Abstract
Background
Valgus high tibial osteotomy (HTO) has been recommended for ligament stability and enhanced function after anterior cruciate ligament (ACL) reconstruction in varus-angulated knees. However, it is not clear whether HTO should be performed in patients undergoing ACL reconstruction who have primary varus knees without medial compartment arthrosis.
Questions/purposes
We therefore asked whether stability and function differed in patients having ACL reconstruction with differing degrees of preoperative alignment.
Patients and Methods
We retrospectively reviewed 201 patients who had primary, single-bundle ACL reconstructions with primary varus knees based on the preoperative mechanical axis deviation (MAD) on preoperative standing hip-knee-ankle radiographs. Patients were categorized into four groups according to the MAD: Group 1: 0 mm to 4 mm, Group 2: 5 mm to 9 mm, Group 3: 10 mm to 14 mm, and Group 4: greater than 15 mm. A total of 201 patients, 67 in Group 1, 53 in Group 2, 38 in Group 3, and 43 in Group 4, were assessed. Ligament stability was determined with the Lachman test, pivot shift test, and KT 2000™ arthrometer. Functional scores were assessed using the Lysholm score and the International Knee Documentation Committee (IKDC) score. The minimum followup was 24 months (mean, 45 months; range, 24–96 months).
Results
We observed no differences in the side-to-side KT 2000™ measurements, Lysholm score, or IKDC functional scores based on the preoperative MAD.
Conclusions
The stability and functional scores after ACL reconstruction were not adversely altered by primary varus alignment. Thus, if there is no medial compartment arthritis or varus thrust, we do not believe a correctional tibial osteotomy is crucial in primary varus knees undergoing ACL reconstruction.
Level of Evidence
Level IV, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Valgus HTO corrects varus alignment, reportedly offloads the medial compartment of the knee [6, 8], and is an established surgical option for treating medial compartment arthritis of varus-angulated knees [1, 13, 23, 25]. The role of HTO has expanded to the treatment of ACL-deficient varus-angulated knees [5, 14, 17, 18, 21, 24]. Noyes et al. classified varus alignment of the knee into three categories: primary, double, and triple varus [19]. Primary varus refers to the overall tibiofemoral varus osseous alignment, including varus alignment secondary to the loss of medial meniscus and articular cartilage. Double varus refers to the tibiofemoral varus osseous alignment and separation of the lateral tibiofemoral compartment owing to a deficiency of lateral soft tissues. Triple varus is attributable to the combination of primary varus, double varus, and increased external rotation and hyperextension caused by posterolateral instability. A high percentage of patients with varus knees and ACL deficiency have a high adduction moment during walking [20]. In the early stance phase after heel-strike, a varus thrust can occur owing to the adduction moment [4]. The varus thrust can increase tension in the ACL graft and lead to failure of the graft. Correction of varus alignment, along with ACL reconstruction, is stressed in a double or triple varus knee [2, 19]. One study showed the mean adduction moment, 35% greater than that of control subjects preoperatively, decreased to less than normal values after HTO with ACL reconstruction [19]. Whether this reduction alters the long-term natural history is not known. It is also unclear whether HTO should be performed in patients undergoing ACL reconstruction who have primary varus knees without medial compartment arthrosis. The decision regarding whether to recommend HTO is clinically important, because the surgery adds risk, rehabilitation would be delayed to permit union of the osteotomized site, and long-term benefits are unknown. One cadaveric study showed that varus alignment in an ACL-deficient knee does not necessarily lead to varus thrust and the authors concluded such knees do not always need HTO [26].
We therefore asked whether stability and function differed in patients having ACL reconstruction with differing degrees of preoperative alignment.
Patients and Methods
We retrospectively reviewed the records of 344 patients who underwent ACL reconstruction between 2000 and 2006. For this study we included patients with: (1) unilateral primary ACL reconstruction, (2) single-bundle ACL reconstruction with autogenous bone-patellar tendon-bone (BPTB) graft or autogenous quadriceps tendon-bone (QTB) graft, and (3) followup greater than 24 months postoperatively. We excluded 143 patients for the following reasons: (1) 31 had ACL insufficiencies combined with any medial instability or varus thrust in lateral or posterolateral instability, (2) eight had ACL avulsion fractures, (3) 25 had medial compartment articular cartilage erosion greater than Grade II according to the Outerbridge classification [22] at the time of surgery, (4) 32 had subtotal or total meniscectomies that resulted in the loss of hoop tension, (5) 12 had knees with valgus angulation, and (6) 35 had generalized joint laxity. Preoperatively, the concomitant instability was evaluated by physical examination in combination with radiologic study. MR images were closely reviewed to detect multiligament injuries. If injuries of medial or lateral ligamentous structures were suggestive, valgus or varus stress radiographs were obtained. The posterolateral instability was evaluated by measuring the thigh-foot angle and identifying the varus thrust. The Beighton and Horan criteria [3], which have gained international acceptance [9], were used to assess the joint laxity. The exclusions left 201 patients (154 males and 47 females). Single-bundle ACL reconstruction with BPTB was performed in 105 patients, whereas single-bundle ACL reconstruction with QTB was performed in 96 patients. Fourteen patients (7%) underwent partial medial meniscectomies before the index surgery. The demographics among the groups are provided (Table 1). The mean age of the patients was 28 years (range, 16–53 years) and the mean duration of symptoms was 14 months. The minimum followup was 24 months (average, 45 months; range, 24–96 months). No patients were lost to followup, and none were recalled specifically for this study; all data were obtained from medical records and radiographs.
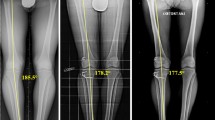
Preoperatively, the standing hip-knee-ankle radiographs were taken and MAD was measured (Fig. 1). To stratify varus alignment, patients were categorized into four groups according to their MAD: Group 1 had a MAD of 0 mm to 4 mm, Group 2 had a MAD of 5 mm to 9 mm, Group 3 had a MAD of 10 mm to 14 mm, and Group 4 had a MAD of 15 mm or greater. The mean MAD was 2.1 mm in Group 1 (67 patients), 7.7 mm in Group 2 (53 patients), 12.7 mm in Group 3 (38 patients), and 25.5 mm in Group 4 (43 patients). Preoperatively, in addition to the mean MAD, there were no differences in the demographics or use of grafts in the groups.
The illustration shows the mechanical axis as the line passing from the center of the hip to the center of the ankle. The mechanical axis deviation (MAD) is the perpendicular segment, measured in millimeters, extending from the axis to the center of the knee. The patients were categorized into four groups according to their MAD.
All the patients were operated on by the senior author (SJK). To avoid graft length mismatch, a BPTB graft was selected if the length of the patellar tendon was less than 4 cm on the MR image. Otherwise, a QTB graft was used. The BPTB graft was harvested with a width of 10 mm. The patellar and tibial bone blocks were trapezoidal, 25 mm long and 8 mm deep. A tibial tunnel was drilled with a 10-mm cannulated reamer. A femoral guide pin was positioned at the 10:30 o’clock position on the right knee or the 1:30 o’clock position on the left knee with the knee flexed 70° to 90°, and the femoral socket was reamed to a depth of 30 mm. The previously prepared BPTB graft was passed through the tibial tunnel, across the joint, and into the femoral socket. After fixation of the femoral bone plug, the graft was pretensioned by pulling it tightly and moving the knee through full ROM 10 times. The graft then was fixed in the tibial tunnel with a bioabsorbable interference screw at 10° to 15° knee flexion. For the QTB graft, a 7-cm long longitudinal midline incision was placed on the proximal site from 1/3 of the patella, extending proximally to provide adequate exposure. The central quadriceps tendon was harvested in a segment 10 mm in width, 6 mm to 7 mm in thickness, and 55 mm in length. The patellar bone block was 10 mm in width, 8 mm in depth, and 25 mm in length. The cut surface of the quadriceps tendon was closed with a Number 2 Ethibond suture (Ethicon, Inc, Somerville, NJ, USA). A 30-mm long suturing was performed on the proximal quadriceps tendon using baseball stitches. A 9-mm diameter Endopearl device (Linvatec, Largo, FL, USA) was fixed to the quadriceps tendon with Number 2 Ethibond sutures. Tibial and femoral tunnels were made in the same manner in BPTB grafts. The prepared graft was passed through the tibial tunnel, across the joint, and into the femoral socket. The quadriceps tendon, with attached Endopearl device, was secured in the femoral socket using an absorbable interference screw. The QTB graft was pretensioned by pulling the graft tightly and moving the knee through full ROM 10 times. The patellar bone plug was secured in the tibial tunnel by an absorbable interference screw at 10° to 15° knee flexion.
All patients followed the same rehabilitation protocol. Full ROM and tolerable weightbearing using crutches were permitted immediately after surgery. Patients were allowed to bear full weight approximately 4 weeks after surgery. By 12 weeks, jogging, swimming, and cycling were permitted. Participation in sports involving jumping, pivoting, or sidestepping was allowed 6 months after surgery.
Postoperatively, patients had regular followups in the outpatient clinic at 2 weeks, 3 months, 6 months, and annually thereafter. Standing anterior to posterior radiographs of both knees were taken annually. Clinical outcomes were assessed before surgery and at the latest followup. Ligament stability was examined with the Lachman test [11, 27], pivot shift test, and the side-to-side difference of anterior translation, measured with a KT 2000™ arthrometer (SSD) (MEDmetric Corp, San Diego, CA, USA) at 30° knee flexion. The Lachman test was graded as 0 (< 3 mm), 1+ (3 mm–5 mm), 2+ (6 mm–10 mm), or 3+ (> 10 mm). The pivot shift phenomenon was graded as 0 (absent), 1+ (subluxation), 2+ (jump), or 3+ (transient locking). Manual examinations were performed by the senior author. Functional scores were assessed using the Lysholm score [15] and the IKDC score [7].
Data were normally distributed and the mean and SD of the SSD and Lysholm score were determined for each group. The differences in the Lachman and pivot shift tests were analyzed using the chi square test. ANOVA was used to analyze the differences in the SSD and Lysholm score. The differences in IKDC scores were analyzed using the chi square test. Statistical analysis was performed using SPSS software (Version 13.0, SPSS Inc, Chicago, IL, USA).
Results
We observed no differences in postoperative knee stability and functional score among the groups with differing varus alignment. At final followup, no differences were found between the groups regarding the percentage of patients in each grade by the Lachman and pivot shift tests with a statistical power of 99% (Table 2). The mean SSD was similar (p = 0.314) in the groups, with a statistical power of 32%: 2.02 mm in Group 1, 2.19 mm in Group 2, 1.58 mm in Group 3, and 2.02 mm in Group 4 (Table 3). The mean Lysholm score was similar (p = 0.511) in the groups with a statistical power of 21%: 92.1 in Group 1, 92.1 in Group 2, 93.9 in Group 3, and 93.2 in Group 4. We found similar (p = 0.569) percentages of Grade A or Grade B IKDC scores in the groups, with a statistical power of 99%: 89.5% in Group 1, 81.1% in Group 2, 89.4% in Group 3, and 86.0% in Group 4. Among the patients who received BPTB grafts, we observed no difference (p = 0.638) in the groups regarding the SSD. Likewise, there were no differences (p = 0.220) in the groups regarding the SSD for patients who received QTB grafts. The QTB grafts provided good stability, comparable to the BPTB grafts in each group.
Discussion
Two clear indications are identified in the literature for the HTO in varus-aligned ACL-deficient knees. One is varus thrust in double or triple varus knees [2, 17, 19], and the other is medial compartment osteoarthritis (OA) in primary varus knees [10, 14, 21, 24]. However, it is unclear whether HTO should be performed in patients undergoing ACL reconstruction who have primary varus knees without medial compartment arthrosis. We therefore asked whether stability and function differed in patients having ACL reconstruction with differing degrees of preoperative alignment.
Our study has some several limitations. First, the followup, with a minimum of 24 months and mean of 45 months, is relatively short. The LCL can become insufficient with time with chronic varus alignment and varus thrust consequently can develop [2]. Although no patients had a clinically apparent varus thrust postoperatively, our short-term findings do not ensure the long-term integrity of the LCL. Additionally, although we observed no patients with substantial arthritis, we cannot ensure these patients will not have progressive medial compartment arthritis develop in the long-term. Stein et al. indicated that patients with ACL insufficiency and malalignment are at risk for the development of early arthritis, and both problems need to be addressed to alter the natural history of progressive OA [24]. However, in their series, all patients had medial compartment arthritis at the time of surgery. The potential risk for progression to medial compartment arthritis is still unknown in the ACL-reconstructed physiologic varus knees with intact hoop tension of the medial meniscus. It was beyond the scope of our study to determine the need for HTO to prevent medial compartment arthritis in this subset of patients. Second, varus alignment of patients included in this study was not over the weightbearing line (WBL) at the medial edge of the tibial plateau (WBL at 100%). Therefore, the conclusion cannot be applicable to severe primary varus-aligned ACL deficiency over the WBL at 100%. Third, two kinds of grafts were used in this study. Kim et al. previously reported that QTB grafts attached with the Endopearl device provided knee stability comparable to the BPTB, but with less kneeling pain [12]. Likewise, there were no differences in outcome variables between the grafts in our current series. Fourth, valgus alignment was not included in our current study. The question remains whether patients with valgus alignment would have had better or worse results. We think more definitive conclusions could have been drawn if patients with valgus alignment had been included in the study. Fifth, the analyses for the SSD and Lysholm score were underpowered. However, even in the case of a Type II error, the SSD and Lysholm score in Group 4 are better than those of Group 1 or 2. Therefore, we believe the conclusion of the current study is supported rather than reversed.
We divided patients according to preoperative MAD and compared the outcome variables. Group 4, with a mean MAD of 25.5 mm, was presumed to represent the primary varus-angulated knees. We observed no differences in terms of postoperative knee stability and functional scores among the groups. These observations suggest that if there is no medial compartment arthrosis, the HTO may not be needed in ACL-deficient primary varus knees. Increasing degrees of varus alignment up to the WBL at 100% does not appear to compromise the results of ACL reconstruction in primary varus knees. van de Pol et al. [26] conducted a cadaveric study in which the strain in the ACL and the lateral joint opening was measured under axial compressive limb loads with three different WBLs. The mechanical axis was set to pass the center of the knee (WBL at 0%), halfway between the medial tibial plateau (WBL at 50%), and WBL at 100%. An obvious varus thrust and substantial increase of lateral joint opening after ACL release were observed only in the WBL at 100%. van de Pol et al. suggested a slight varus alignment did not substantially increase ACL tensions [26]. This is consistent with our result in that every varus-aligned knee does not need HTO if there is no varus thrust. The results of our study were not reversed when separating patients according to the WBL percentage: 173 patients (86%) between a WBL at 0% and a WBL at 50%, versus 28 patients (14%) between a WBL at 50% and a WBL at 100%. However, in clinical situations, varus knees with a WBL less than 50% can have varus thrust develop when chronic ACL deficiency is present. ACL reconstructions in knees with a varus thrust fail if varus alignment is not corrected [16, 20]. Kean et al. [10] reported the peak adduction moment decreased 1 year after simultaneous HTO and ACL reconstruction. Dejour et al. [5] reported the results of simultaneous HTO and ACL reconstruction in 43 patients with chronic anterior instability combined with acquired varus alignment. In their series, 74% of patients had lateral joint opening and 43% had medial chondral lesions. We excluded patients with medial compartment arthritis and 14 patients (7%) had undergone medial meniscectomy before index surgery. Moreover, only two patients in Group 4 previously underwent partial medial meniscectomy. This proportion of a previous meniscectomy is much lower than in previous studies [14, 17–19, 21, 24], in which medial meniscus deficiencies ranged from 73% to 100% and frequently led to medial compartment arthritis in conjunction with chronic anterior instability. Thus, we suspect the varus in our patients reflected a physiologic varus caused by tibiofemoral osseous malalignment.
ACL reconstruction alone in primary varus knees without medial compartment arthritis predicted good anterior stability and functional and radiologic outcomes. Therefore, if there is no medial compartment arthritis, ACL reconstruction without HTO does not preclude maintenance of stability and high functional scores during short- to midterm followup. Longer-term followup is needed to ensure stability and function are maintained and determine whether late OA develops more frequently with greater preoperative malalignment.
References
Akizuki S, Shibakawa A, Takizawa T, Yamazaki I, Horiuchi H. The long-term outcome of high tibial osteotomy: a ten- to 20-year follow-up. J Bone Joint Surg Br. 2008;90:592–596.
Badhe NP, Forster IW. High tibial osteotomy in knee instability: the rationale of treatment and early results. Knee Surg Sports Traumatol Arthrosc. 2002;10:38–43.
Beighton P, Horan F. Orthopaedic aspects of the Ehlers-Danlos syndrome. J Bone Joint Surg Br. 1969;51:444–453.
Bulgheroni P, Bulgheroni MV, Andrini L, Guffanti P, Giughello A. Gait patterns after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1997;5:14–21.
Dejour H, Neyret P, Boileau P, Donell ST. Anterior cruciate reconstruction combined with valgus tibial osteotomy. Clin Orthop Relat Res. 1994;299:220–228.
Dugdale TW, Noyes FR, Styer D. Preoperative planning for high tibial osteotomy: the effect of lateral tibiofemoral separation and tibiofemoral length. Clin Orthop Relat Res. 1992;274:248–264.
Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1:226–234.
Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis: a long-term follow-up study. J Bone Joint Surg Am. 1984;66:1040–1048.
Juul-Kristensen B, Rogind H, Jensen DV, Remvig L. Inter-examiner reproducibility of tests and criteria for generalized joint hypermobility and benign joint hypermobility syndrome. Rheumatology (Oxford). 2007;46:1835–1841.
Kean CO, Birmingham TB, Garland JS, Jenkyn TR, Ivanova TD, Jones IC, Giffin RJ. Moments and muscle activity after high tibial osteotomy and anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 2009;41:612–619.
Kim SJ, Kim HK. Reliability of the anterior drawer test, the pivot shift test, and the Lachman test. Clin Orthop Relat Res. 1995;317:237–242.
Kim SJ, Kumar P, Oh KS. Anterior cruciate ligament reconstruction: autogenous quadriceps tendon-bone compared with bone-patellar tendon-bone grafts at 2-year follow-up. Arthroscopy. 2009;25:137–144.
Koshino T, Yoshida T, Ara Y, Saito I, Saito T. Fifteen to twenty-eight years’ follow-up results of high tibial valgus osteotomy for osteoarthritic knee. Knee. 2004;11:439–444.
Lattermann C, Jakob RP. High tibial osteotomy alone or combined with ligament reconstruction in anterior cruciate ligament-deficient knees. Knee Surg Sports Traumatol Arthrosc. 1996;4:32–38.
Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150–154.
Naudie DD, Amendola A, Fowler PJ. Opening wedge high tibial osteotomy for symptomatic hyperextension-varus thrust. Am J Sports Med. 2004;32:60–70.
Neuschwander DC, Drez D Jr, Paine RM. Simultaneous high tibial osteotomy and ACL reconstruction for combined genu varum and symptomatic ACL tear. Orthopedics. 1993;16:679–684.
Noyes FR, Barber SD, Simon R. High tibial osteotomy and ligament reconstruction in varus angulated, anterior cruciate ligament-deficient knees: a two- to seven-year follow-up study. Am J Sports Med. 1993;21:2–12.
Noyes FR, Barber-Westin SD, Hewett TE. High tibial osteotomy and ligament reconstruction for varus angulated anterior cruciate ligament-deficient knees. Am J Sports Med. 2000;28:282–296.
Noyes FR, Schipplein OD, Andriacchi TP, Saddemi SR, Weise M. The anterior cruciate ligament-deficient knee with varus alignment: an analysis of gait adaptations and dynamic joint loadings. Am J Sports Med. 1992;20:707–716.
O’Neill DF, James SL. Valgus osteotomy with anterior cruciate ligament laxity. Clin Orthop Relat Res. 1992;278:153–159.
Outerbridge RE, Dunlop JA. The problem of chondromalacia patellae. Clin Orthop Relat Res. 1975;110:177–196.
Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis: survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85:469–474.
Stein BE, Williams RJ III, Wickiewicz TL. Arthritis and osteotomies in anterior cruciate ligament reconstruction. Orthop Clin North Am. 2003;34:169–181.
Tang WC, Henderson IJ. High tibial osteotomy: long term survival analysis and patients’ perspective. Knee. 2005;12:410–413.
van de Pol GJ, Arnold MP, Verdonschot N, van Kampen A. Varus alignment leads to increased forces in the anterior cruciate ligament. Am J Sports Med. 2009;37:481–487.
Wiertsema SH, van Hooff HJ, Migchelsen LA, Steultjens MP. Reliability of the KT1000 arthrometer and the Lachman test in patients with an ACL rupture. Knee. 2008;15:107–110.
Acknowledgment
We thank Dong-Su Jang, Research Assistant, Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea, for help with the figures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Kim, SJ., Moon, HK., Chun, YM. et al. Is Correctional Osteotomy Crucial in Primary Varus Knees Undergoing Anterior Cruciate Ligament Reconstruction?. Clin Orthop Relat Res 469, 1421–1426 (2011). https://doi.org/10.1007/s11999-010-1584-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-010-1584-1