Abstract
Background
Bone strength depends on both bone quantity and quality. The former is routinely estimated in clinical settings through bone mineral density measurements but not the latter. Bone quality encompasses the structural and material properties of bone. Although its importance is appreciated, its contribution in determining bone strength has been difficult to precisely quantify partly because it is multifactorial and requires investigation of all bone hierarchical levels. Fourier transform infrared spectroscopy provides one way to explore these levels.
Questions/purposes
The purposes of our review were to (1) provide a brief overview of Fourier transform infrared spectroscopy as a way to establish bone quality, (2) review the major bone material parameters determined from Fourier transform infrared spectroscopy, and (3) review the role of Fourier transform infrared microspectroscopic analysis in establishing bone quality.
Methods
We used the ISI Web of Knowledge database initially to identify articles containing the Boolean term “infrared” AND “bone.” We then focused on articles on infrared spectroscopy in bone-related journals.
Results
Infrared spectroscopy provides information on bone material properties. Their microspectroscopic versions allow one to establish these properties as a function of anatomic location, mineralization extent, and bone metabolic activity. It provides answers pertaining to the contribution of mineral to matrix ratio, mineral maturity, mineral carbonate substitution, and collagen crosslinks to bone strength. Alterations of bone material properties have been identified in disease (especially osteoporosis) not attainable by other techniques.
Conclusions
Infrared spectroscopic analysis is a powerful tool for establishing the important material properties contributing to bone strength and thus has helped better understand changes in fragile bone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Loss of bone mass, measured clinically as change in bone mineral density (BMD), is considered an important risk factor for bone fragility. However, it is not the sole predictor of whether an individual will experience a fracture [8, 43]. Moreover, considerable overlap in BMD exists between populations that do and do not develop fractures [14, 41, 45]. For a given bone mass, an individual’s risk to fracture increases with age [32]. Additionally, numerous reports document mechanical variables directly related to fracture risk are either independent [33] or not solely dependent on bone mass itself [34, 35, 44, 50, 53]. In a recent report analyzing iliac crest biopsies from 54 women (32 with fractures, 22 without) who had lower (compared with normal) spine but not hip BMDs, cortical and cancellous collagen maturity strongly correlated with overall fracture incidence (increased with increased fracture risk) [27], emphasizing the contribution of collagen quality in determining bone strength.
It is becoming evident, in addition to BMD, bone quality should also be considered when assessing bone strength and fracture risk. Bone quality is a broad term encompassing factors affecting the structural and material properties of bone (Fig. 1), both of which mainly depend on bone turnover. Notable potential exceptions (at least as far as material properties are concerned) are cases in which factors directly affecting the physical chemistry of mineral crystallites are involved. Such a case would be bisphosphonates as they adsorb onto the apatitic surfaces, changing the surface properties, and affect the rate of mineral growth and dissolution [29–51]. Other examples would be strontium [40, 71] as it incorporates into the apatitic mineral, changing its dissolution characteristics and crystallite size and shape, and fluoride [21, 23, 69, 70] as it incorporates into the apatitic mineral crystallites, making them larger, and greatly reduces the dissolution rate of these crystallites. As far as collagen properties are concerned, an example would be homocysteine [1, 75] as it interferes with collagen enzymatic posttranslation modifications that occur after it has been synthesized and excreted by the osteoblast.
One of the obstacles to be circumvented when assessing mineral and matrix tissue properties is tissue heterogeneity at the microscopic level. Bone surfaces may be undergoing formation and/or resorption or they may be inactive. These processes, which can be visualized microscopically, occur throughout life in both cortical and trabecular bone [18]. Bone remodeling is a surface phenomenon and in humans occurs on periosteal, endosteal, Haversian canal, and trabecular surfaces [9, 10, 18, 64]. The rate of cortical bone remodeling, as high as 50% per year in the midshaft of the femur during the first 2 years of life, eventually declines to a rate of 2% to 5% per year in the elderly. Rates of remodeling in trabecular bone are proportionately higher throughout life and may normally be five to 10 times higher than cortical bone remodeling rates in the adult [18]. This information is critical when evaluating bone at the microscopic level; thus, variability in tissue age should be accounted for.
Fourier transform infrared (FTIR) spectroscopy provides one way to explore bone quality at multiple bone hierarchical levels. The purposes of our review were to (1) provide a brief overview of FTIR spectroscopy as a way to establish bone quality and explore tissue variability, (2) review the major bone material parameters determined from FTIR spectroscopy, and (3) review the role of FTIR microspectroscopic analysis in establishing bone quality.
Search Strategy and Criteria
We used the ISI Web of Knowledge database initially to identify articles containing the Boolean term “infrared” AND “bone.” This resulted in 1640 hits. To narrow the number of articles identified, we used additional terms such as “aging,” “osteoporosis,” “fracture,” “mineral,” “carbonate,” and “collagen,” which resulted in 99, 49, 80, 711, 216, and 229 hits, respectively. The searches were then focused primarily on infrared spectroscopy articles published in bone-related journals and bone studies published in peer-reviewed chemical spectroscopy and biomedical optical journals.
FTIR Spectroscopy
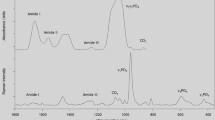
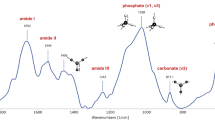
All chemical bonds undergo vibrations that encompass a variety of motions of the atomic groupings within the molecular framework. These include bond stretching, in- and out-of-plane angle bending, wagging (change in angle between a bond and a plane), and twisting (change in angle between two planes). If a particular motion is accompanied by a dipole moment change, when illuminated with infrared radiation, it will absorb at a specific infrared frequency, characteristic of the overall configuration of the atoms and representative of specific functional groups. Moreover, through detailed analysis of the absorption spectra, information may be derived on subtle interactions with the surrounding groups of a molecule. FTIR spectra provide information on all bone tissue components (Fig. 2). The protein and mineral constituents produce intense, structure-sensitive infrared modes. The peaks of interest have been appropriately marked. Information is available from both the mineral (carbonate substituting in the apatite lattice and phosphate from the apatite itself) and collagen (Amide I, II, and III; the first results from peptide bond C=O stretch, the second results from mixed C–N stretch and N–H in-plane bend, and the third also results from mixed C–N stretch and N–H in-plane bend with additional contributions from C–Cα stretch).
FTIR Spectroscopy Major Bone Material Parameters
The most frequently reported parameters of FTIR spectroscopy pertaining to bone material properties are (1) mineral to matrix ratio, (2) mineral maturity/crystallinity, and (3) collagen maturity (expressed as the ratio of two of the major Type I bone collagen crosslinks).
Mineral to Matrix Ratio
The integrated area under an infrared band is directly proportional to the amount of species that generates the band. As a result, the ratio of the integrated phosphate and any of the amide bands (usually Amide I) is representative of the amount of mineral normalized to the amount of collagen present. It is a measure of BMD and correlates with ash weight measurements [6]. Nevertheless, caution should be exercised when the results are considered and compared with conventional BMD parameters such as BMD and BMD distribution (BMDD) because this infrared spectra parameter provides information on the amount of mineral per volume analyzed per amount of collagen present, whereas BMD and BMDD express amount of mineral per volume. It is biologically relevant because it accounts for both the main constituents of bone but suffers from the fact that it is a ratio (for example, an unaltered ratio may be the result of the fact that there is no change in either of the ratio components or a proportionate one in both). It is most informative when combined with results of a technique such as quantitative backscattered electron imaging, which provides information on the amount of mineral distribution at the microscopic level [72]. On the other hand, it may provide a more relevant description of whether bone tissue is either hyper- or hypomineralized because it encompasses both major constituents of bone (mineral and collagen), and because bone is considered a composite material, its mechanical performance depends on the quantity and quality of both.
Mineral Maturity/Crystallinity
FTIR spectroscopy has been extensively used in the analysis of bone mineral [3, 63, 65, 74]. Through spectroscopic and mathematical analysis of the phosphate band by means of techniques such as deconvolution, second-derivative spectroscopy, and curve fitting, spectral regions (underlying peaks) have been identified and correlated with the various chemical environments present in biologic apatites, enabling the monitoring of the calcium phosphate crystal maturity (ionic substitutions in the poorly crystalline apatite lattice, stoichiometry) [2, 3, 66–68, 73]. This mineral characteristic changes as a function of tissue age [54] as a result of the dynamic physical chemistry status of the crystals bathing in biologic fluids, resulting in variable mineral maturity within the same specimen. For example, not only carbonate content but also the type of substitution (Type A represents carbonate in the hydroxyl position of apatite, Type B represents carbonate in the phosphate, and labile represents loosely adsorbed carbonate on the crystal surfaces) changes as a function of tissue age [54], influencing the solubility and crystallinity (shape and size) of apatite crystallites.
Based on physical chemical studies [20, 54], it is known, as bone mineral crystals age, so does the crystallinity of the apatite mineral. As a result, efforts have been made to extrapolate from the direct measure of FTIR spectroscopy, which is mineral maturity, to mineral crystallinity [24, 54]. Nevertheless, caution should be exercised in that the synthetic mineral crystallites used in the in vitro experiments are not subjected to any ordering and/or orientation, unlike bone mineral crystallites. This may be the underlying cause for the paradox that the same spectroscopic parameter in experiments involving powders correlates well with the c-crystallographic axis (crystallite length, determined by xray diffraction analysis), whereas in tissue sections, it correlates with crystallite thickness (determined by small-angle xray spectroscopic analysis) [12, 24, 54]. The important contribution of mineral maturity/crystallinity in determining bone strength may be appreciated in the case of fluoride-treated osteoporotic bone. In that instance, despite gains in BMD, bone eventually becomes fragile because of the crystallites’ increased maturity/crystallinity due to the physical chemical effect of fluoride on biologic apatites [21, 23, 69, 70].
The v1,v3 PO4 3− infrared band in mineralized tissues is a composite one, reflecting the presence of nanocrystalline mineral; the band consists of underlying peaks, each of which is representative of a specific chemical environment. The major underlying bands (as determined through second-derivative spectroscopy and curve-fitting routines) are summarized (Table 1) [54]. The relative distribution of these bands varies as a result of the dynamic nature of poorly crystalline apatitic crystals.
Collagen Maturity and Collagen Crosslinks
The protein Amide I (peptide bond C=O stretch) and Amide II (mixed C–N stretch and N–H in-plane bend) modes near 1650 and 1550 wavenumbers (cm−1) undergo frequency and intensity changes as a result of changes in protein secondary structure [25]. As in the case of mineral analysis, information on protein structure is extracted from broad spectral bands consisting of component peaks resulting from the Amide I modes of various secondary structures by using a spectral resolution technique such as Fourier self-deconvolution, second-derivative spectroscopy, and difference FTIR spectroscopy [15, 25, 36–39, 76]. The major underlying bands and their assignments are summarized (Table 1). Fairly recently, a method was developed enabling the spectral analysis of the Amide I band to isolate underlying peaks corresponding to two of the major Type I bone collagen crosslinks, namely pyridinoline (pyr) and divalent ones [61].
FTIR Microspectroscopy and Imaging
Despite the fact that detailed information on mineral maturity and protein secondary structure is obtainable using FTIR analysis, homogenized bone tissue is a prerequisite, making it impossible to correlate the monitored bone material properties with tissue age and bone metabolic activity, thus greatly dependent on bone turnover.
In the late 1980s, the combination of an optical microscope with an infrared spectrometer offered the opportunity to examine thin bone tissues with a spatial resolution of approximately 20 μm [46]. This, for the first time as far as FTIR analyses were concerned, allowed the selection of anatomic areas to be investigated based on parallel histologically stained sections and enabled the correlation of the spectroscopic results with bone surface metabolic activity (tissue age) [46, 57, 61]. This work was later replicated and expanded by others [11, 16, 19, 30, 31, 47, 48], resulting in a wealth of new information about the mineral component of bone as a function of cellular activity, tissue age, disease, and therapeutic intervention.
Another achievement was the development of spectroscopic parameters corresponding to two of the major mineralizing Type I collagen crosslinks (pyr and dehydrodihydroxynorleucine [DHLNL]) in thin, histologically stained bone sections [61]. This allowed the establishment of the variation in their spatial distribution as a function of anatomic location, cellular activity, and tissue age [61]. As a consequence, it is feasible to describe differences between healthy and diseased bone independent of bone turnover status.
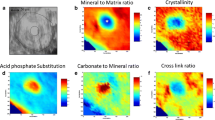
One major drawback of FTIR microspectroscopic analysis using a single infrared detector element (area of analysis of 10 × 10 μm) is the time required for spectral acquisition and further spectral processing (so as to calculate the relevant parameters pertaining to mineral crystallinity and collagen crosslinks) over a large area (so as to obtain statistically meaningful results) of a single biopsy, rendering the efficient analysis of biopsies virtually impossible. This limitation was overcome through the development of infrared microspectroscopic imaging. The approach required the integration of an infrared focal-plane array detector and a FTIR microscope [42]. The advantage of this technique lies in the fact that the spectral acquisition and processing time is shortened by at least 1000-fold compared with conventional FTIR microspectroscopy. Using a step-scanning FTIR spectrometer with an mercury cadmium telluride array detector placed at the image focal plane of the FTIR microscope enables areas of approximately 400 × 400 μm2 to be analyzed in less than 3 to 4 minutes with a spatial resolution of approximately 6.3 μm [42]. Instead of a single FTIR spectrum, 4096 spectra are collected simultaneously in a 64 × 64 array configuration. Through integration of the appropriate bands, high-fidelity chemical images providing information on the spatial distribution of mineral, collagen, and polymethylmethacrylate (embedding medium) may be portrayed (in all images, blue = minimum, red = maximum). Through further spectral and mathematical processing and resolution of the raw bands into their constituent underlying peaks, the spatial distribution of the mineral crystallite maturity/crystallinity and pyr/divalent collagen crosslinks may be calculated and presented (Fig. 3) [28, 57, 59–61]. To date, it has been successfully applied in the analysis of cell cultures and bones from animal models and humans [5, 28].
An example of FTIR analysis of a thin section from a normal human iliac crest biopsy (cortical bone) with two evident osteons is shown. In all images, red corresponds to the highest and blue to the lowest values. Spatial distribution of the amount of (A) mineral, (B) collagen, and (C) polymethylmethacrylate (PMMA; biopsy embedding material) is provided. Additionally, the spatial distribution of (D) mineral to matrix ratio, (E) mineral crystallite maturity/crystallinity, and (F) collagen crosslink ratio (pyr/DHLNL) may be calculated from the raw spectral images. FTIR = Fourier transform infrared.
An Example of Important Findings Based on FTIR Analysis of Bone
Infrared analysis of bone tissue has proven a valuable tool in the field of osteoporosis and other conditions affecting bone quality such as osteopetrosis, osteomalacia, renal osteodystrophy, and osteogenesis imperfecta. Its use (and in particular the fact that it allows for anatomic area selection at the microscopic level) allowed for the detection of differences between normal and osteoporotic human bone both as far as mineral maturity/crystallinity (invariably higher in osteoporotic bone) and collagen crosslink ratio (invariably higher in osteoporotic patients at equivalent anatomic locations) are concerned [4, 59, 60]. These findings, especially the collagen crosslink ratio, strongly suggest factors other than bone turnover alone are partly responsible for the observed changes in material properties of osteoporotic bone, contributing to the clinically manifested fragility. For example, the changes in the collagen crosslink ratio observed in osteoporotic patients compared with normal humans would be consistent with the presence of elevated blood plasma homocysteine levels [1, 59, 60], which has been recently proposed as an additional risk factor in osteoporosis.
Discussion
Bone strength depends on both bone quantity and quality. Bone quality encompasses the structural and material properties of bone. Although its importance is appreciated, its contribution in determining bone strength has been difficult to precisely quantify partly because it is multifactorial and requires investigation of all bone hierarchical levels. FTIR spectroscopy provides one way to explore bone quality at multiple bone hierarchical levels. In this review, we (1) provided a brief overview of FTIR spectroscopy as a way to establish bone quality and explore tissue variability, (2) reviewed the major bone material parameters determined from FTIR spectroscopy, and (3) reviewed the role of FTIR microspectroscopic analysis in establishing bone quality.
We bring to the reader’s attention the following limitations to our review and the literature. Only a fraction of the available papers and specifically those in readily accessible peer-reviewed medical and chemical spectroscopy and biomedical optics journals have been cited. Details of the relevant spectroscopic theory and instrumentation have not been included. The interested reader can find them in advanced analytical chemistry textbooks and specialized books on infrared spectroscopy and its applications. A point the reader should bear in mind is that most studies involving FTIR microspectroscopic analysis of bone employ thin tissue sections. Since there are always microvariations in thickness even within the same section, it is unreliable to report absolute amounts as some of the referenced studies have done. The most commonly used way around this limitation is to report ratios rather than absolute amounts, thus rendering these thickness microvariations unimportant.
Both FTIR microspectroscopic and imaging analyses provide a wealth of otherwise unattainable information, yet we believe neither is well suited as a routine clinical screening tool because a bone biopsy is required. On the other hand, both are powerful research tools, providing unique insights into the pathophysiology of musculoskeletal diseases such as osteoporosis, osteogenesis imperfecta, Paget’s disease, osteomalacia, osteopetrosis, osteosclerosis, and so on [28]. They are particularly useful in the investigation of exceptional clinical cases such as fracturing patients whose “classic” clinical risk indicators such as BMD and biochemical markers are normal [60]. The obtained bone material properties complement ones obtained through histology, histomorphometry, biochemical markers, blood analysis, and BMD measurements, thus offering unique information on the mechanisms that result in healthy and diseased bone. They have also proven useful in evaluating various therapeutic protocols, providing insight into the mechanisms by which the various drugs work and therefore assisting in the design of new and more targeted ones [7, 13, 17, 22, 26, 49, 52, 55, 56, 58, 62, 71].
One of the unique capabilities of FTIR microspectroscopy and imaging is the potential to describe the spatial variation of pyr and DHLNL collagen crosslinks in mineralized thin tissue sections. These are only two of the major collagen crosslinks, and as a result only a partial understanding of the spatial and temporal distribution of collagen properties and their bearing on bone strength has been gained. In the future, spectral and mathematical methods should be developed to derive spectroscopic parameters that describe all of the known collagen crosslinks because they are important both in the mineralization initiation cascade of events and in determining bone strength.
In conclusion, infrared spectroscopic analysis is a powerful tool for establishing the important material properties contributing to bone strength and thus has helped better understand changes in fragile bone.
References
Blouin S, Thaler HW, Korninger C, Schmid R, Hofstaetter JG, Zoehrer R, Phipps R, Klaushofer K, Roschger P, Paschalis EP. Bone matrix quality and plasma homocysteine levels. Bone. 2009;44:959–964.
Blumenthal NC, Betts F, Posner AS. Effect of carbonate and biological macromolecules on formation and properties of hydroxyapatite. Calcif Tissue Res. 1975;18:81–90.
Bohic S, Heymann D, Pouezat JA, Gauthier O, Daculsi G. Transmission FT-IR microspectroscopy of mineral phases in calcified tissues. C R Acad Sci III. 1998;321:865–876.
Boskey AL, DiCarlo E, Paschalis E, West P, Mendelsohn R. Comparison of mineral quality and quantity in iliac crest biopsies from high- and low-turnover osteoporosis: an FT-IR microspectroscopic investigation. Osteoporos Int. 2005;16:2031–2038.
Boskey AL, Mendelsohn R. Infrared spectroscopic characterization of mineralized tissues. Vib Spectrosc. 2005;38:107–114.
Boskey AL, Pleshko N, Doty SB, Mendelsohn R. Applications of FT-IR microscopy to the study of mineralization in bone and cartilage. Cell Materials. 1992;2:209–220.
Boskey AL, Spevak L, Weinstein RS. Spectroscopic markers of bone quality in alendronate-treated postmenopausal women. Osteoporos Int. 2009;20:793–800.
Boyce TM, Bloebaum RD. Cortical aging differences and fracture implications for the human femoral neck. Bone. 1993;14:769–778.
Bullough P. The tissue diagnosis of metabolic bone disease. Orthop Clin North Am. 1990;21:65–79.
Bullough P. Atlas of Orthopaedic Pathology. New York, NY: Gower Medical Publishing; 1992.
Burr DB, Miller L, Grynpas M, Li J, Boyde A, Mashiba T, Hirano T, Johnston CC. Tissue mineralization is increased following 1-year treatment with high doses of bisphosphonates in dogs. Bone. 2003;33:960–969.
Camacho NP, Rinnerthaler S, Paschalis EP, Mendelsohn R, Boskey AL, Fratzl P. Complementary information on bone ultrastructure from scanning small angle x-ray scattering and Fourier-transform infrared microspectroscopy. Bone. 1999;25:287–293.
Childs LM, Paschalis EP, Xing L, Dougall WC, Anderson D, Boskey AL, Puzas JE, Rosier RN, O’Keefe RJ, Boyce BF, Schwarz EM. In vivo RANK signaling blockade using the receptor activator of NF-kappaB:Fc effectively prevents and ameliorates wear debris-induced osteolysis via osteoclast depletion without inhibiting osteogenesis. J Bone Miner Res. 2002;17:192–199.
Cummings SR. Are patients with hip fractures more osteoporotic? Review of the evidence. Am J Med. 1985;78:487–494.
Dong A, Huang P, Caughey WS. Protein secondary structures in water from second-derivative amide I infrared spectra. Biochemistry. 1990;29:3303–3308.
Dumas P, Jamin N, Teillaud JL, Miller LM, Beccard B. Imaging capabilities of synchrotron infrared microspectroscopy. Faraday Discuss. 2004;126:289–302; discussion 303–311.
Durchschlag E, Paschalis EP, Zoehrer R, Roschger P, Fratzl P, Recker R, Phipps R, Klaushofer K. Bone material properties in trabecular bone from human iliac crest biopsies after 3- and 5-year treatment with risedronate. J Bone Miner Res. 2006;21:1581–1590.
Einhorn TA. The bone organ system: form and function. In: Marcus R, Feldman D, Kelsey J, eds. Osteoporosis. New York, NY: Academic Press Inc; 1996.
Federman S, Miller LM, Sagi I. Following matrix metalloproteinases activity near the cell boundary by infrared micro-spectroscopy. Matrix Biol. 2002;21:567–577.
Fratzl P, Gupta HS, Paschalis EP, Roschger P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem. 2004;14:2115–2123.
Fratzl P, Roschger P, Eschberger J, Abendroth B, Klaushofer K. Abnormal bone mineralization after fluoride treatment in osteoporosis: a small-angle x-ray-scattering study. J Bone Miner Res. 1994;9:1541–1549.
Fratzl P, Roschger P, Fratzl-Zelman N, Paschalis EP, Phipps R, Klaushofer K. Evidence that treatment with risedronate in women with postmenopausal osteoporosis affects bone mineralization and bone volume. Calcif Tissue Int. 2007;81:73–80.
Fratzl P, Schreiber S, Roschger P, Lafage MH, Rodan G, Klaushofer K. Effects of sodium fluoride and alendronate on the bone mineral in minipigs: a small-angle x-ray scattering and backscattered electron imaging study. J Bone Miner Res. 1996;11:248–253.
Gadaleta SJ, Paschalis EP, Betts F, Mendelsohn R, Boskey AL. Fourier transform infrared spectroscopy of the solution-mediated conversion of amorphous calcium phosphate to hydroxyapatite: new correlations between x-ray diffraction and infrared data. Calcif Tissue Int. 1996;58:9–16.
George A, Veis A. FTIRS in H2O demonstrates that collagen monomers undergo a conformational transition prior to thermal self-assembly in vitro. Biochemistry. 1991;30:2372–2377.
Gourion-Arsiquaud S, Allen MR, Burr DB, Vashishth D, Tang SY, Boskey AL. Bisphosphonate treatment modifies canine bone mineral and matrix properties and their heterogeneity. Bone. 2010;46:666–672.
Gourion-Arsiquaud S, Faibish D, Myers E, Spevak L, Compston J, Hodsman A, Shane E, Recker RR, Boskey ER, Boskey AL. Use of FTIR spectroscopic imaging to identify parameters associated with fragility fracture. J Bone Miner Res. 2009;24:1565–1571.
Gourion-Arsiquaud S, West PA, Boskey AL. Fourier transform-infrared microspectroscopy and microscopic imaging. Methods Mol Biol. 2008;455:293–303.
Henneman ZJ, Nancollas GH, Ebetino FH, Russell RG, Phipps RJ. Bisphosphonate binding affinity as assessed by inhibition of carbonated apatite dissolution in vitro. J Biomed Mater Res A. 2008;85:993–1000.
Huang RY, Miller LM, Carlson CS, Chance MR. Characterization of bone mineral composition in the proximal tibia of cynomolgus monkeys: effect of ovariectomy and nandrolone decanoate treatment. Bone. 2002;30:492–497.
Huang RY, Miller LM, Carlson CS, Chance MR. In situ chemistry of osteoporosis revealed by synchrotron infrared microspectroscopy. Bone. 2003;33:514–521.
Hui S, Slemenda CW, Johnston CC. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–1809.
Jepsen KJ, Schaffler MB. Bone mass does not adequately predict variations in bone fragility: a genetic approach. Trans Orthop Res Soc. 2001;26:114.
Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1142.
Kann P, Graeben S, Beyer J. Age-dependence of bone material quality shown by the measurement of frequency of resonance in the ulna. Calcif Tissue Int. 1994;54:96–100.
Kennedy DF, Crisma M, Toniolo C, Chapman D. Studies of peptides forming 3(10)- and alpha-helices and beta-bend ribbon structures in organic solution and in model biomembranes by Fourier transform infrared spectroscopy. Biochemistry. 1991;30:6541–6548.
Lazarev YA, Grishkovsky BA, Khromova TB. Amide I band spectrum and structure of collagen and related polypeptides. Biopolymers. 1985;24:1449–1478.
Lazarev YA, Grishkovsky BA, Khromova TB, Lazareva AV, Grechishko VS. Bound water in the collagen-like triple-helical structure. Biopolymers. 1992;32:189–195.
Lazarev YA, Lazareva AV, Shibnev A, Esipova NG. Infrared-spectra and structure of synthetic polytripeptides. Biopolymers. 1978;17:1197–1214.
Li C, Paris O, Siegel S, Roschger P, Paschalis E, Klaushofer K, Fratzl P. Strontium is incorporated into mineral crystals only in newly formed bone during strontium ranelate treatment. J Bone Miner Res. 2010;25:968–975.
Manolagas SC. Corticosteroids and fractures: a close encounter of the third cell kind. J Bone Miner Res. 2000;15:1001–1005.
Marcott C, Reeder RC, Paschalis EP, Tatakis DN, Boskey AL, Mendelsohn R. Infrared microspectroscopic imaging of biomineralized tissues using a mercury-cadmium-telluride focal-plane array detector. Cell Mol Biol (Noisy-le-grand). 1998;44:109–115.
Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259.
McCabe F, Zhou LJ, Steele CR, Marcus R. Noninvasive assessment of ulnar bending stiffness in women. J Bone Miner Res. 1991;6:53–59.
McCreadie RB, Goldstein AS. Biomechanics of fracture: is bone mineral density sufficient to assess risk? J Bone Miner Res. 2000;15:2305–2308.
Mendelsohn R, Hassankhani A, DiCarlo E, Boskey A. FT-IR microscopy of endochondral ossification at 20 mu spatial resolution. Calcif Tissue Int. 1989;44:20–24.
Miller LM, Carlson CS, Carr GL, Chance MR. A method for examining the chemical basis for bone disease: synchrotron infrared microspectroscopy. Cell Mol Biol (Noisy-le-grand). 1998;44:117–127.
Miller LM, Vairavamurthy V, Chance MR, Mendelsohn R, Paschalis EP, Betts F, Boskey AL. In situ analysis of mineral content and crystallinity in bone using infrared micro-spectroscopy of the nu(4) PO(4)(3−) vibration. Biochim Biophys Acta. 2001;1527:11–19.
Monier-Faugere MC, Geng Z, Paschalis EP, Qi Q, Arnala I, Bauss F, Boskey AL, Malluche HH. Intermittent and continuous administration of the bisphosphonate ibandronate in ovariohysterectomized beagle dogs: effects on bone morphometry and mineral properties. J Bone Miner Res. 1999;14:1768–1778.
Mosekilde L, Mosekilde L, Danielsen CC. Biomechanical competence of vertebral trabecular bone in relation to ash density and age in normal individuals. Bone. 1987;8:79–85.
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RG, Ebetino FH. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38:617–627.
Ouyang H, Sherman PJ, Paschalis EP, Boskey AL, Mendelsohn R. Fourier transform infrared microscopic imaging: effects of estrogen and estrogen deficiency on fracture healing in rat femurs. Appl Spectrosc. 2004;58:1–9.
Parfitt AM. Bone remodeling and bone loss: understanding the pathophysiology of osteoporosis. Clin Obs Gynecol. 1987;30:789–811.
Paschalis EP. Fourier transform infrared analysis and bone. Osteoporos Int. 2009;20:1043–1047.
Paschalis EP, Boskey AL, Kassem M, Eriksen EF. Effect of hormone replacement therapy on bone quality in early postmenopausal women. J Bone Miner Res. 2003;18:955–959.
Paschalis EP, Burr DB, Mendelsohn R, Hock JM, Boskey AL. Bone mineral and collagen quality in humeri of ovariectomized cynomolgus monkeys given rhPTH(1–34) for 18 months. J Bone Miner Res. 2003;18:769–775.
Paschalis EP, DiCarlo E, Betts F, Sherman P, Mendelsohn R, Boskey AL. FTIR microspectroscopic analysis of human osteonal bone. Calcif Tissue Int. 1996;59:480–487.
Paschalis EP, Glass EV, Donley DW, Eriksen EF. Bone mineral and collagen quality in iliac crest biopsies of patients given teriparatide: new results from the fracture prevention trial. J Clin Endocrinol Metab. 2005;90:4644–4649.
Paschalis EP, Recker R, DiCarlo E, Doty SB, Atti E, Boskey AL. Distribution of collagen cross-links in normal human trabecular bone. J Bone Miner Res. 2003;18:1942–1946.
Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL. Bone fragility and collagen cross-links. J Bone Miner Res. 2004;19:2000–2004.
Paschalis EP, Verdelis K, Doty SB, Boskey AL, Mendelsohn R, Yamauchi M. Spectroscopic characterization of collagen cross-links in bone. J Bone Miner Res. 2001;16:1821–1828.
Pleiner-Duxneuner J, Zwettler E, Paschalis E, Roschger P, Nell-Duxneuner V, Klaushofer K. Treatment of osteoporosis with parathyroid hormone and teriparatide. Calcif Tissue Int. 2009;84:159–170.
Posner AS. Bone mineral on the molecular level. Fed Proc. 1973;32:1933–1937.
Raisz LG, Kream BE. Regulation of bone formation. N Engl J Med. 1983;309:29–35.
Rey C, Collins B, Goehl T, Dickson IR, Glimcher MJ. The carbonate environment in bone mineral: a resolution-enhanced Fourier transform infrared spectroscopy study. Calcif Tissue Int. 1989;45:157–164.
Rey C, Miquel JL, Facchini L, Legrand AP, Glimcher MJ. Hydroxyl groups in bone mineral. Bone. 1995;16:583–586.
Rey C, Renugopalakrishnan V, Collins B, Glimcher MJ. Fourier transform infrared spectroscopic study of the carbonate ions in bone mineral during aging. Calcif Tissue Int. 1991;49:251–258.
Rey C, Shimizu M, Collins B, Glimcher MJ. Resolution-enhanced Fourier transform infrared spectroscopy study of the environment of phosphate ion in the early deposits of a solid phase of calcium phosphate in bone and enamel and their evolution with age. 2. Investigations in the nu3PO4 domain. Calcif Tissue Int. 1991;49:383–388.
Rinnerthaler S, Roschger P, Jakob HF, Nader A, Klaushofer K, Fratzl P. Scanning small angle x-ray scattering analysis of human bone sections. Calcif Tissue Int. 1999;64:422–429.
Roschger P, Fratzl P, Klaushofer K, Rodan G. Mineralization of cancellous bone after alendronate and sodium fluoride treatment: a quantitative backscattered electron imaging study on minipig ribs. Bone. 1997;20:393–397.
Roschger P, Manjubala I, Zoeger N, Meirer F, Simon R, Li C, Fratzl-Zelman N, Misof B, Paschalis E, Streli C, Fratzl P, Klaushofer K. Bone material quality in transiliac bone biopsies of postmenopausal osteoporotic women after 3 years strontium ranelate treatment. J Bone Miner Res. 2010;25:891–900.
Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42:456–466.
Termine JD, Lundy DR. Hydroxide and carbonate in rat bone mineral and its synthetic analogues. Calcif Tissue Res. 1973;13:73–82.
Termine JD, Posner AS. Infrared analysis of rat bone: age dependency of amorphous and crystalline mineral fractions. Science. 1966;153:1523–1525.
Thaler R, Spitzer S, Rumpler M, Fratzl-Zelman N, Klaushofer K, Paschalis EP, Varga F. Differential effects of homocysteine and beta aminopropionitrile on preosteoblastic MC3T3-E1 cells. Bone. 2010;46:703–709.
Weis MA, Wilkin DJ, Kim HJ, Wilcox WR, Lachman RS, Rimoin DL, Cohn DH, Eyre DR. Structurally abnormal type II collagen in a severe form of Kniest dysplasia caused by an exon 24 skipping mutation. J Biol Chem. 1998;273:4761–4768.
Author information
Authors and Affiliations
Corresponding author
Additional information
One or more of the authors (EPP) has received consultancies from Procter & Gamble Pharmaceuticals (Cincinnati, OH), Eli Lilly & Co (Indianapolis, IN), and Novartis AG (Basel, Switzerland) and is a member of the scientific board of Crescent Diagnostics Ltd (London, UK).
About this article
Cite this article
Paschalis, E.P., Mendelsohn, R. & Boskey, A.L. Infrared Assessment of Bone Quality: A Review. Clin Orthop Relat Res 469, 2170–2178 (2011). https://doi.org/10.1007/s11999-010-1751-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-010-1751-4