Abstract
Risedronate is used in osteoporosis treatment. Postmenopausal women enrolled in the Vertebral Efficacy with Risedronate Therapy trial received either risedronate (5 mg/day) or placebo for 3 years. Subjects received calcium and vitamin D supplementation if deficient at baseline. Lumbar spine bone mineral density (BMD) was measured at baseline and at 3 years. Quantitative back-scattered electron imaging (qBEI) was performed on paired iliac crest biopsies (risedronate, n = 18; placebo, n = 13) before and after treatment, and the mineral volume fraction in the trabecular bone was calculated. Combining dual-energy X-ray absorptiometric values with the mineral volume fraction for the same patients allowed us to calculate the relative change in trabecular bone volume with treatment. This showed that the effect on BMD was likely to be due partly to changes in matrix mineralization and partly due to changes in bone volume. After treatment, trabecular bone volume in the lumbar spine tended to increase in the risedronate group (+2.4%, nonsignificant) but there was a significant decrease (−3.7%, P < 0.05) in the placebo group. Calcium supplementation with adequate levels of vitamin D led to an ∼3.3% increase in mineral content in the bone material independently of risedronate treatment. This increase was larger in patients with lower matrix mineralization at baseline and likely resulted from correction of calcium/vitamin D deficiency as well as from reduced bone remodeling. Combining BMD and bone mineralization density distribution data show that in postmenopausal osteoporosis 3-year treatment with risedronate preserves or may increase trabecular bone volume, unlike placebo. This analysis also allows, for the first time, separation of the contributions of bone volume and matrix mineralization to the increase in BMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Osteoporosis is a skeletal disorder characterized by compromised bone strength and increased risk of fracture [1]. In postmenopausal osteoporosis, bone loss results from increased bone turnover associated with a negative bone balance (bone resorption exceeding bone formation). Antiresorptive agents are the predominant class of currently available osteoporosis therapeutic agents. Among them, the nitrogen-containing bisphosphonates alendronate and risedronate were shown to reduce the risk of both vertebral and nonvertebral fractures and are widely used in clinical practice [2]. The results from large epidemiological studies and clinical trials have shown that antiresorptive agents reduce fracture risk [3]. The magnitude of the reduction in fracture risk is suggested to be proportional more to the magnitude of reduction in markers of bone turnover [4–6] than to the net gain in bone mineral density (BMD). Indeed, while it is widely accepted that a reduced BMD is associated with an increased fracture risk, it has recently emerged that the degree of fracture risk reduction after antiresorptive therapy is poorly related to an increase in BMD [7–10]. One of the difficulties in interpreting BMD outcomes is that BMD measures the total amount of mineral in a volume of bone tissue and therefore combines two effects, the amount of mineral per bone volume and the amount of bone material per tissue volume. In addition, BMD measured by dual-energy X-ray absorptiometry (DXA) is influenced by bone geometry (or macroarchitecture) of the site measured [11].
At the material level, the biomechanical competence of the collagen/mineral composite is determined by properties of the two components (organic matrix and mineral) and by their structural organization [12]. Normal bone mineralization density distribution (BMDD) of trabecular bone can be defined [13] and used to identify states of hypomineralization, where bone becomes weak and may deform before actually breaking, or of hypermineralization, where bone becomes stiff and brittle [14]. BMDD reflects the amount of mineral within the matrix and can be measured by quantitative back-scattered electron imaging (qBEI) [13]. The amount of mineral depends on the age of various bone packets and is therefore distributed heterogeneously throughout the matrix due to the constant remodeling. As a consequence, qBEI does not give just a simple number reflecting the mean degree of matrix mineralization but a whole distribution of mineral contents (BMDD), reflecting the coexistence of bone packets with different tissue ages [15]. BMDDs can be measured routinely with high precision on transiliac biopsies and used for the quantitative assessment of the mineral content and the variability of mineral content distribution. It has been shown that BMDD of normal human trabecular bone is independent of anatomic location, age, sex, or racial origin [16].
While BMD reflects a combination of bone volume and mean matrix mineralization, BMDD gives direct information on the second quantity alone. Here, we show how BMD measurements can be combined with BMDD measurements on biopsies from the same patient to obtain information about changes in bone volume and in matrix mineralization separately. Our data are based on a combined analysis of BMD and BMDD results from individual women with postmenopausal osteoporosis after 3-year treatment with placebo or risedronate.
Methods
General Considerations Concerning Bone Density Measurements
The physical principle of a bone density measurement by the DXA method is shown schematically in Figure 1. An area (A) of the bone is irradiated with X-rays, and its attenuation through the thickness (H) of this bone is measured. Use of two different X-ray wavelengths allows separation of the absorption in organic tissue and absorption in mineral, giving a measure of the amount of mineral encountered by the beam. The total amount of mineral per unit area A is the areal BMD and is measured in grams per centimeters squared [17]. Knowing the thickness of bone, areal BMD can be converted into volumetric BMD from BMD/H. Volumetric BMD can also be determined directly by quantitative computed tomography (qCT) [18].
Schematic representation of BMD measurement using the DXA method. The bone area A is irradiated by X-rays, which are attenuated corresponding to the amount of mineral in the tissue volume (bone material + soft tissue). Subsequently, the attenuated X-ray intensities are measured by the X-ray detector
Calculation of the Volume Fraction of Bone Material
As mineral is encountered only within the bone material (Fig. 1) and not in the marrow space, we can write the volumetric bone density (BMD/H) as a product of three terms,
where ρM (= 3.18 g/cm3) is the density of plain mineral (carbonated hydroxyapatite), Φ is the matrix mineralization, and Ψ is the volume fraction of bone material within the whole volume of bone tissue. The quantity Ψ is the bone volume per tissue volume (BV/TV) in the context of bone morphometry. The quantity Φ can be determined by qBEI [13, 16, 19, 20] or microradiography [21, 22] and is related by a simple equation to the weight fraction of calcium in bone material, as reported in earlier work [23]. Both methods require biopsies and cannot be used for noninvasive clinical evaluation. Nevertheless, it has been shown that the mineral concentration as determined by qBEI is strongly conserved across skeletal sites and among normal individuals even of different geographical or ethnic origin [13]. This means that the matrix mineralization determined by qBEI in an iliac crest biopsy should be representative even for cancellous bone at the skeletal site where the BMD evaluation was performed in the same patient.
In clinical studies of osteoporosis treatments in which bone biopsies are collected, two of the three parameters in equation 1 can be measured directly. Hence, treatment-induced changes of bone material volume fraction (as measured by Ψ) and of mineral content in bone material (as measured by Φ) can be determined separately. One major problem in analyzing treatment effects on these parameters is the inherent variation of the bone volume fraction between different individuals. Hence, statistically significant treatment effects can only be expected either for very large patient groups or in paired evaluations within the same patients before and after treatment. For this purpose, the fundamental equation 1 can be rewritten in terms of treatment-induced changes within a given patient as follows:
where δ denotes the change from before to after treatment and where we assume there is no notable change in the bone thickness (this assumption would have to be reconsidered if growing children were to be evaluated, e.g.). In these notations, δBMD/BMD, δΦ/Φ, and δΨ/Ψ represent the relative changes in BMD, in mineral concentration (within the bone material), and in bone volume, respectively. As Φ is typically determined in cancellous bone from iliac crest biopsies, it is essential to use BMD values from sites with the largest contribution from cancellous bone, such as the lumbar spine. Treatment-induced changes in Ψ may then be estimated via equation 2, when paired biopsies are available.
Bone Samples
In the present work, we analyzed BMDD (Φ) and BMD data obtained from individual subjects in a previous study examining the effects of risedronate in postmenopausal osteoporosis [24, 25]. Paired biopsies were collected from postmenopausal women at baseline (pretreatment) and after 3-year treatment with placebo tablets (n = 13) or risedronate sodium (Actonel, 5 mg/day orally; n = 18). All 31 subjects also received calcium 1,000 mg/day, and those with low serum 25-hydroxyvitamin D levels at baseline (<40 nmol/L) received vitamin D (cholecalciferol) supplementation up to 500 IU/day. The paired biopsies were investigated by qBEI to determine the calcium content in the bone material (wt% Ca) [13, 16]. Lumbar spine BMD as well as histological and micro-CT data on the biopsies were available for each subject at baseline and after 3-year treatment [26, 27]. In the present study, the wt% Ca data were converted into Φ values as described above [23]. Relative changes in Φ and BMD were calculated from the published values and inserted in equation 2 to obtain the treatment-induced variation of the bone volume per tissue volume, Ψ, in the lumbar spine.
Statistical Analysis
Linear regression analysis, as well as paired t-tests and Wilcoxon’s signed rank tests were performed with the software package Sigmastat (Systat Software Inc., Richmond, CA).
Results
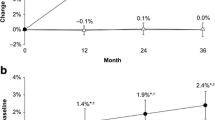
There were no significant differences between the two treatment groups in baseline demographics (Table 1). Baseline values for lumbar spine BMD and iliac crest bone biopsy parameters (matrix mineralization [Φ], mineralising surface per bone surface (MS/BS) from histomorphometry, and BV/TV determined by histomorphometry and by qCT) are given for all patients in Table 2, together with percent changes after 3 years of placebo or risedronate treatment. Mean values of the changes of BMD, matrix mineralization, and BV/TV are plotted in Figure 2 (as well as in Table 2). Several points are evident: BMD increased significantly with risedronate (about 8%) while remaining constant in the placebo group [24, 28]. In contrast, the mineral concentration in the matrix increased significantly in both risedronate- (5.0%) and placebo- (3.3%) treated subjects, as already reported [25] (see Fig. 2). From equation 2, the relative change of BV/TV is the difference between the relative changes in BMD and matrix mineralization. With this analysis, a small increase (about +2.4%, not significant) was observed for the vertebral bone volume (Ψ) with risedronate treatment. In the placebo treatment group, however, there was a significant decrease (−3.7%) in bone volume after 3 years, which when combined with the increase in matrix mineralization resulted in a net zero effect in BMD. The bone biopsy BV/TV values determined by histomorphometry and by qCT are also shown in Table 2. The changes measured by histomorphometry showed large scatter and were not significant. The changes measured by micro-CT were consistent with the changes calculated here but were also not significant (see Fig. 2 and Table 2).
Changes in BMD and matrix mineralization (min), as determined by DXA and qBEI, respectively, and in bone volume per tissue volume (BV/TV), as derived from histomorphometry (histo), from qCT and from equation 2 (Ψ). All data are shown for risedronate- (RIS) as well as placebo- (PLA) treated patients. Error bars indicate standard error of mean (18 and 13 patients for risedronate and placebo, respectively). Both values and error bars are expressed in percent of the mean value of the corresponding parameter at baseline. ***P < 0.001, **P < 0.01, *P < 0.05 give the significance at 3 years of treatment with respect to baseline (see Table 1)
These data suggest that the risedronate treatment effect on BMD results from a small increase in bone volume and a larger increase in mineral concentration. To test this hypothesis, we plotted the changes in BMD as a function of the changes in matrix mineralization in Figure 3. Though the linear regression analyses did not show a significant dependence between the two parameters, there is clearly a tendency for larger BMD increases at larger increases of matrix mineralization. In those specimens where data points are above the broken line in Figure 3, there should be a net increase in bone volume by tissue volume (according to equation 2); and for points below the line, there should be a net decrease. Generally speaking, most of the points for the controls are below the line, while the majority of the points for risedronate-treated patients are above.
Relationship between the relative changes (%) in BMD (y axis) and the relative changes in matrix mineralization (Φ) (x axis) during risedronate (full circles) and placebo (open triangles) treatments. Broken line shows a slope of 1, where the increase in BMD corresponds exactly to the increase in matrix mineralization
Clearly, bone volume is controlled by the remodeling process, which, although reduced in the placebo control group, still produced significant bone loss as estimated by equation 2 (about −3.7%, Fig. 2). This is reversed with even a slight increase in bone mass (+2.4%) with risedronate treatment. The increase in mineral concentration was 5% in the risedronate group and 3.3% in the placebo group (Fig. 2 and Table 2). This suggests that in these subjects the increase in mineral concentration was dependent on calcium and vitamin D intake as well as on the reduction of the remodeling rate. The greater reduction in remodeling with risedronate did appear to produce an additional increase in mineralization, but a larger contribution might have been expected. It is clear that increased remodeling activity, e.g., as induced by parathyroid hormone treatment [29, 30], leads to prevalence of younger, less mineralized bone packets [15]. Hence, increased remodeling activity in combination with anabolic effects reduces the average mineral concentration in the matrix.
One possible explanation is that the bone matrix in these subjects had a low matrix mineralization at baseline, due possibly to low dietary calcium/vitamin D intake or to high turnover rates at baseline. This calcium/vitamin D deficiency was then corrected by the supplementation during the study. If true, the increase in matrix mineralization should be inversely related to the mineralization at baseline. Correlation analysis shows this to be the case (Fig. 4b). Most interestingly, there was also a strong correlation of matrix mineralization at baseline with turnover rates (MS/BS) at baseline (Fig. 4a), indicating that the low calcium content in the bone matrix at baseline could be the result of enhanced turnover after menopause. In fact, the turnover rate decreased significantly for both treatment groups (see Table 2). The treatment-induced increase in mineral concentration was larger the lower the mineral concentration at baseline. The correlation was significant in both the risedronate treatment group (P = 0.005) and the placebo control group (P = 0.022). The dependence is stronger for risedronate treatment, and this difference is most likely the effect of the reduction in remodeling (which was greater with risedronate) on mineral concentration.
Remodeling rate MS/BS at baseline (upper panel) and treatment-induced increase of matrix mineralization (lower panel) as a function of matrix mineralization (Φ) at baseline. Solid and broken lines show linear regressions with significance of correlation. Separate regressions for risedronate (RIS, full circles) and placebo (PLA, open triangles) are shown in the lower panel. Upper panel shows a regression without distinguishing risedronate and placebo
Discussion
At present it is unclear whether antiresorptive treatments have a beneficial effect on bone volume. Histomorphometry on transiliac bone biopsies typically fails to show an effect on bone volume despite significant treatment-related increases in spine and/or hip BMD [28, 31]. The difficulty in interpreting changes in BMD arises from the fact that the measured density values represent bone volume as well as mineralization density of the bone matrix (BMDD). In the present study, we used a combination of both BMDD (measured by qBEI) and BMD (measured by DXA) data from individual patients. This allowed us to separate effects of treatment on bone volume and matrix mineralization (i.e., allowed separation of increases in bone volume and matrix mineralization; equation 2). The underlying assumptions are (1) that bone tissue mineralization in cancellous bone is roughly site-independent and (2) that BMD from the lumbar spine is dominated by cancellous bone. The first assumption is strongly supported by previous comparative qBEI investigations [16]. The second assumption is somewhat more problematic as several studies [32, 33] have demonstrated that changes in spine areal BMD by DXA after alendronate or teriparatide treatment differ from qCT-based assessments of trabecular volumetric BMD. However, since qCT-based assessments of trabecular volumetric BMD are not available in the clinical routine, we believe that areal BMD from DXA is the best available approximation for our purpose.
The determination of BV/TV in iliac crest biopsies (Table 2) did not show statistically significant changes, probably because of the well-known intraindividual variability [34] and the fact that only a comparatively small tissue volume is investigated compared to BMD measurements on the lumbar spine. Moreover, the iliac crest does not experience the same type of mechanical stimulus as the lumbar spine, where osteoporotic fractures are likely to occur.
The data used in the present study, corrected for changes in matrix mineralization, show that bone volume remained nearly constant in the risedronate-treated group (+2.4%, nonsignificant), while there was a significant loss (−3,7%, P < 0.05) in the placebo group. The latter result is consistent with a recent study of iliac crest biopsies by micro-CT [26]. Furthermore, our analyses of matrix mineralization showed that patients who had low matrix mineralization at baseline had large increases in matrix mineralization density due to calcium/vitamin D supplementation. The increase was greater the more severe the hypomineralization was at baseline, reaching a plateau of normal mineralization values [25].
These results underline the importance of the maintenance of adequate levels of calcium and vitamin D for proper bone mineralization and support their role as a vital component of disease management for the prevention and treatment of osteoporosis. Data analyses of the present study suggest an interesting “decoupling” between the effects of calcium/vitamin D supplementation, on the one hand, and of risedronate, on the other. A > 3% increase in matrix mineralization was seen in the placebo-treated group, suggesting that the calcium/vitamin D supplementation increased matrix mineralization (without affecting BV/TV) irrespective of the bisphosphonate treatment. This likely resulted from correction of calcium deficiency as well as reduced bone remodeling even in the placebo group.
It is well documented that both mineral concentration and bone volume fraction have a profound effect on the mechanical properties of bone. The stiffness of bone material increases in a nonlinear way with matrix mineralization [14, 35, 36]. In addition, the stiffness of a highly porous material, such as cancellous bone, increases in a nonlinear, typically quadratic fashion with the bone volume per tissue volume [37]. Thus, increases in either or both parameters can contribute to increased stiffness. The situation is less clear concerning the influence of these parameters on fracture incidence. In particular, the mineral concentration is believed to have an optimal value, which compromises between brittleness at high mineral content and low stiffness at low mineral content [38]. Moreover, it is very likely that the quality of the organic matrix plays an essential role in bone strength and fracture risk [12]. A systematic analysis of the two independent parameters, matrix mineralization and bone volume per tissue volume, determined by a combination of DXA measurements and qBEI analysis of biopsies would provide more clarity and a better understanding of fracture risk and the origin of fracture. For example, treatment with the bisphosphonates risedronate and alendronate results in a reduction in heterogeneity of bone matrix mineralization associated with prolonged secondary mineralization [25, 39, 40]. It is clear that other techniques are needed to explore the changes in bone material qualities that contribute to bone strength and fracture risk but which cannot be determined by BMD [10].
In conclusion, the combined analysis of BMD (from DXA) and BMDD (from qBEI of paired bone biopsies) allows separation of changes in bone volume and in bone mineralization. The effect of 3-year treatment with risedronate in a group of postmenopausal osteoporosis subjects was analyzed in this way, and the following was found:
-
1.
Treatment with risedronate influenced bone volume compared to untreated controls. The analysis presented here suggests that it maintained or even produced a moderate increase in bone volume compared to a 3.7% loss of bone volume in the placebo group over 3 years.
-
2.
Calcium/vitamin D supplementation led to a significant increase in mineralization in both the placebo and risedronate groups.
-
3.
The increase in the degree of bone mineralization was larger the greater the degree of undermineralization at baseline, which also correlated with the turnover at baseline. This suggests that available mineralization sites in the bone matrix are filled with mineral when there is increased calcium intake in association with adequate levels of vitamin D and/or due to a reduction of bone turnover.
-
4.
The increase in mineralization was greater with risedronate treatment, probably due to the greater reduction in bone turnover.
The combination of DXA measurements and qBEI data from bone biopsies has provided new insights into the effect of anticatabolic treatment on bone by allowing for the first time a separation between the effects on bone volume and on matrix mineralization. Lumbar spine BMD values after risedronate therapy in women with postmenopausal osteoporosis show an overlap of effects on bone volume and matrix mineralization. Indeed, using paired biopsies from placebo- and risedronate-treated subjects, we have shown that up to two-thirds of the observed BMD increase from baseline was due to calcium deposition in the bone material as a result of calcium supplementation with adequate vitamin D, and the remainder of the increase in BMD resulted from an increase or better maintenance of bone volume due to risedronate therapy. Future studies are required to determine the impact of these findings on bone quality and the assessment of individual fracture risk.
References
NIH (2001) NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7–29, 2000. Highlights of the conference. South Med J 94:569–573
McClung M (2003) Use of highly potent bisphosphonates in the treatment of osteoporosis. Curr Osteoporos Rep 1:116–122
Epstein S (2005) The roles of bone mineral density, bone turnover, and other properties in reducing fracture risk during antiresorptive therapy. Mayo Clin Proc 80:379–388
Bjarnason NH, Sarkar S, Duong T, Mitlak B, Delmas PD, Christiansen C (2001) Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int 12:922–930
Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD (2003) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 18:1051–1056
Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, Thompson DE, Ewing SK, Delmas PD (2004) Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res 19:1250–1258
Delmas PD, Seeman E (2004) Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with anti-resorptive therapy. Bone 34:599–604
Watts NB, Cooper C, Lindsay R, Eastell R, Manhart MD, Barton IP, van Staa TP, Adachi JD (2004) Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom 7:255–261
Miller PD, Hochberg MC, Wehren LE, Ross PD, Wasnich RD (2005) How useful are measures of BMD and bone turnover? Curr Med Res Opin 21:545–554
Watts NB, Geusens P, Barton IP, Felsenberg D (2005) Relationship between changes in BMD and nonvertebral fracture incidence associated with risedronate: reduction in risk of nonvertebral fracture is not related to change in BMD. J Bone Miner Res 20:2097–2104
Miller PD, McClung M (1996) Prediction of fracture risk. I: Bone density. Am J Med Sci 312:257–259
Fratzl P, Gupta H, Paschalis E, Roschger P (2004) Structure and mechanical quality of the collagen-mineral nano-composite in bone. J Mater Chem 14:2115–2123
Roschger P, Fratzl P, Eschberger J, Klaushofer K (1998) Validation of quantitative backscattered electron imaging for the measurement of mineral density distribution in human bone biopsies. Bone 23:319–326
Currey JD (2002) Bones - Structure and Mechanics. Princeton University Press, Princeton
Misof BM, Roschger P, Cosman F, Kurland ES, Tesch W, Messmer P, Dempster DW, Nieves J, Shane E, Fratzl P, Klaushofer K, Bilezikian J, Lindsay R (2003) Effects of intermittent parathyroid hormone administration on bone mineralization density in iliac crest biopsies from patients with osteoporosis: a paired study before and after treatment. J Clin Endocrinol Metab 88:1150–1156
Roschger P, Gupta HS, Berzlanovich A, Ittner G, Dempster DW, Fratzl P, Cosman F, Parisien M, Lindsay R, Nieves JW, Klaushofer K (2003) Constant mineralization density distribution in cancellous human bone. Bone 32:316–323
Kanis JA (2000) An update on the diagnosis of osteoporosis. Curr Rheumatol Rep 2:62–66
Cann CE (1988) Quantitative CT for determination of bone mineral density: a review. Radiology 166:509–522
Boyde A, Maconnachie E, Reid SA, Delling G, Mundy GR (1986) Scanning electron microscopy in bone pathology: review of methods, potential and applications. Scanning Electron Microsc Pt 4:1537–1554
Roschger P, Plenk H Jr, Klaushofer K, Eschberger J (1995) A new scanning electron microscopy approach to the quantification of bone mineral distribution: backscattered electron image grey-levels correlated to calcium K alpha-line intensities. Scanning Microsc 9:75–88
Eschberger J, Eschberger D (1986) Microradiography. In: Von Recum AF (ed) Handbook of biomaterials evaluation. Macmillan, New York, pp 461–500
Boivin G, Meunier PJ (2002) The degree of mineralization of bone tissue measured by computerized quantitative contact microradiography. Calcif Tissue Int 70:503–511
Zizak I, Roschger P, Paris O, Misof BM, Berzlanovich A, Bernstorff S, Amenitsch H, Klaushofer K, Fratzl P (2003) Characteristics of mineral particles in the human bone/cartilage interface. J Struct Biol 141:208–217
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
Zoehrer R, Roschger P, Paschalis EP, Hofstaetter JG, Durchschlag E, Fratzl P, Phipps R, Klaushofer K (2006) Effects of 3- and 5-year treatment with risedronate on bone mineralization density distribution in triple biopsies of the iliac crest in postmenopausal women. J Bone Miner Res 21:1106–1112
Borah B, Dufresne TE, Ritman EL, Jorgensen SM, Liu S, Chmielewski PA, Phipps RJ, Zhou X, Sibonga JD, Turner RT (2006) Long-term risedronate treatment normalizes mineralization and continues to preserve trabecular architecture: sequential triple biopsy studies with micro-computed tomography. Bone 39:345–352
Eriksen EF, Melsen F, Sod E, Barton I, Chines A (2002) Effects of long-term risedronate on bone quality and bone turnover in women with postmenopausal osteoporosis. Bone 31:620–625
Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY (2000) Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN Study Group. J Clin Endocrinol Metab 85:1895–1900
Qin L, Raggatt LJ, Partridge NC (2004) Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab 15:60–65
Whitfield JF, Morley P, Willick GE (2002) Parathyroid hormone, its fragments and their analogs for the treatment of osteoporosis. Treat Endocrinol 1:175–190
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11:83–91
Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215
McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF (2005) Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165:1762–1768
Parisien MV, McMahon D, Pushparaj N, Dempster DW (1988) Trabecular architecture in iliac crest bone biopsies: infra-individual variability in structural parameters and changes with age. Bone 9:289–295
Jager I, Fratzl P (2000) Mineralized collagen fibrils: a mechanical model with a staggered arrangement of mineral particles. Biophys J 79:1737–1746
Gao H, Ji B, Jager IL, Arzt E, Fratzl P (2003) Materials become insensitive to flaws at nanoscale: lessons from nature. Proc Natl Acad Sci USA 100:5597–5600
Gibson LA, MF. (1999) Cellular Solids, Structure and Properties. Cambridge University Press, Cambridge
Currey JD (1999) The design of mineralised hard tissues for their mechanical functions. J Exp Biol 202:3285–3294
Roschger P, Fratzl P, Klaushofer K, Rodan G (1997) Mineralization of cancellous bone after alendronate and sodium fluoride treatment: a quantitative backscattered electron imaging study on minipig ribs. Bone 20:393–397
Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaushofer K (2001) Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone 29:185–191
Acknowledgement
We thank Gerda Dinst, Sabrina Thon, and Daniela Gabriel (Bone Material Laboratory of the Ludwig Boltzmann Institute of Osteology, Vienna, Austria) for the sample preparation. This work was supported in part by the FWF—Fonds zur Förderung der wissenschaftlichen Forschung (Austrian Science Fund) (grant P16880-B13) and by a research grant from The Alliance for Better Bone Health. Support was also received from the Austrian Social Insurance for Occupational Risk and the Social Health Insurance Vienna.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fratzl, P., Roschger, P., Fratzl-Zelman, N. et al. Evidence that Treatment with Risedronate in Women with Postmenopausal Osteoporosis Affects Bone Mineralization and Bone Volume. Calcif Tissue Int 81, 73–80 (2007). https://doi.org/10.1007/s00223-007-9039-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-007-9039-8