aggressive treatment. However, we found that some patients necessitating surgical treatment could be underestimated.
When comparing the Catterall and Herring et al. classifications on the presence of metaphyseal hyperintensity on DWI, we found that all patients with this sign were furthermore classified as Catterall grade 3 and subsequently as Herring et al. type B or C with a poor outcome, while those without this sign were classified as Catterall grade 2 and subsequently as Herring type A, thus with a good outcome (Figs. 1, 2, 3, 4). For this MRI sign, we had no discrepancies with the Catterall and Herring et al. classifications, and it was a perfect precocious prognostic criterion (Table 1).
When comparing the Stulberg et al. classification to the presence of metaphyseal hyperintensity on DWI, we found that the prediction of the final results was correct in all cases except one: in this case, the DWI indicated a poor prognosis, but, in spite of the lack of surgical treatment, the outcome in the long term was good (Table 2).
In detail, the hyperintensity of the metaphysis near to physis was a better indicator of predictability than the lateral pillar enhancement on diffusion MRI, which also remains a good indicator.
The pathological significance of this sign remains to be elucidated: animal models suggest that fibro-vascular tissue has resorbed and replaced the metaphyseal bone and that this tissue can be responsible for the increased ADC value [10, 19].
This study carries some limitations. First, it’s a retrospective study. Moreover, due to the limited number of enrolled children, the statistical power of our study is weak: for this reason, we did not estimate cut-off values of enhancement intensity and ADC measurement because it would not be reliable. However, these preliminary results are encouraging: further investigations are needed in order to reliably correlate a more sophisticated MRI measurement for simple, visual, and objective signs promptly at use to all paediatric radiologist in their everyday routine practice.
In conclusion, it is the authors’ opinion that MRI DWI in children with LCP disease and mild femoral head involvement is helpful in the early detection of the potential for poorly evolving hips. In particular, the correlation appears to be strong if hyperintensity of the metaphysis adjacent to physis is detected on DWI MR. However, it seems better to compare this sign to the lack of enhancement of the lateral pillar on DGS MR: having both indicators on MRI appears to be particularly helpful for the paediatric orthopaedic surgeon dealing with patients with LCP disease, as it would help in the early treatment decision-making.
References
Ibrahim T, Little DG (2016) The pathogenesis and treatment of Legg–Calvé–Perthes disease. JBJS Rev 4(7). https://doi.org/10.2106/JBJS.RVW.15.00063
Wiig O, Terjesen T, Svenningsen S (2008) Prognostic factors and outcome of treatment in Perthes’ disease: a prospective study of 368 patients with five-year follow-up. J Bone Joint Surg Br 90(10):1364–1371
Stulberg SD, Cooperman DR, Wallensten R (1981) The natural history of Legg–Calvé–Perthes disease. J Bone Joint Surg Am 63(7):1095–1108
Herring JA, Neustadt JB, Williams JJ, Early JS, Browne RH (1992) The lateral pillar classificatn of Legg–Calvé–Perthes disease. J Pediatr Orthop 12(2):143–150
Comte F, De Rosa V, Zekri H et al (2003) Confirmation of the early prognostic value of bone scanning and pinhole imaging of the hip in Legg–Calvé–Perthes disease. J Nucl Med 44(11):1761–1766
Tsao AK, Dias LS, Conway JJ, Straka P (1997) The prognostic value and significance of serial bone scintigraphy in Legg–Calvé–Perthes disease. J Pediatr Orthop 17(2):230–239
Conway JJ (1993) A scintigraphic classification of Legg–Calvé–Perthes disease. Semin Nucl Med 23(4):274–295
Lamer S, Dorgeret S, Khairouni A et al (2002) Femoral head vascularisation in Legg–Calvé–Perthes disease: comparison of dynamic gadolinium-enhanced subtraction MRI with bone scintigraphy. Pediatr Radiol 32(8):580–585
Jaramillo D, Connolly SA, Vajapeyam S et al (2003) Normal and ischemic epiphysis of the femur: diffusion MR imaging study in piglets. Radiology 227(3):825–832
Nonomura Y, Yasumoto M, Yoshimura R et al (2001) Relationship between bone marrow cellularity and apparent diffusion coefficient. J Magn Reson Imaging 13(5):757–760
Kim HK, Kaste S, Dempsey M, Wilkes D (2013) A comparison of non-contrast and contrast-enhanced MRI in the initial stage of Legg–Calvé–Perthes disease. Pediatr Radiol 43(9):1166–1173
Li X, Qi JJ, Xia L et al (2008) Diffusion MRI in ischemic epiphysis of the femoral head: an experimental study. J Magn Reson Imaging 28(2):471–477
Le Bihan D, Mangin JF, Poupon C et al (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13(4):534–546
Menezes NM, Connolly SA, Shapiro F et al (2007) Early ischemia in growing piglet skeleton: MR diffusion and perfusion imaging. Radiology 242(1):129–136
Merlini L, Combescure C, De Rosa V, Anooshiravani M, Hanquinet S (2010) Diffusion-weighted imaging findings in Perthes disease with dynamic gadolinium-enhanced subtracted (DGS) MR correlation: a preliminary study. Pediatr Radiol 40(3):318–325
Yoo WJ, Kim YY-J, Menezes NM, Cheon J-E, Jaramillo D (2011) Diffusion-weighted MRI reveals epiphyseal and metaphyseal abnormalities in Legg–Calvé–Perthes disease: a pilot study. Clin Orthop Relat Res 469(10):2881–2888
Yoo WJ, Choi IHI, Cho T-J et al (2016) Risk factors for femoral head deformity in the early stage of Legg–Calvé–Perthes disease: MR contrast enhancement and diffusion indexes. Radiology 279(2):562–570
Sebag G, Ducou Le Pointe HH, Klein IH et al (1997) Dynamic gadolinium-enhanced subtraction MR imaging—a simple technique for the early diagnosis of Legg–Calvé–Perthes disease: preliminary results. Pediatr Radiol 27(3):216–220
Baunin C, Sanmartin Viron D, Accadbled F et al (2014) Prognosis value of early diffusion MRI in Legg–Perthes–Calvé disease. OTSR 100(3):317–321
Kim HK, Burgess J, Thoveson A, Gudmundsson P, Dempsey M, Jo CC-H (2016) Assessment of femoral head revascularization in Legg–Calvé–Perthes disease using serial perfusion MRI. J Bone Joint Surg Am 98(22):1897–1904
Boutault JR, Baunin CJ, Bérard EJ et al (2013) Diffusion MRI of the neck of the femur in Legg–Calve–Perthes disease: a preliminary study. Diagn Interv Imaging 94(1):78–83
Castaneda P (2016) Can we solve Legg–Calvé–Perthes disease with better imaging technology? Commentary on an article by Harry K.W. Kim, MD, MS, et al.: “Assessment of Femoral Head Revascularization in Legg-Calvé–Perthes Disease Using Serial Perfusion MRI. J Bone Joint Surg Am 98(22):e103-e
Catterall A (1971) The natural history of Perthes’ disease. J Bone Joint Surg Br 53(1):37–53
Canavese F, Dimeglio A (2008) Perthes’ disease: prognosis in children under six years of age. J Bone Joint Surg Br 90(7):940–945
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the research.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
No patients were involved. This is a retrospective study of patient’s data, and an IRB approval was obtained.
Rights and permissions
About this article
Cite this article
De Rosa, V., Laurent, M., Canavese, F. et al. A simple, precocious, and reliable way to assess future clinical outcome in children with Perthes disease and mild femoral head involvement: correlation between MRI with diffusion-weighted and dynamic gadolinium-enhanced subtraction and Catterall and Herring classifications. Eur J Orthop Surg Traumatol 28, 1283–1290 (2018). https://doi.org/10.1007/s00590-018-2209-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-018-2209-8