Abstract
Background
Biopsy is a critical step in the diagnosis of musculoskeletal malignancy. As an alternative to open biopsy, percutaneous core needle biopsy techniques have been developed. As many studies combine office-based, image-guided, and operative biopsies, the accuracy of office-based core needle biopsy is not well documented.
Question/purposes
We asked whether (1) office-based core needle biopsy for the diagnosis of malignant musculoskeletal neoplasms would have few complications and diagnostic and accuracy rates comparable to those cited in the literature for core needle biopsy, (2) diagnostic errors related to office-based core needle biopsy would result in surgical treatment errors, and (3) tissue core quantity and tumor type would affect accuracy.
Patients and Methods
We retrospectively reviewed 234 patients with 252 core needle biopsies of malignant bone and soft tissue neoplasms at one institution between 1999 and 2007. Biopsy accuracy and errors were determined on the basis of histologic evaluation of prior or subsequent biopsies and/or resected specimens, when available. We eliminated 19 patients who had needle biopsies: three had the core needle biopsy completed in the operating room and 16 had insufficient documentation or followup, leaving 233 for study.
Results
Of the 233 core needle biopsies, 212 (91%) were diagnostic and accurate for malignancy. Fourteen (6%) biopsies were nondiagnostic. Major errors, defined as a benign diagnosis in a malignant tumor, occurred in seven cases (3%). Minor errors, defined as errors in histopathologic diagnosis or grade, occurred in 24 biopsies (10%). All nondiagnostic and major core needle biopsy errors were identified and addressed with either a diagnostic open biopsy or definitive wide local excision, resulting in no surgical treatment errors. Accuracy was not influenced by core number; however, myxoid lesions showed a correlation with biopsy error. There were no biopsy-related complications.
Conclusions
Office-based core needle biopsy for diagnosis of malignant musculoskeletal neoplasms has high diagnostic and accuracy rates without associated complications.
Level of Evidence
Level II, diagnostic study. See the Guidelines for Authors for a complete description of the level of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The biopsy is a critical step in the diagnosis of neoplastic, inflammatory, infectious, and reactive lesions of the musculoskeletal system. Although an open, incisional technique traditionally has been considered the gold standard, it requires an incision, an operative suite, and frequently necessitates general anesthesia [7, 10]. The overall diagnostic accuracy of open biopsies ranges from 91% to 96% [4, 10, 20]. Complications of any biopsy include seroma, hematoma, infection, wound dehiscence with tumor fungation, and fracture, and tend to occur more frequently after open or excisional biopsies, with the complication rate of percutaneous techniques ranging from 0% to 1% [3, 13, 17, 20, 25] and surgical open biopsies from 4% to 19% [4, 10, 11, 22]. Nondiagnostic open biopsies occur in approximately 5% of cases [4, 20], despite being theoretically superior to percutaneous techniques in this regard. Additionally, open biopsy errors related to incision placement may alter treatment options and negatively influence outcomes in patients with malignant neoplasms, with such errors occurring three to eight times more frequently when the biopsy is performed at the nontreating, referring facility [4, 10, 11].
As an alternative to open biopsy, percutaneous techniques, including core needle biopsy (CNB) and fine needle aspiration, have been developed. These techniques can be performed in the office under local anesthesia when the lesion or pertinent landmarks are palpable or in the radiology suite using fluoroscopy, CT, MRI, or ultrasound [2, 5, 13, 16, 17, 19, 23, 25, 27–29]. Advantages of office CNB over open or image-assisted biopsies include decreased cost, expediency owing to the avoidance of scheduling delays, lower complication rates, and smaller incisions that may be easier to incorporate into the definitive surgical resection [10, 11, 20, 24]. Potential disadvantages may include decreased diagnostic accuracy and tumor sampling error. However, many of the published series regarding CNB combine office-based and image-guided biopsy results [12, 14, 18, 25, 26], whereas others have combined office-based CNB with CNB performed in the operating room [13, 21]. Additionally, some studies excluded inadequate or nondiagnostic biopsies from their statistical analysis, which may falsely elevate accuracy rates [3, 8, 9]. Therefore, the putative limitations of CNB are not clearly substantiated or refuted.
We asked whether (1) office-based CNB for the diagnosis of malignant musculoskeletal neoplasms would have few complications with diagnostic biopsy and accuracy rates comparable to those reported in the literature for CNB, (2) diagnostic errors related to office-based CNB would result in subsequent surgical treatment errors, and (3) specific biopsy-related factors, including tissue core quantity and tumor type, would affect biopsy accuracy.
Patients and Methods
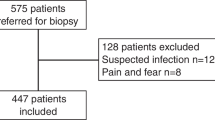
We retrospectively reviewed the medical records of 497 patients from our two institutions who had percutaneous, office-based CNB of bone or soft tissue between 1999 and 2007 and identified 515 biopsies. We excluded 282 patients: 263 for benign diagnoses, three for CNB completion in the operating room, and an additional 16 for insufficient documentation or followup. Followup was considered adequate if any of the following criteria were fulfilled: (1) confirmatory histologic specimens were available from previous histologic specimens in a patient with a known malignancy, or from an additional subsequent diagnostic biopsy, or from the definitive surgical resection; (2) there was a minimum of 6 months’ clinical followup; or (3) the patient died of their tumor with less than 6 months’ clinical followup. Of the 515 CNBs, 252 were performed on lesions ultimately determined to be malignant. This left 233 patients with malignancies of bone and soft tissue, 198 with comparative confirmatory histologic specimens (85%). Ninety-eight (42%) of the patients were female and 135 (58%) were male, with a mean age of 48 years (range, 10–95 years). Of the 233 biopsies, 196 CNBs were for primary malignant diagnoses and 37 were evaluations for local recurrence; 217 (93%) of the malignancies were soft tissue and 16 (7%) were osseous. The distributions of lesion location and diagnoses (Table 1) were highly variable. We had prior institutional review board approval.
After radiographic review and clinical examination, the risks, benefits, and alternatives of CNB were discussed with the patients and the patients formally consented to CNB. All biopsies were performed by a fellowship-trained orthopaedic oncologist (HTT, JDP). We prepared and draped the area in sterile fashion followed by infiltration of 1% lidocaine for local anesthesia. A Number 11 blade was used to incise the skin and the core needle device was advanced into the mass. We used Temno®, Tru-Cut®, or Jamshidi® needles (Allegiance Healthcare Corp/Cardinal Health, McGaw Park, IL). Multiple CNB specimens (range, 1–10) were obtained using the single incision, with the absolute number of cores determined on an individual basis by the treating surgeon. The fresh cores were transported directly to the pathology suite. Specimens were processed routinely for hematoxylin and eosin staining for permanent and, in many cases, frozen section analysis, with subsequent special confirmatory or diagnostic staining and immunohistochemical studies performed at the discretion of the interpreting pathologist and the availability of adequate additional fresh tissue. The remainder of the specimen then was placed in neutral-buffered formalin for permanent pathologic analysis. Owing to the long time of the study, multiple pathologists were responsible for pathologic specimen processing, review, and interpretation.
CNBs were considered diagnostic if the pathologist was able to establish a histopathologic diagnosis and assign a histopathologic grade. Diagnostic biopsies with available comparative specimens were analyzed for accuracy with respect to detection of malignancy, histopathologic diagnosis, and grade. The diagnosis and grade of biopsies without comparative specimens were considered accurate if they were concordant with the clinical behavior, radiographic characteristics, and patient outcome and clinical course.
CNB errors were analyzed and divided into major and minor errors. Major errors included any benign CNB diagnosis for tumors later found to be malignant. Minor errors were errors in histopathologic diagnosis or grade that potentially could influence treatment. Discrepancies in histologic subtype alone (chondroblastic versus osteoblastic) were not considered errors if the histopathologic diagnosis and grade were concordant. Although analysis of CNBs on benign neoplasms was not a goal of our study, these charts were reviewed for major errors and we identified only one case of a malignant CNB diagnosis in a benign neoplasm. In this case, a benign histiocytic soft tissue mass received a diagnosis of a malignant reticulohistiocytic sarcoma on CNB and was treated with wide local excision without neoadjuvant therapy.
On chart review, the following patient data were collected: history of malignancy, location of CNB site, previous biopsy at the CNB site, previous surgical procedures and surgical margins at the CNB site, history of radiotherapy at the CNB site, and history of systemic chemotherapy. Other data recorded included additional biopsies at the CNB site and the method of biopsy; medical, surgical, and adjuvant treatments used after the CNB; any reported complications after CNB; the number of cores obtained at the time of CNB; and whether the CNB was for an evaluation for primary diagnosis or local recurrence. The size of the lesion at the time of CNB as measured clinically or according to radiographic reports was collected in terms of greatest linear dimension in centimeters. Pathology reports were reviewed for histopathologic diagnosis, grade (benign, low, intermediate, or high grade, or malignancy with further grade unspecified), and specific tissue types (myxoid, lipomatous, cartilaginous).
Descriptive statistics were used for overall CNB diagnostic accuracy rates. Chi square analysis or Fisher’s exact test, as appropriate, were used for comparing diagnostic error rates between groups based on tissue type (ie, myxoid, nonmyxoid cartilage, nonmyxoid fat versus other) and CNB core number (ie, < 4 versus ≥ 5 cores). Post hoc power analysis showed this study had an 88% power to detect a significant difference between myxoid tissue types and others, but less than 10% power to detect a difference between nonmyxoid cartilaginous or fatty tissue types owing to the nearly identical diagnostic error rates between the latter groups and the cohort as a whole (excluding myxoid tissue types). Likewise, as the diagnostic error rate between the low (< 4) and high (≥ 5) core number cohorts was nearly identical across all tissue types at 20% and 19%, respectively, this study had only a 5% power to detect a difference between these two groups, although no clinically relevant difference was apparent based on the aforementioned error percentages. Statistical analysis was performed using SPSS® Version 15.0 (SPSS Inc, Chicago, IL, USA) and power analysis using PASS 2008 (NCSS, Kaysville, UT, USA). All reported p values are two-tailed.
Results
Of the 233 CNBs meeting the inclusion criteria, 212 (91%) were diagnostic and accurate for malignancy, representing a 97% accuracy rate for malignancy when nondiagnostic biopsies were eliminated. Furthermore, 188 (80.7%) of the CNBs were diagnostic and accurate for histopathologic diagnosis and grade. The diagnostic biopsy rate was 94%, with 14 (6%) nondiagnostic CNBs. Four patients with nondiagnostic CNBs had additional open diagnostic biopsies followed by wide local excision. The remaining 10 cases were considered highly suspicious for malignancy by the treating surgeon and the patients subsequently were treated with wide local excision without repeat biopsy. Thus, none of the 14 nondiagnostic biopsies resulted in errors in surgical treatment (Table 2).
There were 31 total errors (13.3%), with seven (3.0%) considered major and 24 (10.3%) considered minor. Of the seven patients with major errors, four were followed with a diagnostic open biopsy and wide local excision for definitive surgical treatment. The remaining three patients were considered highly suspicious for malignancy by the treating surgeon and the patients were treated with wide local surgical excision without repeat biopsy. Therefore, none of the major CNB errors resulted in errors in definitive surgical treatment (Table 3). Of the 24 patients who had CNBs with minor errors, two had additional open biopsies that were both inaccurate. For one patient, the CNB and open biopsy diagnoses supported malignancy (although neither established the definitive diagnosis), and the patient was treated appropriately with wide local excision (Table 4, Patient 24). The patient with the other lesion was treated with a marginal excision based on an inaccurate benign diagnosis on the open biopsy and ultimately required a tumor bed excision for local disease control. The CNB specimen supported a malignant diagnosis and the correct histopathologic diagnosis; however, the subsequent open biopsy erroneously suggested a benign entity and led to inappropriate initial surgical treatment (Table 4, Patient 24). The remaining 22 patients were treated with wide local excision without repeat biopsy. Therefore, none of the CNB minor errors resulted in errors in definitive surgical treatment. The one surgical treatment error that did occur in this group was attributable to an error in an open biopsy diagnosis. There were no local complications from any office-based CNB. Specifically, there were no instances of local infection, hematoma, substantive bleeding, or inadvertent neurovascular injury.
The only studied tissue type with an increased (p = 0.021) rate of CNB error was a myxoid tissue type. The number of cores obtained at the time of biopsy was highly variable (mean = 4; range, 1–10); however, we found no relationship (p = 0.77) between core number and CNB errors.
Discussion
The optimal technique for the biopsy of malignant neoplasms of soft tissue and bone remains controversial and often is dictated by the preference of the operating surgeon. Although CNB has become more widely used in recent years, the literature for diagnostic yield and accuracy remains quite variable and the current literature does not address whether CNB error leads to surgical treatment error. We asked whether (1) office-based CNB for the diagnosis of malignant musculoskeletal neoplasms would be associated with few complications and have diagnostic accuracy rates comparable to those reported in the literature for CNB, (2) diagnostic errors related to office-based CNB would result in subsequent surgical treatment errors, and (3) specific biopsy-related factors (ie, core number, tumor type) would affect biopsy yield and accuracy.
Our study has several limitations. First, we lacked confirmatory pathologic tissue in a subset of 35 patients. In the patients with malignant lesions for whom confirmatory diagnostic tissue was not available, we used additional studies such as immunohistochemistry and cytogenetics to confirm the histopathologic interpretation and concordant clinical course and patient followup to determine the accuracy of the biopsy diagnosis. Second, owing to the retrospective nature of the study, multiple pathologists were involved in the interpretation of CNBs which precluded a detailed analysis of the effect, if any, of pathologist experience and training on diagnostic accuracy. Third, there was high variability in the number of biopsy cores obtained, and biopsies were completed by two orthopaedic oncologists. Both surgeons use the same biopsy technique, practice in the same institutions, and use the same multidisciplinary conference approach to biopsy interpretation and surgical decision making. Finally, although our study highlights the importance of multidisciplinary consultation and musculoskeletal oncologist involvement in each step of the treatment process, including biopsy, our results may not be generalizable to others who treat malignancies infrequently or practice in relative isolation. Despite these limitations, we believe this study provides important and clinically relevant information regarding CNB.
The few dedicated series analyzing outpatient CNB show an overall diagnostic accuracy ranging from 84% to 98% [3, 8, 9, 20]. Skrzynski et al. [20] prospectively analyzed 62 patients undergoing outpatient CNB and reported an overall diagnostic accuracy of 84%, with 13% of biopsies considered nondiagnostic, compared with 96% accuracy in a contemporary cohort of patients undergoing open biopsy. In a series of CNBs, Heslin et al. [8] obtained adequate biopsy samples in 93% and established a correct diagnosis of malignancy in 95%, histology in 75%, and grade in 88%, results comparable to their open biopsy results for malignancy and grade. In an analysis by Ball et al. [3] of 52 CNBs, the overall accuracy was 98%, but inadequate or nondiagnostic biopsy specimens were excluded from the statistical analysis. We found office-based CNB has a 97% accuracy rate for malignancy and is diagnostic and accurate for histopathologic diagnosis and grade in 81% of cases. Our findings thus are consistent with the aforementioned office-based CNB studies, but we believe our data provide additional useful information by including nondiagnostic biopsy specimens in the analysis. Finally, we found patients who had office-based CNBs to have no complications in our series. Previous studies also have reported very low complication rates after CNB [3, 17, 25, 27], a substantial benefit over reported complication rates after open biopsy ranging from 4% to 19% [4, 10, 11]. We did not specifically address the issue of cost savings for office-based CNB in this study, but this has been reported at a savings of $3000 to $6000 per biopsy versus open techniques [1, 20, 24].
We also analyzed the definitive surgical treatment rendered in all nondiagnostic CNBs and CNB errors. We divided CNB errors into two groups, major and minor errors, as we believe these two groups are substantively different regarding treatment implications. Major errors, defined as a benign diagnosis in a malignant lesion, have the potential to substantially alter treatment and patient outcome if not recognized and adequately addressed. The impact of minor errors, defined as errors in histopathologic diagnosis or grade that could influence treatment, is more difficult to quantify owing to institutional variations in the administration of neoadjuvant and adjuvant chemotherapy and radiotherapy. We found CNB errors resulted in no operative treatment errors. These results must be viewed in the context of the treating surgeons and institutions, as all biopsies in this series were performed by a fellowship-trained orthopaedic oncologist at a tertiary referral center and routinely reviewed in a multidisciplinary oncology conference. Therefore, all CNB errors in the series were recognized and treated appropriately based on multidisciplinary analysis and correlation of the available pathologic specimen with clinicoradiographic data. Clinicoradiographic correlation in biopsy interpretation is essential to all biopsy techniques as has been recommended by multiple authors regarding open biopsy interpretation [4, 6, 10, 11].
We analyzed several variables to identify factors that may increase the error rate in office-based CNB. Skrzynski et al. [20] and Ogilvie et al. [15] advised caution in CNB analysis of myxomatous tumors, with the latter reporting only 11% of myxoid lesion biopsies were useful, compared with 80% for nonmyxoid lesions. Our data likewise indicate a myxoid neoplasm may increase CNB error risk. Conversely, we found the number of tissue cores obtained at the time of biopsy did not correlate with nondiagnostic biopsies or CNB error in our study. In a prospective study of 151 image-guided CNBs, Wu et al. [27] reported diagnostic yield was increased with the number of specimens obtained and with longer specimen length, with a plateau noted at three specimens in bone lesions and four in soft tissue lesions. Our findings thus seem somewhat surprising, as lack of tissue volume for diagnosis is one specific concern with respect to CNB. However, we believe this may be explained by surgeon technique, as the number of cores obtained for any given biopsy is dependent on subjective core quality, sample heterogeneity based on variance of needle trajectory, and overall tissue volume.
Our data suggest office-based CNB of malignant neoplasms is an accurate method of diagnosis, with negligible complication rates, in the hands of fellowship-trained orthopaedic oncologists. Our findings must be viewed in the context of the treating surgeons and institutions, as all biopsies in this series were performed by a fellowship-trained orthopaedic oncologist at a tertiary referral center and routinely reviewed in a multidisciplinary oncology conference. Therefore, all CNB errors in the series were recognized and treated appropriately based on multidisciplinary analysis and correlation of the available tissue specimens with clinical and imaging data. In a multidisciplinary setting of established treating centers for musculoskeletal neoplasms, however, nondiagnostic CNBs and CNB errors can be identified and addressed appropriately without resultant surgical errors. Specific attention should be given to the analysis of myxoid neoplasms.
References
Ashford RU, McCarthy SW, Scolyer RA, Bonar SF, Karim RZ, Stalley PD. Surgical biopsy with intra-operative frozen section: an accurate and cost-effective method for diagnosis of musculoskeletal sarcomas. J Bone Joint Surg Br. 2006;88:1207–1211.
Ayala AG, Ro JY, Fanning CV, Flores JP, Yasko AW. Core needle biopsy and fine-needle aspiration in the diagnosis of bone and soft-tissue lesions. Hematol Oncol Clin North Am. 1995;9:633–651.
Ball AB, Fisher C, Pittam M, Watkins RM, Westbury G. Diagnosis of soft tissue tumours by Tru-Cut biopsy. Br J Surg. 1990;77:756–758.
Boriani S, Ruggieri P, Sudanese A. Biopsy: considerations on surgical technique derived from a study of 749 cases of bone tumour. Ital J Orthop Traumatol. 1984;10:489–499.
Carrino JA, Khurana B, Ready JE, Silverman SG, Winalski CS. Magnetic resonance imaging-guided percutaneous biopsy of musculoskeletal lesions. J Bone Joint Surg Am. 2007;89:2179–2187.
Clasby R, Tilling K, Smith MA, Fletcher CD. Variable management of soft tissue sarcoma: regional audit with implications for specialist care. Br J Surg. 1997;84:1692–1696.
Heare TC, Enneking WF, Heare MM. Staging techniques and biopsy of bone tumors. Orthop Clin North Am. 1989;20:273–285.
Heslin MJ, Lewis JJ, Woodruff JM, Brennan MF. Core needle biopsy for diagnosis of extremity soft tissue sarcoma. Ann Surg Oncol. 1997;4:425–431.
Kissin MW, Fisher C, Carter RL, Horton LW, Westbury G. Value of Tru-cut biopsy in the diagnosis of soft tissue tumours. Br J Surg. 1986;73:742–744.
Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am. 1982;64:1121–1127.
Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am. 1996;78:656–663.
Mitsuyoshi G, Naito N, Kawai A, Kunisada T, Yoshida A, Yanai H, Dendo S, Yoshino T, Kanazawa S, Ozaki T. Accurate diagnosis of musculoskeletal lesions by core needle biopsy. J Surg Oncol. 2006;94:21–27.
Moore TM, Meyers MH, Patzakis MJ, Terry R, Harvey JP Jr. Closed biopsy of musculoskeletal lesions. J Bone Joint Surg Am. 1979;61:375–380.
Oetgen ME, Grosser DM, Friedlaender GE, Lindskog DM. Core needle biopsies of musculoskeletal tumors: potential pitfalls. Orthopedics. 2008;31. pii: orthosupersite.com/view.asp?rID = 32927.
Ogilvie CM, Torbert JT, Finstein JL, Fox EJ, Lackman RD. Clinical utility of percutaneous biopsies of musculoskeletal tumors. Clin Orthop Relat Res. 2006;450:95–100.
Puri A, Shingade VU, Agarwal MG, Anchan C, Juvekar S, Desai S, Jambhekar NA. CT-guided percutaneous core needle biopsy in deep seated musculoskeletal lesions: a prospective study of 128 cases. Skeletal Radiol. 2006;35:138–143.
Ray-Coquard I, Ranchere-Vince D, Thiesse P, Ghesquieres H, Biron P, Sunyach MP, Rivoire M, Lancry L, Meeus P, Sebban C, Blay JY. Evaluation of core needle biopsy as a substitute to open biopsy in the diagnosis of soft-tissue masses. Eur J Cancer. 2003;39:2021–2025.
Serpell JW, Pitcher ME. Pre-operative core biopsy of soft-tissue tumours facilitates their surgical management. Aust NZ J Surg. 1998;68:345–349.
Shin HJ, Amaral JG, Armstrong D, Chait PG, Temple MJ, John P, Smith CR, Taylor G, Connolly BL. Image-guided percutaneous biopsy of musculoskeletal lesions in children. Pediatr Radiol. 2007;37:362–369.
Skrzynski MC, Biermann JS, Montag A, Simon MA. Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 1996;78:644–649.
Stoker DJ, Cobb JP, Pringle JA. Needle biopsy of musculoskeletal lesions: a review of 208 procedures. J Bone Joint Surg Br. 1991;73:498–500.
Thomas JM. Surgical biopsy techniques and differential diagnosis of soft tissue tumours. Recent Results Cancer Res. 1995;138:25–29.
van der Bijl AE, Taminiau AH, Hermans J, Beerman H, Hogendoorn PC. Accuracy of the Jamshidi trocar biopsy in the diagnosis of bone tumors. Clin Orthop Relat Res. 1997;334:233–243.
Ward WG Sr, Kilpatrick S. Fine needle aspiration biopsy of primary bone tumors. Clin Orthop Relat Res. 2000;373:80–87.
Welker JA, Henshaw RM, Jelinek J, Shmookler BM, Malawer MM. The percutaneous needle biopsy is safe and recommended in the diagnosis of musculoskeletal masses. Cancer. 2000;89:2677–2686.
Woon DT, Serpell JW. Preoperative core biopsy of soft tissue tumours facilitates their surgical management: a 10-year update. ANZ J Surg. 2008;78:977–981.
Wu JS, Goldsmith JD, Horwich PJ, Shetty SK, Hochman MG. Bone and soft-tissue lesions: what factors affect diagnostic yield of image-guided core-needle biopsy? Radiology. 2008;248:962–970.
Yao L, Nelson SD, Seeger LL, Eckardt JJ, Eilber FR. Primary musculoskeletal neoplasms: effectiveness of core-needle biopsy. Radiology. 1999;212:682–686.
Zornoza J, Bernardino ME, Ordonez NG, Thomas JL, Cohen MA. Percutaneous needle biopsy of soft tissue tumors guided by ultrasound and computed tomography. Skeletal Radiol. 1982;9:33–36.
Acknowledgments
We thank Zoraida Moore and Nancy Garcia for assistance with this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at University of Miami Miller School of Medicine.
About this article
Cite this article
Adams, S.C., Potter, B.K., Pitcher, D.J. et al. Office-based Core Needle Biopsy of Bone and Soft Tissue Malignancies: An Accurate Alternative to Open Biopsy with Infrequent Complications. Clin Orthop Relat Res 468, 2774–2780 (2010). https://doi.org/10.1007/s11999-010-1422-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-010-1422-5



