Abstract
Background
Several mechanical studies suggest locking plate constructs may inhibit callus necessary for healing of distal femur fractures. However, the rate of nonunion and factors associated with nonunion are not well established.
Questions/purposes
We (1) determined the healing rate of distal femur fractures treated with locking plates, (2) assessed the effect of patient injury and treatment variables on fracture healing, and (3) compared callus formation in fractures that healed with those that did not heal.
Patients and Methods
We retrospectively reviewed 82 patients treated with 86 distal femur fractures using lateral locking plates. We reviewed all charts and radiographs to determine patient and treatment variables and then determined the effects of these variables on healing. We quantitatively measured callus at 6, 12, and 24 weeks. The minimum time for telephone interviews and SF-36v2TM scores was 1 year (mean, 4.2 years; range, 1–7.2 years).
Results
Fourteen fractures (20%) failed to unite. Demographics and comorbidities were similar in patients who achieved healing compared with those who had nonunions. There were more empty holes in the plate adjacent to fractures that healed; comminuted fractures failed to heal more frequently than less comminuted fractures. Less callus formed in fractures with nonunions and in patients treated with stainless steel plates compared with titanium plates. Complications occurred in 28 of 70 fractures (40%), 19 of which had additional surgery.
Conclusions
We found a high rate of nonunion in distal femur fractures treated with locking plates. Nonunion presented late without hardware failure and with limited callus formation suggesting callus inhibition rather than hardware failure is the primary problem. Mechanical factors may play a role in the high rate of nonunion.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Locking plates for internal fixation of distal femur fractures have largely replaced intramedullary nails, blade plates, and condylar screws [11]. Reported clinical nonunion rates after treatment of distal femur fractures with locking plates vary between 0% and 10% [6, 7, 11, 16, 20, 27, 29]. Difficulties with fracture healing in the distal femur may present clinically as delayed union, hardware failure, loss of alignment, or an established nonunion. Some patients have a secondary procedure such as bone grafting. Currently, factors that influence healing of distal femur fractures treated with locking plates are unknown. However, biomechanical studies suggest locking plates may be too stiff to promote bridging callus between fracture fragments [5, 7, 9]. If a bridging callus does not form in a comminuted fracture, regardless of device strength, the construct eventually will fail [2, 32].
We therefore posed three questions: (1) What is the healing rate with distal femur fractures treated with locking plates? (2) Do any of numerous patient or injury variables (diabetes, age, smoking, open versus closed injury, Injury Severity Score [ISS], Orthopaedic Trauma Association [OTA] classification) or treatment variables (alignment, plate material, plate length, unfilled holes, bridge span length, infection) influence healing rates? (3) Does the volume of callus differ in fractures that healed compared with those that had nonunion develop, or differ with varying features of the construct?
Patients and Methods
We retrospectively reviewed 82 adult patients with 86 distal femur fractures treated with fixed-angle lateral locking plates between 2003 and 2008 at two institutions, The University of Iowa Hospitals and Clinics (73 fractures) and the Slocum Center for Orthopaedics (13 fractures). We excluded 11 patients without radiographs at a minimum of 12 weeks after injury, leaving 71 study patients with 75 fractures. Two of these 11 patients died in the early postoperative period, four had documented transfer of care out of state, and the remaining five had limited followup of less than 12 weeks. Forty-six of the 71 patients (64%) had clinical and radiographic followup at 1 year or longer after injury. Twenty-five additional patients in the group of 71 had clinical and radiographic followup at greater than 12 weeks but less than 1 year after injury and were contacted by telephone. They were asked the following questions: Have you had any other operations on your knee? If yes, what kind? Have you seen an orthopaedic surgeon for your knee? Do you use any walking aids specifically for your knee? Are you currently working or back to recreational activities? If not, why? The questions were designed to identify any issues that would be expected in patients with failure of fracture healing after their injury. Based on the responses to this questionnaire, five additional patients were eliminated because the healing outcome of their femur fracture was considered uncertain. This patient questionnaire at longer-term followup was used as a surrogate for observing fracture healing on radiographs as the expense, inconvenience, and radiation exposure for patients to achieve long-term followup radiographs years after their injury could not be justified. The final study population was 66 of the 82 patients (80%) with 70 fractures. The time of followup for telephone interviews and SF-36v2TM scores was a minimum of 1 year (mean, 4.2 years; range, 1–7.2 years). There were 10 periprosthetic fractures, all of which were Rorabeck Type II fractures with well-fixed components [26]. The study was approved by the Institutional Review Board at both institutions.
The procedures were performed by or under the supervision of an orthopaedic trauma surgeon at both institutions. The time from injury to applying the locking plate ranged from 0 to 10 days, with an average of 2.8 days. Nine patients had external fixators placed before definitively fixing the fracture, and 18 had irrigation and débridement for an open fracture. Plates were placed through small 4- to 6-cm incisions, and the metaphyseal portion of the fracture was reduced closed in 52 fractures and open in 18 fractures. No acute bone grafts were performed. None of the periprosthetic fractures required revision of components.
Patients were mobilized wearing a hinged knee brace on the first postoperative day. Deep venous thrombosis prevention measures were provided, most commonly aspirin and mechanicals.
Patients typically were seen for followup at 2 weeks, 6 weeks, 3 months, 6 months, and 1 year after injury with clinical examination and radiographs performed each time with the exception of the initial postoperative visit. We reviewed the medical records and radiographs of all patients and recorded demographic data, injury mechanism, time to weightbearing, ability to bear weight, and complications. Charts were reviewed for smoking history by answering yes or no. Patients were contacted by telephone or mail to obtain SF-36v2TM scores [33]. These scores were compared with published US age-matched normative data [33]. SF-36v2TM surveys were obtained for 44 of the 66 patients (67%), and the average physical component score (PCS) and mental component score (MCS) were calculated. All outcomes scores were for healed fractures; two patients who initially had nonunions were included because they had been treated successfully with revision surgery and had achieved healing. Surgical site infections were determined according to the Centers for Disease Control and Prevention definition as superficial or deep [10]. The specific plate used and the construct material (stainless steel versus titanium) were identified (Table 1).
Radiographs were assessed for fracture classification, alignment, change in alignment, healing, and callus formation. All fractures were classified on injury radiographs using the OTA/AO Universal fracture classification [24], and open fractures were classified by the Gustilo and Anderson system [8] (Table 2). The fractures were divided into those without substantial metaphyseal comminution (33A1, A2, and C1; n = 33) and those with metaphyseal comminution (33A3, C2, and C3; n = 42).
One of us (CEH) measured alignment of the fracture on postoperative AP and lateral radiographs of the femur and knee. Normal coronal alignment was considered 5° to 7° valgus, and normal sagittal alignment was neutral. Malalignment was defined as greater than 5° deviation from normal coronal or sagittal alignment [25]. Loss of alignment was defined as greater than a 3°-change in angular measurements between postoperative and followup radiographs.
Six-week postoperative radiographs were assessed for plate length, bridge span length, and number of holes left unfilled adjacent to the fracture or area of comminution. The bridge span length was defined as the distance (millimeters) between screws adjacent to the fracture; two groups were formed by comparing those with above-average bridge spans (> 69 mm, n = 26) with those with below-average bridge span length (< 69 mm, n = 44). A screw hole was counted as unfilled adjacent to the fracture if, at the site of an empty hole, a perpendicular screw path would encounter only intact cortical bone avoiding any comminution. Two groups were formed for analysis, those with zero holes left unfilled (n = 27) versus those with two or more holes left unfilled (n = 13). Fractures with only one unfilled hole were not included in this analysis to allow comparison between two groups with different mechanical properties. The ratio of bridge span to plate length also was compared between groups.
Serial orthogonal radiographs taken at 6 weeks, 3 months, and 6 months after the fracture were reviewed by one observer (CEH) who was not a treating surgeon, to assess union by identifying bridging callus of at least two cortices [12, 22, 23]. Patient charts were reviewed for documentation of the ability to bear weight without pain. The formation of bridging callus on radiographs and the ability to bear weight without pain are the most frequently used criteria for fracture union in reported clinical series and were used in this study [3, 4]. Cortical bridging observed on radiographs is reportedly the most reliable assessment of fracture healing [35]. Nonunion was defined by pain with weightbearing and the absence of progressive fracture healing or bridging callus at the medial cortex on serial radiographs.
The amount of callus formed on the anterior, posterior, and medial sides of the fracture opposite the locking plate was measured on radiographs at 6, 12, and 24 weeks after injury in 63 fractures. To measure callus, the serial radiographs had to meet strict criteria such as consistent rotation, which resulted in eliminating seven fractures. An established algorithm was used to objectify the measurement of callus size [18, 19]. Briefly, custom software extracted the size of periosteal callus from plain radiographs without the need for manual tracing of callus boundaries. Callus size was converted to metric area using a length standard based on implant features. The algorithm was determined to measure callus area in surrogate models with an error less than 5% [18, 19]. For clinical oversight, three clinicians independently inspected the demarcation of cortical bone and periosteal callus in every analyzed image in a validation protocol [18, 19]. The actual callus measurements for the radiographs in this study were made by an independent observer (TJL) not involved in patient care.
The primary outcome was presence or absence of fracture healing determined by clinical and radiographic review. The individual values of patient age, ISS, bridge span length, and number of unfilled holes adjacent to the fracture were obtained and means were calculated [1]. Fractures were divided into two groups based on whether they were healed. Comparisons then were performed using Fisher’s exact test for categorical variables such as smoking status and Student’s t test for continuous variables such as patient age. All tests were two-tailed. The effect of factors on time to weightbearing and time to bridging callus was assessed with Fisher’s exact test. The effect of factors on callus size was determined with ANOVA (SPSS statistics, SPSS Inc, Chicago, IL, USA).
Results
Fourteen of the 70 fractures (20%) failed to heal. Nine of these 14 were treated with subsequent surgery to achieve union, at an average time of 9 months, and five were treated nonoperatively. In 10 nonunions, the hardware remained intact, but the fracture did not radiographically progress to union. In two of the nonunions, the implant failed, with the plate pulling off proximally at 2 and 10 months after fracture, while in another two, the distal screws broke at 10 and 13 months after injury. The average time from injury to diagnosis of nonunion was 8.5 months after the initial operation.
Healed fractures had more (p = 0.01) unfilled holes than those that did not heal (Table 3). Ten of 14 (71%) nonunions had zero unfilled holes adjacent to the fracture area and the remaining four nonunions had only one unfilled hole. More (p = 0.01) nonunions occurred in the more comminuted OTA fracture classification group (33A3, C2, C3) compared with the less comminuted group. There were no other differences between the patients with healed fractures and those with nonunions (Table 3). Ten of 12 (83%) fractures that changed alignment went on to heal (Table 4). The majority of fractures in which nonunions developed maintained excellent alignment, which did not change over serial radiographs. Only two of the 14 (14%) fracture nonunions were malaligned (12º and 16º valgus) and two changed alignment greater than 3° (Table 4).
Fractures that failed to unite had less callus area than fractures that healed at 6 weeks (p = 0.03), 12 weeks (p = 0.002), and 24 weeks (p = 0.003) (Fig. 1). There was more callus in fractures treated with titanium plates compared with fractures treated with stainless steel plates at 6 weeks (p = 0.03), 12 weeks (p = 0.03), and 24 weeks (p = 0.09). No difference was found in callus formed in fractures treated with plates with zero holes unfilled compared with those with two or more holes unfilled adjacent to the fracture. Fractures with a longer bridge span had an increased callus size at the medial cortex at 6 weeks (p = 0.02), but there was no correlation at 12 or 24 weeks at the medial, anterior, or posterior cortices.
There were complications in 28 of 70 fractures (40%). Nineteen of these required secondary operative intervention (27% of the total number of fractures and 68% of the total number with complications). Seven (10%) of the 70 fractures had infections, four of which were superficial and resolved after oral antibiotics and wound care and three of which were deep and required operative intervention. The average US norms for SF-36v2TM PCS and MCS were higher (p = 0.0001 and 0.007, respectively) than the average for our femur fracture population as a whole and divided by age group (Table 5).
Discussion
Locking plates have become the most commonly used method to fix fractures of the distal femur [17]. However, factors that lead to successful fracture healing have not been carefully studied. We evaluated the rate of healing of a consecutive case series of distal femur fractures treated with these plates and assessed the effect of multiple patient and treatment variables. Callus was measured quantitatively to assess whether the healing outcome and construct stiffness affect the amount of callus formed.
Our study is limited by some factors. First, some patients were eliminated secondary to loss of followup. Second, the radiographic followup for some of the included patients was short (minimum, 12 weeks) and radiographs were reviewed for bridging callus by one observer. However, by contacting all patients in the study with less than 1 year of radiographic followup (at an average of 4.2 years), we attempted to minimize the chance of missing healing complications. If there were patients we included and classified as healed who actually did not heal, this would further raise the rate of nonunion in this study. Third, the patient group was heterogeneous in that there was considerable variability of patient demographics, fracture types, and differences in fracture plate constructs among the study population. We analyzed the effect on healing of some factors that could be modified by the surgeon. The type of fracture or mechanical environment could confound variables we studied, such as the number of empty holes adjacent to a fracture. There are other potentially important factors we did not have data to analyze, including bone quality, size of screws, unicortical versus bicortical, strain, and strength of the lock of screws to the plate, to name a few. There are limitations with the callus measurement technique, including using a two-dimensional projection to measure a three-dimensional biologic process and inability to measure internal callus in the fracture [18, 19]. Finally, although we observed variation in callus formation based on mechanical differences, clinical nonunion rates were not affected by these differences, possibly representing a type II statistical error.
We found a high rate of nonunion, 20% (14 of 70), in our study. We eliminated 16 fractures from this study secondary to inadequate followup. If we speculated all these 16 fractures healed, the study nonunion rate would decrease to 14 of 86 (16%). Although some authors have reported a 100% union rate in small series [6, 16, 31, 34, 35], most larger series have found a substantial rate of healing difficulties [6, 20, 27–29, 32]. When nonunion, delayed union, the need for secondary surgery, and hardware failure are considered, the rate of healing difficulties reported in the literature is comparable to the rate in our study (Table 6) [6, 7, 11, 14–16, 20, 27–29, 31, 32, 34, 35]. A systematic review by Zlowodzki et al. [37] showed an increased risk of fixation failure and revision surgery with locking plates compared with conventional techniques. In another study, there was an increased rate of complications, malunions, and the need for secondary procedures when locking condylar plates were compared with angled blade plates [32].
There were no differences in patient and biologic factors between fractures that healed and those that had nonunions. This suggests there may be mechanical factors contributing to failure to heal. We found bridging constructs with more open holes adjacent to the fracture had a better healing rate than constructs with fewer open holes (Fig. 2). Gaines et al. [7] reported the nonunion rate after treatment with stainless steel locking plates was 23% compared with 7% for titanium locking plates. In our study, there was a greater percentage of nonunions in fractures treated with stainless steel plates. Stoffel et al. [30] found increasing the plate span by omitting one screw hole on either side of the fracture decreased the stiffness of a locked plating construct in compression and torsion by 50%. In our study, 10 of 14 (71%) nonunions had zero open holes adjacent to the fracture and the remaining four had only one open hole, suggesting the better healing rate in our cases with longer open spans may have been from more interfragmentary motion. The majority of our nonunions maintained fixation with no change in alignment until revision or late hardware failure.
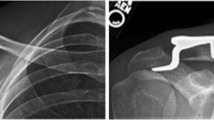
(A) A 6-month postoperative radiograph shows a stiff construct with cortical contact medially, multiple locking screws, and a very short bridge span at the fracture site. There is little peripheral callus seen. (B) In contrast, a 6-month postoperative radiograph of a comminuted fracture shows a long bridge span and multiple unfilled holes adjacent to the fracture resulted in substantial callus formation.
The stiffness of a fracture fixation construct affects callus formation, and interfragmentary motion in the millimeter range is known to induce bone healing that does not occur with less motion [3, 13]. Comminuted fractures treated with bridge plating must heal with external callus. Compared with external fixators, locking plate constructs can be considerably more stiff, given the proximity of the plate to the bone [17, 21], and they may act like extremely rigid internal fixators preventing callus from forming [17]. Two studies have reported locking plates for distal femur fractures that mechanically failed and healing occurred after medial collapse (Fig. 3) [9, 32]. Locking plate constructs failed late after a long period of maintained fixation [2, 32]. Callus measurement provides a tool to further understand the mechanical environment of a fracture and its effect on union. The fractures in our patients that did not heal formed less callus at 6, 12, and 24 weeks than those that did heal, and more flexible titanium plates had more callus than stainless steel plates. Lujan et al. [18] suggested distal femur fractures with periarticular locking plates have asymmetric callus, with the majority of callus on the medial cortex, where interfragmentary motion is greatest.
(A) A postoperative radiograph shows 5° valgus alignment. (B) This alignment was maintained at 6 months despite delayed union with little callus formation. (C) A radiograph taken 11 months after injury shows the distal screws have fractured and there has been medial collapse into 2° varus. The fracture went on to heal.
Our study, when combined with published studies, indicates some patients experience healing difficulties, including nonunion, delayed union, and hardware failure, when fractures of the distal femur are treated with locking plates. Fractures that fail to heal usually maintain alignment and form less callus, suggesting callus inhibition rather than hardware failure is the primary problem. Mechanical factors such as implant and construct stiffness may play a role in callus inhibition, but at this stage, the optimal mechanical environment for a distal femur fracture treated with locking implants remains uncertain.
References
Baker SP, O’Neill B, Haddon W Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196.
Button G, Wolinsky P, Hak D. Failure of less invasive stabilization system plates in the distal femur: a report of four cases. J Orthop Trauma. 2004;18:565–570.
Claes LE, Heigele CA, Neidlinger-Wilke C, Kaspar D, Seidl W, Margevicius KJ, Augat P. Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res. 1998;355(suppl):S132–S147.
Corrales LA, Morshed S, Bhandari M, Miclau T 3rd. Variability in the assessment of fracture-healing in orthopaedic trauma studies. J Bone Joint Surg Am. 2008;90:1862–1868.
Egol KA, Kubiak EN, Fulkerson E, Kummer FJ, Koval KJ. Biomechanics of locked plates and screws. J Orthop Trauma. 2004;18:488–493.
Fankhauser F, Gruber G, Schippinger G, Boldin C, Hofer HP, Grechenig W, Szyszkowitz R. Minimal-invasive treatment of distal femoral fractures with the LISS (Less Invasive Stabilization System): a prospective study of 30 fractures with a follow up of 20 months. Acta Orthop Scand. 2004;75:56–60.
Gaines RJ, Sanders R, Sagi HC, Haidukewych GJ. Titanium versus stainless steel locked plates for distal femur fractures: is there any difference? OTA abstract. 2008: Paper Number 55.
Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58:453–458.
Henderson CE, Bottlang M, Marsh JL, Fitzpatrick DC, Madey SM. Does locked plating of periprosthetic supracondylar femur fractures promote bone healing by callus formation? Two cases with opposite outcomes. Iowa Orthop J. 2008;28:73–76.
Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606–608.
Kayali C, Agus H, Turgut A. Successful results of minimally invasive surgery for comminuted supracondylar femoral fractures with LISS: comparative study of multiply injured and isolated femoral fractures. J Orthop Sci. 2007;12:458–465.
Keating JF, O’Brien PJ, Blachut PA, Meek RN, Broekhuyse HM. Locking intramedullary nailing with and without reaming for open fractures of the tibial shaft: a prospective, randomized study. J Bone Joint Surg Am. 1997;79:334–341.
Kenwright J, Richardson JB, Goodship AE, Evans M, Kelly DJ, Spriggins AJ, Newman JH, Burrough SJ, Harris JD, Rowley DI. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;2:1185–1187.
Kregor PJ, Hughes JL, Cole PA. Fixation of distal femoral fractures above total knee arthroplasty utilizing the Less Invasive Stabilization System (L.I.S.S). Injury. 2001;32(suppl 3):SC64–SC75.
Kregor PJ, Stannard J, Zlowodzki M, Alonso J. Distal femoral fracture fixation utilizing the Less Invasive Stabilization System (L.I.S.S.): the technique and early results. Injury. 2001;32(suppl 3):SC32–SC47.
Kregor PJ, Stannard JA, Zlowodzki M, Cole PA. Treatment of distal femur fractures using the less invasive stabilization system: surgical experience and early clinical results in 103 fractures. J Orthop Trauma. 2004;18:509–520.
Kubiak EN, Fulkerson E, Strauss E, Egol KA. The evolution of locked plates. J Bone Joint Surg Am. 2006;88(suppl 4):189–200.
Lujan TJ, Henderson CE, Madey SM, Fitzpatrick DC, Marsh JL, Bottlang M. Locked plating of distal femur fractures leads to inconsistent and asymmetrical callus formation. J Orthop Trauma. 2010;24:156–162.
Lujan TJ, Madey SM, Fitzpatrick DC, Byrd GD, Sanderson JM, Bottlang M. A computational technique to measure fracture callus in radiographs. J Biomech. 2010;43:792–795.
Markmiller M, Konrad G, Sudkamp N. Femur-LISS and distal femoral nail for fixation of distal femoral fractures: are there differences in outcome and complications? Clin Orthop Relat Res. 2004;426:252–257.
Marti A, Fankhauser C, Frenk A, Cordey J, Gasser B. Biomechanical evaluation of the less invasive stabilization system for the internal fixation of distal femur fractures. J Orthop Trauma. 2001;15:482–487.
Moed BR, Watson JT, Goldschmidt P, van Holsbeeck M. Ultrasound for the early diagnosis of fracture healing after interlocking nailing of the tibia without reaming. Clin Orthop Relat Res. 1995;310:137–144.
Morshed S, Corrales L, Genant H, Miclau T 3rd. Outcome assessment in clinical trials of fracture-healing. J Bone Joint Surg Am. 2008;90(suppl 1):62–67.
Orthopaedic Trauma Association Committee for Coding and Classification. Fracture and dislocation compendium. J Orthop Trauma. 1996;10(suppl 1):v–ix, 1–154.
Paley D. Principles of Deformity Correction. Berlin, Germany: Springer-Verlag; 2002.
Rorabeck CH, Taylor JW. Periprosthetic fractures of the femur complicating total knee arthroplasty. Orthop Clin North Am. 1999;30:209–214.
Schandelmaier P, Partenheimer A, Koenemann B, Grun OA, Krettek C. Distal femoral fractures and LISS stabilization. Injury. 2001;32(suppl 3):SC55–SC63.
Schutz M, Muller M, Krettek C, Hontzsch D, Regazzoni P, Ganz R, Hass N. Minimally invasive fracture stabilization of distal femoral fractures with the LISS: a prospective multicenter study. Results of a clinical study with special emphasis on difficult cases. Injury. 2001;32(suppl 3):SC48–SC54.
Schutz M, Muller M, Regazzoni P, Hontzsh D, Krettek C, Van der Werken C, Hass N. Use of the less invasive stabilization system (LISS) in patients with distal femoral (AO33) fractures: a prospective multicenter study. Arch Orthop Trauma Surg. 2005;125:102–108.
Stoffel K, Dieter U, Stachowiak G, Gachter A, Kuster MS. Biomechanical testing of the LCP: how can stability in locked internal fixators be controlled? Injury. 2003;34(suppl 2):B11–B19.
Syed AA, Agarwal M, Giannoudis PV, Matthews SJ, Smith RM. Distal femoral fractures: long-term outcome following stabilisation with the LISS. Injury. 2004;35:599–607.
Vallier HA, Hennessey TA, Sontich JK, Patterson BM. Failure of LCP condylar plate fixation in the distal part of the femur: a report of 6 cases. J Bone Joint Surg Am. 2006;88:846–853.
Ware JE, Kosinski M, Dewey JE. How to Score Version 2 of the SF-36 Health Survey. Lincoln, RI: QualityMetric Inc; 2000.
Weight M, Collinge C. Early results of the less invasive stabilization system for mechanically unstable fractures of the distal femur (AO/OTA types A2, A3, C2, and C3). J Orthop Trauma. 2004;18:503–508.
Whelan DB, Bhandari M, McKee MD, Guyatt GH, Kreder HJ, Stephen D, Schemitsch EH. Interobserver and intraobserver variation in the assessment of the healing of tibial fractures after intramedullary fixation. J Bone Joint Surg Br. 2002;84:15–18.
Wong MK, Leung F, Chow SP. Treatment of distal femoral fractures in the elderly using a less-invasive plating technique. Int Orthop. 2005;29:117–120.
Zlowodzki M, Bhandari M, Marek DJ, Cole PA, Kregor PJ. Operative treatment of acute distal femur fractures: systematic review of 2 comparative studies and 45 case series (1989 to 2005). J Orthop Trauma. 2006;20:366–371.
Acknowledgments
We thank Todd O. Mckinley for his contribution to this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
One or more of the Authors (MB, DF) have a commercial association with a company (Zimmer) that introduced MotionLoc technology.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at University of Iowa Hospitals and Clinics and the Slocum Center for Orthopaedics.
About this article
Cite this article
Henderson, C.E., Lujan, T.J., Kuhl, L.L. et al. 2010 Mid-America Orthopaedic Association Physician in Training Award: Healing Complications Are Common After Locked Plating for Distal Femur Fractures. Clin Orthop Relat Res 469, 1757–1765 (2011). https://doi.org/10.1007/s11999-011-1870-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-011-1870-6