Abstract
Purpose
The purpose of this study was to identify the impact of tibial reamer size and placement on the position of femoral tunnel placement via a transtibial approach for anterior cruciate ligament (ACL) reconstruction.
Methods
Eight cadaveric knee specimens were fixed to a stationary table at 90° of flexion and neutral rotation. After removing the anterior capsule and patella, native joint anatomy was recorded with a digitizer (MicroScribe™; CNC Services, Amherst, VA) accurate to 0.05 mm. Tibial and femoral tunnels were drilled via a transtibial ACLR technique using the optimal tibial starting point described by Piasecki et al. On the tibial side, tunnels were drilled progressively with 6-, 7-, 8-, 9-, 10-, and 11-mm reamers. After each reaming, a beath pin was placed in the posterior aspect of the tibial tunnel, positioned relative to the native anatomic ACL femoral footprint, and digitized. Rhino software (McNeel, Seattle, WA) was used to geometrically determine the center of the native femoral footprint and measure in millimeters the relationship of this point with the femoral position achieved using a 7-mm offset femoral guide with each tibial tunnel size. The surface areas of each tibial and femoral insertion were measured using the insertional periphery data recorded with the digitizer. Statistical analysis of continuous variable data was performed with t tests; P values below 0.05 were deemed significant.
Results
The center of the femoral ACL footprint was reached with a 9-mm tibial tunnel in six knees, and with an 8-mm tunnel in two knees. A 6- or 7-mm tibial tunnel did not allow for anatomic positioning in any specimen, with femoral positioning significantly more superior than that achieved with a 9-, 10-, or 11-mm tibial tunnel (P < 0.01). The 6- and 7-mm tunnels produced errors of 4.6 ± 1.6 and 2.9 ± 0.5 mm, respectively. After use of the 11-mm tibial reamer, the average tibial tunnel length was 32.1 ± 2.6 mm.
Conclusions
Limitations of a transtibial ACLR technique may result in non-anatomic femoral tunnel placement with tibial tunnel sizes smaller than 9 mm. However, tibial tunnels placed in the proximal entry position with at least a 9-mm tunnel size allowed anatomic femoral tunnel placement via a transtibial approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As anterior cruciate ligament (ACL) anatomy becomes better understood, a more anatomic approach to ACL reconstruction is increasingly being pursued [1]. Despite improvements in native ligament anatomy as well as advances in surgical approaches and instrumentation, optimal surgical technique in ACL reconstruction remains uncertain. Small variations in femoral and tibial tunnel positioning can drastically change ACL length, tensioning patterns, as well as alter ACL force vectors and joint kinematics [1–5]. Grafts placed higher on the femoral wall in ACL reconstruction—a less coronally oblique orientation—less effectively opposes rotatory loads as compared to grafts placed lower on the femoral wall [5]. Decreased sagittal plane obliquity has also been implicated, largely because such an orientation incompletely resists anterior translational loads as compared to the native ACL [1, 2, 6–8]. Regardless of which ACL reconstruction technique is utilized, a growing body of the literature supports the notion that a more anatomic reconstruction better restores knee kinematics than non-anatomic reconstructions [9, 10].
The modified transtibial endoscopic single bundle ACL reconstruction has been demonstrated to have equal efficacy in improving knee joint biomechanical stability as ACL reconstructions performed via an anteromedial portal technique and an outside-in technique [11]. The most limiting aspect of this technique is the reliance of femoral tunnel positioning on tibial tunnel orientation and position; because the femoral tunnel is drilled through the tibial tunnel, the tibial tunnel represents a potentially unforgiving linear constraint to instrumenting the femur. The ideal scenario for transtibial reconstruction is one where the tibial tunnel is collinear with a line connecting the centers of both femoral and tibial ACL insertions. Such geometry has been shown to be impractical, however. As noted by Heming et al. [12], a guide pin drilled through the center of both insertions will consistently exit the tibia within millimeters of the joint line—in fact, Piasecki et al. recently quantified this distance to be 11.10 mm [13]. A tibial tunnel created with this proximal of a starting point likely would compromise tibial graft fixation and create significant graft-tunnel mismatch problems if a bone–tendon–bone graft was employed. If a more distal, traditional tibial starting position is employed instead, the resultant tunnel will be less aligned with the native ligament and will result in less-than-anatomic femoral tunnel positioning [13].
In a previous cadaveric study, it was noted that tibial and femoral tunnels can be created in a highly anatomic manner using a transtibial technique but requires a fairly proximal, carefully chosen tibial starting position [13]. In that study, however, an 11-mm tibial reamer was utilized in all specimens which afforded great flexibility in placing the offset femoral guide through the 11-mm-wide tibial tunnel onto an anatomic position on the femoral ACL footprint. At the present time, a large proportion of bone–tendon–bone and soft tissue grafts used are smaller than 11 mm. It is possible that smaller tibial reamers would not allow for such precise anatomic femoral tunnel placement using a transtibial technique because the resultant smaller tibial tunnel would limit flexibility in placing an offset femoral guide and transtibial guidewire. If this notion were proved true, a femoral-independent drilling technique may need to be pursued for select cases in which a narrow tibial tunnel is anticipated.
One of the clinical concerns with the transtibial approach to ACL reconstruction is the potential difficulty with establishing an anatomic femoral tunnel position. The purpose of this study is to identify the impact of tibial reamer size on the ability to place anatomic femoral tunnels via a transtibial approach. It is hypothesized that there is a threshold for tibial tunnel size, under which, the surgeon will be unable to obtain anatomic femoral tunnel placement using a transtibial technique.
Materials and methods
Eight fresh-frozen adult knee specimens (six males, two females; four right, four left) with an average age of 47.2 ± 5.6 years (range 36–53 years) without ligamentous injury or significant degenerative joint disease were thawed over 24 h (mid-femoral diaphysis to mid-tibial diaphysis). Taking care to preserve soft tissues about the knee joint, skin, muscle, and subcutaneous tissue were removed from tibial and femoral diaphyses. Specimens were then mounted in 90° of flexion on a custom mount stationary on a laboratory table stabilized to floor. The mount comprised of two bars perpendicular in the sagittal plane and parallel in the coronal plane with respect to one another. Clamps were then fixated in a similar orientation on the bars and attached to the femoral and tibial diaphysis. In this position, each specimen was secure from external forces affecting alignment. This flexion angle was chosen as it is the most common position of the knee during transtibial reconstruction techniques. In order to ensure that the necessary exposures of the ACL insertions did not destabilize the knee and result in aberrant motion of the tibia and femur, a three-point coordinate system was arbitrarily defined on each specimen by choosing and marking a point on the femur, tibia, and laboratory table. The x, y, z coordinates of each of these points were measured and repeatedly referenced throughout the study to assure a static relationship between the femur, tibia, and digitizer (MicroScribe™; CNC Services, Amherst, VA) accurate to 0.05 mm.
After fixing the specimen on the custom designed mount, the lateral femoral condyle was further secured to the lateral tibial plateau with two divergent K-wires. Extra-articular soft tissues about the knee joint were then dissected off and the intact nature of the articular cartilage, meniscal attachments, and cruciate ligaments were confirmed. The superior border of the pes anserinus and anterior edge of the medial collateral ligament (MCL) was marked on the proximal tibia prior to removal. The length of the central third of the patellar tendon was measured with a ruler for each specimen, from distal patella to tendo-osseous junction on the tibial tubercle prior to removal.
To allow a post hoc three-dimensional analyses, the knee joint’s surface femoral and tibial anatomy was then recorded using the digitizer to log extensive point cloud arrays of both bones. In addition to articular surfaces and bony landmarks, soft tissue structures such as the anterior horn of the lateral meniscus, the medial meniscus, and the anterior face of the posterior cruciate ligament (PCL) at the posterior edge of the tibial plateau were also digitized to better appreciate the anatomic relationship of the ACL to these structures.
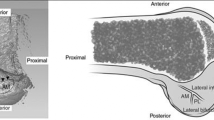
Using an oscillating saw, the medial femoral condyle was then carefully removed with great caution taken to avoid damage to the femoral ACL insertion (Fig. 1). The ACL was then sharply divided transversely and removed with care to allow the tibial and femoral footprints to be digitized after being marked with a pen. The x, y, z coordinates of the three arbitrary points on the tibia, femur, and laboratory were measured once again to confirm the static relationship between the femur, tibia, and digitizer had not changed.
a Anterior view of right knee after capsule, patella, patellar tendon, and ligamentum mucosum have been removed. b Using an oscillating saw, the medial femoral condyle was then carefully removed with great caution taken to avoid damage to the femoral ACL insertion. The probe is on the ACL in Fig. 2
Surgical technique
As shown by Piasecki et al. [13] there is an optimal tibial tunnel starting point (15.9 mm below the medial plateau, 9.8 mm posteromedial to the medial margin of the tibial tubercle) which best allows for anatomic femoral tunnel drilling using a transtibial technique. Using this idealized tibial tunnel starting point, a guide pin was drilled using a standard ACL tibial aimer (Smith and Nephew Endoscopy, Andover, MA) to the center of the marked tibial footprint (Fig. 2). To ensure the tibial tunnel center point remained constant during sequential reaming, the guide pin was advance into the femoral epiphysis. Cannulated straight tibial reaming was then sequentially performed over the guide wire beginning with a 6-mm full-fluted reamer and proceeding to an 11-mm full-fluted reamer (Smith and Nephew Endoscopy, Andover, MA). After each tibial reaming, a 7-mm offset femoral guide was placed through the tibial tunnel to a point as low on the femoral wall and central in the femoral ACL insertion’s anteroposterior distance as possible. This position on the femur was recorded for each reamer size via the digitizer, and the distance from this point to the native femoral insertion center was calculated. After final straight reaming with an 11-mm reamer, the digitizer was then used to register the periphery of this tibial tunnel entrance within the joint.
Using this idealized tibial tunnel starting point, described by Piasecki et al. [13], a guide pin was drilled using a standard ACL tibial aimer (Smith and Nephew Endoscopy, Andover, MA) to the center of the marked tibial footprint
The digitizer was used to register the apertures and dimensions of tibial tunnels, and to measure the tunnel location in relation to the native ACL tibial and femoral footprint anatomy. Once these measurements were taken, the guide wire was replaced, and the femoral tunnel was reamed with a standard fluted 10-mm reamer through the tibial tunnel. The digitizer was once again utilized to analyze the tibial and femoral tunnel dimensions, and tunnel location compared with the native anatomy. This study was classified as exempt from the Rush University Medical Center Institutional Review Board.
Data and statistical analysis
A number of subsequent analyses were performed using the spatial information recorded with the digitizer. Rhino software (McNeel, Seattle, WA) was used to geometrically determine the center of the native femoral footprint and measure in millimeters the relationship of this point with the femoral position achieved with each tibial tunnel size using an offset femoral guide and transtibial guidewire. The surface areas of each tibial and femoral insertion were measured using the insertional periphery data recorded with the digitizer. Similar surface areas were calculated for the recorded peripheries of the intra-articular tibial tunnel exit and femoral tunnel. The percentage overlap of the tibial tunnel surface area with that of the native tibial insertion was then directly calculated. Throughout the study, all recorded and calculated data points were carried out to the nearest tenth for significant figures.
Statistical analysis of continuous variable data was performed with t tests with alpha set to 0.05 using GraphPad Software (La Jolla, CA); P values below this were deemed significant. A pre hoc sample size for comparison of tibial tunnel sizes was determined by an a priori power analysis (G*Power 3.0, Dusseldorf, Germany) based on a previously published study with similar methodology conducted in the same laboratory [13]. Assuming a 50 % increase in tunnel size and a standard deviation of 0.25 the mean value, to achieve a power of 0.80 with a two-tailed analysis, a minimum of six specimens were required.
Results
In all cases, the coordinates used to refer the tibia, femur, and laboratory table remained within 0.1 mm as measured by the digitizer throughout the testing protocol.
After use of an 11-mm tibial reamer, tibial tunnel length was 32.1 ± 2.6 mm, and tibial-articular ACL footprint area was 111.5 ± 16.4 mm2, compared to the native landmark size of 151.5 ± 29.0 mm2. After use of a 6-mm tibial reamer, tibial tunnel length was 31.6 ± 1.8 mm. Initial tibial tunnel reaming of 6-, 7-, 8-, 9-, and 10-mm full-cannulated reamers produced increasing areas of the tibial-articular ACL footprints with 35.1 ± 8.1, 47.3 ± 8.7, 60.0 ± 9.5, 78.4 ± 13.2, and 96.3 ± 10.5 mm2, respectively. Upon tibial tunnel reaming, no specimen showed compromise of the proximal bone bridge or medial tibial plateau. Of note, upon reaming the femoral tunnel with a 7-mm offset femoral guide centered on native femoral insertion and a 10-mm full-cannulated reamer, the tibial-articular aperture increased in size to 189.8 ± 22.1 mm2.
In six knees, a 9-mm tibial tunnel was necessary for the center of the femoral ACL footprint to be reached. In two knees, the center of the femoral footprint was reached with an 8-mm tunnel. After reaching the anatomic center of the femoral ACL footprint in these specimens, it was shown that subsequent reaming with 10 and 11 mm (in six knees) or 9, 10, and 11 mm (in two knees) allowed placement of the guided pin not only on the native center, but also distal and slightly anterior or posterior to the native insertion on the condylar wall as needed (Fig. 3). Comparisons between guide tip positions in 9-, 10-, and 11-mm tibial tunnels was significantly more distal than guide tip positions in 6-, 7-, and 8-mm tunnels as shown in Table 1. A 6- or 7-mm tibial tunnel did not allow for anatomic positioning in any specimen (Fig. 4). The 6- and 7-mm tunnels produced errors that were proximal and slightly posterior to the native femoral ACL center with an average elevation distance of 4.4 ± 1.8 and 2.9 ± 0.5 mm, respectively (Table 1).
Native femoral ACL footprint (blue), reamed femoral tunnel (brown) with locations of positions achieved with various tibial tunnel sizes using a 7-mm offset femoral guide on a single specimen. Rhino software (McNeel, Seattle, WA) was used to geometrically determine the center of the native femoral footprint and measure in millimeters the relationship of this point with the guide pin positions
Close-up view of cadaver native femoral ACL footprint (blue outline) with locations of 7-mm offset femoral guide positions (beath pin without guide used for demonstration purposes) achieved. As shown here, the tibial tunnel represents a potentially unforgiving linear constraint to instrumenting the femur. a With a 6-mm tibial tunnel, placement of a femoral tunnel will be too high (too proximal) and anterior relative to the native femoral footprint center. b On the other hand, an 11-mm tibial tunnel in the same specimen affords great flexibility, easily allowing the anatomic femoral position to be achieved
In comparing the location of the ACL femoral-articular footprint in relation to joint anatomy, distances from each landmark were digitized from the center and periphery of the native and 10-mm full-cannulated reamed footprints (Table 2). The native ACL femoral footprint had an area that measured 107.8 ± 37.3 mm compared to that of the 10-mm reamed femoral tunnel intra-articular aperture, which digitized to 115.3 ± 8.6 mm. The percentage of native femoral footprint overlapped by the reamed femoral intra-articular aperture (tunnel aperture area overlapping with footprint/ACL footprint total area) was 76.2 ± 10.5 % (Table 3). While the center of the native footprint was digitized to be 18.5 ± 1.7 mm from the anterolateral corner of the PCL footprint on the femur (“notch distance”), the center of the footprint of the 10-mm full reamer measured 18.9 ± 2.6 mm from the PCL.
Finally, the distance from the center of the ACL’s native femoral footprint to the inferior intra-articular cartilage surface measured 7.6 ± 1.9 mm, compared to 7.7 ± 1.3 mm from the 10-mm reamed center. Additionally, measurements were taken to the “back wall” of the femur from the center and posterior aspect of each footprint; while the native was digitized at 9.8 ± 2.3 mm (from center) and 3.6 ± 1.8 mm (from posterior), the 10 mm full reamer measured 9.7 ± 2.2 and 3.0 ± 1.6 mm, respectively. Of note, throughout the entirety of the testing protocol in no specimen was there an observation of compromise to the “back wall” or intra-articular surface on the femur. Additional values demonstrating the anatomic relationships of the ACL femoral footprints are provided in Table 2.
Discussion
The principle findings of this study suggest that limitations necessitated by a transtibial ACLR technique may result in non-anatomic femoral tunnel placement with tibial tunnel diameters smaller than 8 or 9 mm. Our hypothesis that there is a threshold for a tibial tunnel size, under which the surgeon will be unable to obtain anatomic femoral tunnel placement using a transtibial technique has been proven by the primary result of this study.
With regard to restoring joint biomechanical stability, the modified transtibial ACLR technique has been shown to be equally efficacious to ACL reconstructions performed using other techniques. In a cadaveric laboratory study, Sims et al. used a robotic testing system to place uniform anteroposterior loads on knees with reconstructed ACLs using one of the three endoscopic approaches [11]. The authors showed that the modified transtibial technique, the anteromedial portal technique, and the outside-in technique were all biomechanically comparable in their ability to restore normal knee kinematics [11]. Such results validate the utility of the modified transtibial approach in producing ACL reconstructions that mimic native anatomic function based on anatomic graft placement, particularly for surgeons well versed in its technique.
As demonstrated by this study, transtibial femoral reaming through smaller (6 and 7 mm) tibial tunnels produced errors in femoral tunnel positioning, resulting in femoral tunnels proximal to the native femoral ACL center. Loh et al. showed that grafts placed higher on the femoral wall in ACL reconstruction—a less coronally oblique orientation—less effectively resists rotatory loads as compared to grafts placed lower on the femoral wall [5]. More recently, decreased sagittal plane obliquity has also been implicated, predominantly because such an orientation less effectively and less efficiently opposes anterior translational loads as compared to the native ACL [2, 6–8].
Overall, it appears that at least a 9-mm tibial tunnel should be used in all transtibial ACL reconstructions in order to assure that anatomic femoral positioning can be reached utilizing a 7-mm offset femoral guide. While an 8-mm tibial tunnel did allow for anatomic femoral placement in two specimens, it is difficult to predict which anatomic circumstances would be more forgiving to allow this. Depending on graft choice and fixation methods, a 9-mm tibial tunnel may not be practical in certain situations. In these scenarios, a femoral-independent ACL reconstruction technique may be a more suitable option to allow a lower, more anatomic femoral tunnel position to be achieved.
The primary strength of this study is the application of precise digitization technology—accurate to 0.5 mm—for comparisons between ACL footprint and tunnel anatomy. To the best of the authors knowledge, this is the first time such technology has been applied in such a manner to identify the effect of tibial tunnel width on femoral tunnel positioning. This study also had several limitations. This study’s controlled laboratory study design using static cadaveric specimens inherently limits in vivo or dynamic biomechanical conclusions from being drawn regarding the effects of femoral tunnel positioning. The knee flexion was set at 90°; although this is a very common position during ACL reconstruction surgery, most ACL reconstruction techniques allow surgeons to alter this flexion angle as needed during the procedure. Moreover, the global surgical accuracy of producing a native femoral footprint in this study cannot be elucidated as it was conducted with only one surgeon and at only a single time point. Additionally, subtle anatomic differences between cadaveric specimens were not clarified. Thus, it is unclear why some specimens allowed anatomic transtibial femoral tunnel positioning with an 8-mm tunnel while most other specimens required a 9-mm tibial tunnel to achieve the same result. Additional studies may be necessary to further delineate such findings.
Based on the findings of this study, it appears that at least a 9-mm tibial tunnel should be used in all transtibial ACL reconstructions in order to assure that anatomic femoral positioning can be reached. Although an 8-mm tibial tunnel allowed anatomic femoral positioning to be reached in two specimens, it is difficult to predict what anatomic circumstances would be more forgiving to allow this. Depending on graft choice and fixation methods, a 9-mm tibial tunnel may not be practical in certain situations. In these scenarios, a femoral-independent ACL reconstruction technique may be a better choice to allow a lower, more anatomic femoral tunnel position to be achieved.
Conclusions
Limitations created by a transtibial ACLR technique may result in non-anatomic femoral tunnel placement with tibial tunnel sizes <9 mm. However, tibial tunnels placed via a proximal entry position with at least a 9-mm tunnel size allowed anatomic femoral tunnel placement via a transtibial approach. One of the clinical concerns with the transtibial approach to ACL reconstruction is the potential difficulty with establishing an anatomic femoral tunnel position. The results from this study demonstrate that when using a tibial tunnel of at least 9 mm, an anatomic position on the femur can be adequately achieved.
References
Bedi A, Maak T, Musahl V, Citak M, O’Loughlin PF, Choi D, Pearle AD (2011) Effect of tibial tunnel position on stability of the knee after anterior cruciate ligament reconstruction: Is the tibial tunnel position most important? Am J Sports Med 39(2):366–373
Abebe ES, Kim JP, Utturkar GM, Taylor DC, Spritzer CE, Moorman CT III, Garrett WE, DeFrate LE (2011) The effect of femoral tunnel placement on ACL graft orientation and length during in vivo knee flexion. J Biomech 44(10):1914–1920
Abebe ES, Moorman CT III, Dziedzic TS, Spritzer CE, Cothran RL, Taylor DC, Garrett WE Jr, DeFrate LE (2009) Femoral tunnel placement during anterior cruciate ligament reconstruction: an in vivo imaging analysis comparing transtibial and 2-incision tibial tunnel-independent techniques. Am J Sports Med 37(10):1904–1911
Hefzy MS, Grood ES (1986) Sensitivity of insertion locations on length patterns of anterior cruciate ligament fibers. J Biomech Eng 108(1):73–82
Loh JC, Fukuda Y, Tsuda E, Steadman RJ, Fu FH, Woo SL (2003) Knee stability and graft function following anterior cruciate ligament reconstruction: Comparison between 11 o’clock and 10 o’clock femoral tunnel placement. 2002 Richard O’Connor Award paper. Arthroscopy 19(3):297–304
Abebe ES, Utturkar GM, Taylor DC, Spritzer CE, Kim JP, Moorman CT 3rd, Garrett WE, DeFrate LE (2011) The effects of femoral graft placement on in vivo knee kinematics after anterior cruciate ligament reconstruction. J Biomech 44(5):924–929
Brophy RH, Pearle AD (2009) Single-bundle anterior cruciate ligament reconstruction: a comparison of conventional, central, and horizontal single-bundle virtual graft positions. Am J Sports Med 37(7):1317–1323
Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ (2006) Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am 88(8):1826–1834
Pinczewski LA, Salmon LJ, Jackson WF, von Bormann RB, Haslam PG, Tashiro S (2008) Radiological landmarks for placement of the tunnels in single-bundle reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br 90(2):172–179
Zantop T, Diermann N, Schumacher T, Schanz S, Fu FH, Petersen W (2008) Anatomical and nonanatomical double-bundle anterior cruciate ligament reconstruction: importance of femoral tunnel location on knee kinematics. Am J Sports Med 36(4):678–685
Sim JA, Gadikota HR, Li JS, Li G, Gill TJ (2011) Biomechanical evaluation of knee joint laxities and graft forces after anterior cruciate ligament reconstruction by anteromedial portal, outside-in, and transtibial techniques. Am J Sports Med 39(12):2604–2610
Heming JF, Rand J, Steiner ME (2007) Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med 35(10):1708–1715
Piasecki DP, Bach BR Jr, Orias AAE, Verma NN (2011) Anterior cruciate ligament reconstruction: Can anatomic femoral placement be achieved with a transtibial technique? Am J Sports Med 39(6):1306–1315
Conflict of interest
There are no conflicts of interest relevant to the content of this manuscript. Dr. Cole receives royalties from Arthrex and DJ Orthopedics; is a paid consultant for Arthrex, Zimmer, and Regentis; has stock options in Carticept and Regentis; receives support form Elsevier, Lippincott, S&N, and WB Saunders; and receives support from Johnson&Johnson, Medipost, and Zimmer. Dr. Verma receives royalties from Arthrex and DJ Orthopedics; is a paid consultant for Arthrex, Zimmer, and Regentis; has stock options in Carticept and Regentis; receives support form Elsevier, Lippincott, S&N, and WB Saunders; and receives support from Johnson&Johnson, Medipost, and Zimmer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhatia, S., Korth, K., Van Thiel, G.S. et al. Effect of tibial tunnel diameter on femoral tunnel placement in transtibial single bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 24, 51–57 (2016). https://doi.org/10.1007/s00167-014-3307-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-014-3307-8