Abstract
Background
Osteonecrosis is a major treatment complication of pediatric leukemias owing to its potential to cause joint deterioration. Because of potential long-term effects of osteonecrosis on joints, information regarding its progression and collapse in different patients can be used to identify high-risk groups, advise the patients and parents of this complication, and potentially consider the risk for development of osteonecrosis in planning primary treatment.
Questions/purposes
We therefore determined: (1) the incidence of joint collapse and/or pain in young patients with hematologic malignancies diagnosed with ON of the knee; (2) risk factors associated with collapse; and (3) the relationship between size and location of osteonecrotic knee lesions and the likelihood of joint collapse.
Patients and Methods
We retrospectively reviewed 109 patients with hematologic malignancies and MRI-confirmed knee osteonecrosis. The median age was 11.5 years (range, 2.3–18.8 years) at primary diagnosis of hematologic malignancy and a median age of 13.4 years (range, 2.7–23.3 years) at diagnosis of osteonecrosis of the knee. For analyses, we used the first and last MR images. Minimum clinical followup was 2.3 years after diagnosis of knee osteonecrosis (median, 6 years; range, 2.3–7.17 years).
Results
Joint collapse occurred in 22% (24 of 109). Older age, pain at osteonecrosis presentation, and lesions extending to the articular surface of distal femoral epiphyses were associated with joint collapse.
Conclusions
Younger patients and those without extensive femoral epiphyseal involvement have a better prognosis for osteonecrosis of the knee.
Level of Evidence
Level II, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteonecrosis (ON) has become a frequent and major complication of treatment for pediatric hematologic malignancies, such as acute lymphoblastic leukemia (ALL) [1, 5, 8, 14], the cure rate for which recently reached 90% [10]. One series using routine MRI of the lower extremities of children treated for ALL revealed an incidence of ON of 38% (in all locations) [9]. Frequency of symptomatic ON in any location reportedly is greater among adolescents and patients receiving higher doses of dexamethasone, and reportedly is as much as 21% in some age groups [8]. In one study, 55% of the knees with ON among young patients with hematologic malignancies were asymptomatic [4, 6]. Clinical symptoms often lag behind MRI presentation of ON and may occur late in the disease [6]. In patients with ALL, ON is mostly an iatrogenic complication that has been attributed to increased use of glucocorticosteroids, asparaginase, and high-dose methotrexate and cyclophosphamide. Proposed etiologies include (1) hypercoagulable state with development of microthrombi, (2) suppression of osteoblasts and apoptosis of osteocytes, and (3) stimulation of intramedullary lipocyte proliferation and hypertrophy resulting in reduced blood flow [7].
Better understanding of ON progression, including risk factors and frequency of joint collapse, is important for advising patients and in selecting the best treatment and patient followup. Knowing ON progression will contribute to a more comprehensive picture of long-term health issues of pediatric cancer survivors [5]. This knowledge should be useful to identify areas of pressing need for refinement of current cancer treatment protocols (eg, adjusting the dose of steroids to minimize ON incidence while maintaining high survival rates) and for finding alternative treatment methods. Studies describing ON progression may establish reference data for future efforts directed at developing effective strategies for treatment and prevention of ON. Whether and how often patients with asymptomatic knee ON experience symptomatic or progressive joint destruction is not well established.
We therefore determined: (1) the incidence of joint collapse and/or pain in young patients with hematologic malignancies diagnosed with ON of the knee; (2) risk factors associated with collapse; and (3) the relationship between size and location of osteonecrotic knee lesions and the likelihood of joint collapse.
Patients and Methods
We retrospectively reviewed the medical records of 168 patients with hematologic malignancy and an MRI diagnosis of ON of the knee made between 1994 and 2003. Beginning in 1994, our institution began acquiring routine MRI to study the development of bony abnormalities. The end year of 2003 was selected to allow sufficient followup from the MRI-confirmed diagnosis of ON. Patients with these MRI studies participated in research protocols that monitored the development of bony abnormalities during or after treatment of the malignancy and routinely underwent MRI regardless of symptoms. At the same time, the patients were asked routinely about the presence of bone and joint symptoms and, if those were present, were referred to a special ON clinic, where they were evaluated by an orthopaedic surgeon. Patients were questioned about the presence or absence of knee pain, mechanical symptoms such as popping, locking, sense of joint instability, limitations in daily activities, use of analgesics, and use of an assist device. Fifty-one of these patients had been referred for MRI in advance of routine imaging for clinical indications (eg, persistent knee pain). We included only patients having followup imaging studies of the knee for at least 12 months after the diagnosis of ON. For analyses, the first and last MR images were used, regardless of the total number of MR images the patient had. The information regarding treatment and clinical symptoms was extracted from the medical records. Exclusion of patients with no followup or less than 12 months followup left 109 of the 168 patients (65%): 57 males (52%), 91 Caucasians (83%). Eighty-four of the 109 patients (77%) had bilateral knee involvement. Their median age was 11.5 years (range, 2.3–18.8 years) at the time of diagnosis of the malignancy and 13.4 years (range, 2.7–23.3 years) at diagnosis of ON of the knee. The primary diagnoses were ALL (97 patients), nonHodgkin lymphoma (seven), and acute myeloid leukemia (five). The minimum clinical followup was 2.3 years after diagnosis of ON of the knee (median, 6 years; range, 2.3–7.17 years). No patients were recalled specifically for this study; all data were obtained from the medical records or images. We obtained prior Institutional review board approval.
From the records we determined whether the patients (1) were asymptomatic with no pain or functional limitations of the knee, (2) were symptomatic, (3) had core decompression, and (4) had arthroscopy.
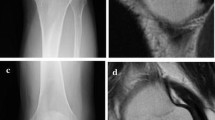
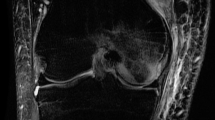
All studies were interpreted by one pediatric radiologist (SCK), blinded to clinical factors. MRI of the knees consisted of coronal noncontrast T1-weighted, coronal short tau inversion recovery, and sagittal FLASH (fast low-angle shot) two-dimensional imaging. All MRI was performed on either a Siemens 1.5-T or a 3-T scanner (Helicon, Vision, or Symphony; Siemens, Erlangen, Germany). The ON lesions were characterized based on (1) the presence or absence of collapse, (2) lesion location (nonepiphyseal or located in distal femoral or proximal tibial epiphysis), and (3) size (determined only for lesions extending to the articular surface and coded as a percentage of the articular surface involved with the ON lesion: < 25%, 25%–50%, and > 50%) [4, 6]. We previously investigated interobserver agreement when using this method of assessing lesion size in the knee, and the agreement was substantial (overall proportion of agreement [Po] = 83%, κ = 0.66) [4]. The interim images of the patients who had subsequent collapse after diagnosis of ON were reviewed to determine the timing of first radiographic signs of collapse. MR images and radiographs were used to determine the presence of subchondral collapse. Stevens et al. investigated the sensitivity and specificity of MRI and plain radiography in comparison to CT for detecting the presence of collapse in the capital femoral epiphysis and found MRI to have sensitivity and specificity of 38% and 100%, respectively, and radiography had sensitivity and specificity of 71% and 97%, respectively [12].
Fisher’s exact and Fisher-Freeman-Halton exact tests were used to investigate frequency of collapse between groups of patients. Patient demographic factors that were significant at the univariate .10 alpha level then were entered in a backward selection first-order multiple logistic regression model as predictors of collapse. The final model included factors significant at the .05 alpha level. All calculations were performed with the statistical package SAS, Version 9.1 (SAS Institute Inc, Cary NC).
Results
At last followup 67 of the 109 patients (61%) were asymptomatic. Among the 42 symptomatic patients, 19 had modest knee pain that required occasional nonnarcotic analgesia; four patients had modest pain, with regular use of nonnarcotic analgesics; four had intermittently disabling pain, requiring intermittent narcotics; two underwent core decompression; and seven had arthroscopy; we had no clinical information regarding the remaining six patients. The two patients who underwent core decompression were symptomatic in both knees and had bilateral surgery at 2.6 and 8.3 years after diagnosis of ON. Collapse of the articular surface occurred 3.5 years after surgery in one of these two patients. At the last followup (4 years and 1 year after the surgeries, respectively), the patients had only minimal pain with activity, not requiring analgesia. Seven patients underwent arthroscopic surgeries (débridement and removal of loose bodies) of 10 knees at a median of 24 months (range, 1–71 months) after the diagnosis of ON. Three patients had bilateral procedures. Six of these seven patients had collapse of the articular surface(s). Two of seven patients died from primary disease. Of the five living patients, three were asymptomatic and two had minimal pain with vigorous activity, occasionally requiring analgesia with NSAIDs at 5.14 years (range, 3.7–5.2 years) after arthroscopic surgery. No patients had undergone arthroplasty at last followup.
Older age at primary diagnosis and diagnosis of ON and knee pain were associated with increased frequency of collapse (Table 1). We found no relationship between the frequency of collapse and other factors: body mass index, gender, primary diagnosis, and administration of steroids after ON diagnosis. The final model showed a 32% increase in odds of collapse associated with age at diagnosis with each unit increase in year and a 361% increase in odds of collapse associated with knee pain at initial ON diagnosis compared with asymptomatic patients (Fig. 1).
Lesions extending to the articular surface were associated with development of collapse (Table 2). Typically, the size and amount of articular surface involvement of ON lesions did not change noticeably between the initial diagnosis of ON and last followup imaging. Twenty-four patients had no epiphyseal lesions at presentation of ON, and 30 patients had epiphyseal lesions not extending to the articular surface. In five patients, the largest lesion occupied less than 25% of the articular surface, two patients had a maximum lesion of 25% to 50%, and 48 patients had a maximum lesion greater than 50%. Of 109 patients, four had radiographic collapse of the articular surface at the first study and 24 (22%) had collapse at the second study (last imaging study before any surgeries). The median time between the diagnosis of ON of the knee and collapse was 12 months (range, 2.1–97.1 months). All collapses of articular surfaces involved the femoral surfaces. Fourteen patients had collapse in both knees.
Discussion
Treatment-induced ON of weightbearing joints has been widely recognized as a major complication of treatment for pediatric hematologic malignancies. Recent years have seen increased attention paid to ON, and the prevalence of ON, the relationship between clinical and radiographic manifestations, and possible ON etiologies were studied [7]. However, the development and progression of ON are still poorly understood. Study of clinical and radiographic progression of ON will assist in understanding the disease progression by identifying frequency of joint collapse and risk factors for joint deterioration, contribute to refinement of protocols for treatment of hematologic malignancies by potential adjustment of steroid regimens, and help healthcare providers advise patients and plan interventions to improve and ameliorate ON. We therefore focused on the following questions: (1) what is the incidence of joint collapse and/or pain in young patients with hematologic malignancies diagnosed with ON of the knee; (2) what risk factors are associated with collapse; (3) is there a relationship between size and location of osteonecrotic knee lesions and the likelihood of joint collapse.
We note several limitations. First, because the interval between the primary malignancy diagnosis and ON diagnosis may be as long as several years (median, < 2 years), it is difficult to ensure all patients who ultimately had ON of the knee develop were included. However, as this study covered a 10-year period, it is likely the number of such patients is too small to noticeably affect the analysis. Second, owing to the relatively small numbers of patients, the variability of steroid use, and our dependence on documentation of steroid dosages in the records, we were unable to ascertain the effect of discontinuing steroids after ON diagnosis on the progression of ON.
Our data show a substantial proportion (22%) of patients diagnosed with knee ON experience joint collapse after the diagnosis. Among patients younger than 11.5 years at primary diagnosis (median age of our cohort), only two (3.6%) experienced joint collapse, whereas collapse occurred in 22 (40.7%) patients older than 11.5 years. The difference is striking and merits additional investigation. One previous report suggests adolescent tissue has a substantial reduction in the fracture toughness compared with immature or mature tissues [2]. Thus, we speculate adolescent patients may be especially susceptible to ON progression. Another possibility is that increased frequency of collapse is associated with skeletal maturity, ie, patients who are skeletally mature are more likely to experience collapse. Although much of the available literature regarding rates of articular surface collapse about the knee describes spontaneous ON of the knee, some reports suggest that in adults with corticosteroid-induced knee ON the rates of collapse are lower than in our series [11, 13]. For example, Sakai et al. reported only a 10% collapse rate (four of 40) at 21 months of followup of the adult knees involved with ON, mostly secondary to steroid use [11]. At the time of last followup, all our patients with knee collapse were treated without arthroplasty. This contrasts with patients having hip ON, in whom hip arthroplasties are quite common. We previously reported a frequency of THAs of 29% (23/80) among patients with pediatric hematologic malignancies and ON of the femoral head at a median of 40.6 months of followup [5]. Long-term outcomes of knee ON remain unknown, and additional investigation is warranted.
We found symptomatic patients were more likely to have collapse and pain than those who were asymptomatic at the time of the initial MRI; collapse occurred in 37% of patients with knee pain versus 9% among asymptomatic patients. We previously reported symptomatic knees are associated with ON lesions occupying larger portions of the articular surface [6]. In the current study, we found femoral epiphyseal lesions extending to the articular surface of the knees also are associated with higher frequency of collapse. In nearly all cases, collapse occurred when ON lesions involved greater than 50% of the articular surface (Table 2). In most patients, the size of ON lesions and involvement of the articular surface did not change noticeably between diagnosis of ON and last available followup. Typical radiographic patterns of ON progression in pediatric patients, that illustrate radiographic signs discussed here, were reported previously [3]. Early radiographic signs appear to be useful predictors of ON progression.
Older age, knee pain, and presence of lesions extending to the articular surface were associated with more frequent collapse. As many as 40% of adolescent patients diagnosed with knee ON experienced collapse, whereas less than 4% of younger patients did so. These findings assist in predicting the clinical progression of treatment-induced ON in pediatric patients with hematologic malignancies.
References
Aricò M, Boccalatte MF, Silvestri D, Barisone E, Messina C, Chiesa R, Santoro N, Tamaro P, Lippi A, Gallisai D, Basso G, De Rossi G; Associazione Italiana di Ematologia ed Oncologia Pediatrica. Osteonecrosis: an emerging complication of intensive chemotherapy for childhood acute lymphoblastic leukemia. Haematologica. 2003;88:747–753.
Flachsmann R, Broom ND, Hardy AE, Moltschaniwskyj G. Why is the adolescent joint particularly susceptible to osteochondral shear fracture? Clin Orthop Relat Res. 2000;381:212–221.
Karimova EJ, Kaste SC. MR imaging of osteonecrosis of the knee in children with acute lymphocytic leukemia. Pediatr Radiol. 2007;37:1140–1146.
Karimova EJ, Rai SN, Deng X, Ingle DJ, Ralph AC, Neel MD, Kaste SC. MRI of knee osteonecrosis in children with leukemia and lymphoma: Part 1, observer agreement. AJR Am J Roentgenol. 2006;186:470–476.
Karimova EJ, Rai SN, Howard SC, Neel M, Britton L, Pui CH, Kaste SC. Femoral head osteonecrosis in pediatric and young adult patients with leukemia or lymphoma. J Clin Oncol. 2007;25:1525–1531.
Karimova EJ, Rai SN, Ingle D, Ralph AC, Deng X, Neel MD, Howard SC, Pui CH, Kaste SC. MRI of knee osteonecrosis in children with leukemia and lymphoma: Part 2, clinical and imaging patterns. AJR Am J Roentgenol. 2006;186:477–482.
Kerachian MA, Séguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol. 2009;114:121–128.
Mattano LA Jr, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. J Clin Oncol. 2000;18:3262–3272.
Ojala AE, Lanning FP, Paakko E, Lanning BM, Osteonecrosis in children treated for acute lymphoblastic leukemia: a magnetic resonance imaging study after treatment. Med Pediatr Oncol. 1997;29:260–265.
Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu M, Coustan-Smith E, Kun LE, Jeha S, Cheng C, Howard SC, Simmons V, Bayles A, Metzger ML, Boyett JM, Leung W, Handgretinger R, Downing JR, Evans WE, Relling MV. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741.
Sakai T, Sugano N, Ohzono K, Matsui M, Hiroshima K, Ochi T. MRI evaluation of steroid- or alcohol-related osteonecrosis of the femoral condyle. Acta Orthop Scand. 1998;69:598–602.
Stevens K, Tao C, Lee SU, Salem N, Vandevenne J, Cheng C, Neumann G, Valentin-Opran A, Lang P. Subchondral fractures in osteonecrosis of the femoral head: comparison of radiography, CT, and MR imaging. AJR Am J Roentgenol. 2003;180:363–368.
Takao M, Sugano N, Nishii T, Miki H, Yoshikawa H. Spontaneous regression of steroid-related osteonecrosis of the knee. Clin Orthop Relat Res. 2006;452:210–215.
Werner A, Jäger M, Schmitz H, Krauspe R. Joint preserving surgery for osteonecrosis and osteochondral defects after chemotherapy in childhood. Klin Padiatr. 2003;215:332–337.
Acknowledgments
We thank Sandra Gaither, Lunetha Britton, RN, and Kimberly Johnson for help with manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
One or more of the authors (SCK) have received funding from grants P30 CA-21765 from the National Institutes of Health, a Center of Excellence grant from the State of Tennessee, and the American Lebanese Syrian Associated Charities (ALSAC).
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at St Jude Children’s Research Hospital.
About this article
Cite this article
Karimova, E.J., Wozniak, A., Wu, J. et al. How Does Osteonecrosis About the Knee Progress in Young Patients with Leukemia?: A 2- to 7-year Study. Clin Orthop Relat Res 468, 2454–2459 (2010). https://doi.org/10.1007/s11999-010-1358-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-010-1358-9