Abstract
The current review explicitly describes the phosphorus-based flame-retardant (FR) coating materials derived from the bio-resources. It segregates the coatings according to their polymeric backbone type and correlates their structure with the end-application properties. The review will provide a readership to understand different chemistries of FR systems and implement similar in developing the bio-based FR systems for coatings. Furthermore, the review targets to brief the various mechanisms of phosphorus-based FR coating systems depending upon the resin type, such as epoxy, phenolic, polyurethane. The synergistic effects of phosphorus and other FR moieties are also discussed, along with their synthesis chemistries and the structural impact on the properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flame retardants (FRs) are chemicals added or incorporated into plastics, coatings, textiles, etc., to impart flame retardancy.1, 2 In the last couple of decades, the use of polymers in our daily lives has increased significantly. Polymer-based coatings became an indispensable part of many things ranging from furniture, buildings, automobiles, metal constructions, etc., due to their ability to protect metals from corroding, reduce damage due to external forces, and improve the aesthetic appeal. Polymer-based materials, in some instances, have even replaced metals and wood in furniture, construction, etc., owing to their excellent mechanical strength and them being light in weight. However, due to the inherent flammability of these polymeric materials (organic or petroleum-based), there is always a high risk of fire hazards associated with these materials. Thus, there is an ever-increasing demand to reduce the risk of fire hazards and increase the safety of these materials for the consumers. This, in turn, led to the use of FRs in these polymeric materials that act as an efficient means to reduce their flammability and meet the required safety standards.3 FRs become active in the presence of an ignition source. They prevent the growth of fire or inhibit the combustion of polymeric substrates either by forming a protective layer on the surface of the polymeric substrates or by suppressing the chemical reactions that cause the breakdown of the long polymer chains that produce flammable gases and radicals that in turn further aid the breakdown of more polymer chains. However, some FRs have been removed from the market because they do not degrade quickly or they bioaccumulate in environments.4, 5 FR products can be incinerated or landfilled when they are no longer used,6 while they can also be recycled, which increases their application in various areas.
There are several ways to classify flame retardants. Based on the means of incorporation, they can be classified as either additive or reactive. Based on their mode of action, they can be classified as gas phase acting or condensed phase acting. FRs can also be classified based on their chemical composition as halogenated, mineral-based, nitrogen-based, silicon-based, and phosphorus-based; some even show synergistic effects in specific combinations. Brominated and chlorinated flame retardants dominate the halogenated FRs, which act in the vapor or gas phases.7, 8 However, they are now being replaced due to harmful health effects and toxicity of halogenated FRs (especially brominated).9,10,11 Aluminum trihydroxide and magnesium hydroxide are two of the most widely used mineral-based FRs.12, 13 They act by two means (a) the endothermic degradation mechanism utilizes the heat from the fire, thereby reducing the total heat energy of the system, and (b) the dilution of the flammable gas phase aided by the water evolved during the degradation of the metal hydroxides. However, they have a problem of leaching out over time, and thus their effectiveness reduces drastically with time. Nitrogen-based FRs include pure melamine, melamine derivatives, and melamine homologs. They act in the gas phase by diluting flammable gases produced from the degradation of polymeric chains. They also generate less smoke and no corrosive gases when compared to their halogen-based counterparts.14 Phosphorous-based FRs are a class of FRs that predominantly work in the condensed phase by forming a protective char layer over the burning material, thereby inhibiting the pyrolysis reactions that feed the flames with flammable gases and eventually dousing the flames. Nitrogen-based FRs are combined with phosphorus-based FRs due to their thermal stability and synergism, leading to higher flame retardancy and low toxic gas generation.15 Silicon-based FRs, such as polydimethylsiloxane (PDMS), are another type of FRs widely used for their inherent bond strength, flexibility of chains, and high thermal stability.16 Synergistic FRs like the combination of nitrogen and phosphorus-based materials and other such combinations show comparatively lower flame retardancy effects individually but enhance flame retardancy and performance when used in tandem.17,18,19 Depending upon the chemical structures of the FR compound, they follow different mechanisms to prevent the spread of fire and retard it.
However, no matter what type of FR coatings are used for protection against fire accidents, they are always derived by either adding into or modifying a base organic resin with specific chemicals that impart the FR coatings with their unique properties. It is the nature and the source of these base organic resins that is gaining the attention of researchers in recent times. Organic resins commercially available in the market are extracted from petroleum sources. The increasing demand and expectations to meet such rising demands have led to incredible levels of pollution and the destruction of the ecosystem. For this reason, the focus has shifted to finding alternate ways to obtain these base organic resins from environment-friendly sources like plants, trees, and other naturally available materials. Later these bio-based organic resins can be modified chemically to impart FR properties. This review covered the study on various bio-based thermoset resins like epoxy, polyurethane, acrylate, and phenolics. The various environment-friendly raw materials used for their synthesis have been discussed, and the different modifications carried out on them to make them FR have also been mentioned.
Mechanism of FRs
When a combustible material comes in contact with an ignition source, it undergoes either pyrolysis or thermo-oxidative degradation leading to the breakdown of the molecular chain and release of volatile flammable gases and energy into the atmosphere. Thus, the system gets divided into two phases, i) the condensed phase, where the pyrolysis or the degradation reactions continue and generate more fuel for the fire, and ii) the gas phase, where there is a mixture of flammable gases. In the gas phase, these gases then keep mixing with oxygen. After a specific temperature is reached (autoignition temperature), this explosive gas mixture self-ignites with the release of more energy in the form of heat. The material is then said to have caught fire. Various suppression systems, such as chemical agents, inert gases, and liquids, are used to prevent the spread of such fires and reduce the temperature. These systems use wet chemicals (potassium acetate/carbonate/citrate) or dry chemicals (sodium bicarbonate, potassium bicarbonate, monoammonium phosphate) to reduce the spread of fire. Water, carbon dioxide, and dry and wet chemicals are also used in extinguishers. Water and foam suppression systems are widely known for fires involving flammable liquids in tanks or processing areas (underground) due to their high efficiency and low toxicity.20, 21 These suppression systems offer a certain degree of protection in the long run due to their ease of use and ability to detect fires when they are small-scale and react swiftly. However, these systems do not work efficiently when there is any evolution of corrosive gases leading to corrosion of metal substrates or where there is a possibility of a chain reaction leading to the breakdown of substrates and causing large-scale fires. Also, large amounts of chemicals would be required to suppress the flames in scenarios where the fire is difficult to control. Due to these reasons, FRs are increasing rapidly as they provide higher efficiency in comparatively smaller quantities for all types of fires, meeting the flammability standards. The use of FRs is so flexible that they can be added to the materials either during or after the manufacture of the materials. Their efficiency is so high that they can prevent the break out of a fire long enough to provide enough time for evacuation of the people. They even emit less smoke than other suppression systems, which aids the safe evacuation. In smaller fires, they can even protect the substrate from severe fire damage.
There are three essential components for a substrate to ignite and lead to a fire (called a fire triangle)—the fuel source (in this case, long-chain polymers), air (oxygen source), and energy in the form of heat (for the initial ignition and to raise the temperature). These three components form a fire triangle, represented schematically in Fig. 1. When we eliminate any of the three components, we can achieve a certain degree of control over the fire and try to control the situation by avoiding further damage or catastrophe. Thus, by stopping the evolution of flammable vapors, or by quenching the flames with chemical extinguishers (inhibiting), or removing the supply of air (oxygen) to the fire (smothering), we can control fires. After ignition, the flame spreads over the surface, and the penetration of the flames into the bulk of the fuel affects the burning rate. The gas phase flame-retardant mechanism follows the endothermic degradation mechanism, wherein the FR compounds thermally degrade by absorbing the heat from the system, thereby cooling down the system (hindering the pyrolysis reaction) and diluting the explosive gas mixture with nonflammable gaseous by-products (e.g., H2O) formed due to their degradation. For accidental fires or gas leakages, the gas phase radical quenching mechanism works where the FR compounds undergo thermal degradation and react with the radicals in the flame, thereby preventing the radicals from further breaking down the fuel source and inhibiting the propagation of fire. The condensed phase flame-retardant mechanism follows the protective layer formation approach, wherein flame retardants react with a material layer to form a protective char layer over the subsequent material. This protective char layer acts as a shield preventing the oxygen from coming in contact with the material below the char layer, thereby preventing the pyrolysis reaction. This layer also acts as a thermal barrier preventing the further transfer of heat energy that causes the thermal degradation of the material. A thermal shielding mechanism is followed for electrical wires where we observe char formation, forming a barrier-like layer over the fuel source and slowing down the heat transfer to fuel.
Any chemical compound with functionality that reacts with oxygen, curbs the fuel supply, or reduces the heat energy can be regarded as an FR. Compounds that show the presence of atoms such as chlorine, bromine, nitrogen, phosphorous, or silicon, can be considered potential FRs. Compounds that do not exhibit any flame retardancy can be incorporated with these functionalities to synthesize FRs. These FRs can be physically added or chemically bonded to the system via chemical reactions. FRs added physically into the polymeric systems are called additive type, while compounds chemically incorporated into the polymer backbone are called reactive-type FRs. The properties of the overall FR system will be significantly affected by the structures of the FR compounds.
FR systems
Halogen-based FRs and metal hydroxides, as mentioned earlier, though widely used as additives, have many drawbacks like toxicity, negative impact on mechanical property, lack of consistency in performance, and the tendency to leach out. Moreover, their activity is restricted to fire below 500°C.7, 8 Now, reactive FRs can overcome the shortcomings associated with additive-type FRs like leaching out (as they are chemically bonded to the system), high concentration requirements (as they are uniformly distributed throughout the system, and their chemical structures can be tailored), inconsistent performance (as they do not leach out and perform even after the surface layer is consumed as the subsequent layers also possess the same FR compound in their structure), etc. But, as discussed before, all the commercially available additive- and reactive-type FRs are petroleum-based. They either consist of elements like halides, or they thermally degrade to form toxic and nonbio-degradable compounds which cause damage to the environment and health of living beings. Hence, bio-based reactive FR systems are being synthesized predominantly to make a safer process and to negate the shortcomings like toxicity, short-term usage, low activity at low concentration, etc., associated with the current FRs readily available in the markets. Bio-based FRs refer to compounds obtained from natural or biological sources. In theory, bio-based reactive FRs should give us the flexibility to incorporate FR properties into the system without increasing the toxicity of the system while at the same time increasing the bio-degradability of the system. Figure 2 shows the different categories of bio-based FR materials under which naturally available and newly developed materials are classified.
As mentioned earlier, the structure of FR plays a vital role in determining how efficiently it can perform when in action. Bio-based materials are also characterized by their molecular structure and stability for determining their use as FRs. Their structure and functionality variations have led to various FR systems being developed with reduced toxicity and smoke generation. Cyclo aliphatic and aromatic structures like tannic acid, phytic acid, isosorbide, diphenolic acid, lignin, etc., have been studied more enthusiastically for use as FRs.22,23,24 This is due to their higher thermal stability, structural rigidity, and flexibility.25,26,27 Not only this but even deoxyribonucleic acid (DNA) is being studied as a potential FR due to the presence of phosphate-rich backbone and nitrogen-containing bases. Another reason for using bio-based materials for developing FRs is because these bio-based materials tend to produce thermally stable species on decomposition.28, 29 These bio-sourced materials can be modified to FRs by incorporating atoms such as sulfur, boron, nitrogen, silicon, or phosphorous into their structures and have been shown to achieve flame-retardant properties matching those or even surpassing the commercially available FRs. However, phosphorus-based FRs are gaining prominence due to various modifications and high flame retardancy efficiency. Also, phosphorus chemistry belongs to the older, well-established chemistry class, and it is rapidly advancing and developing newer means to improve the safety and sustainability of the chemical processes. The phosphorous-based FRs exhibit great versatility: (1) the structure of the phosphorous-based FRs varies from inorganic to organic; (2) the phosphorous content in these molecules varies as well (e.g., from almost 100% for red P to 14.33% for DOPO); (3) the phosphorous atom has different oxidation states, from 0 to + 5, thus paving the way for the development of different FR mechanisms (both in the gas and condensed phase).30 These variations make phosphorous a unique prospect for the design of flame retardants with tailored properties, such as density or glass transition temperatures (Tgs), by changing the binding pattern (e.g., from alkyl to phenyl groups).30
Phosphorous-based FRs include phosphates, phosphinates, phosphonates, and red phosphorous, an element.31 Halogenated phosphates and phosphinates are also available, but since they have halogens, they also tend to liberate small amounts of toxic gases, though not as much as fully halogen-based FRs.32 Though phosphorous-based FRs predominantly act in the condensed phase to inhibit the flames, they also act in the gas phase, or both phases together, depending on the structure of the phosphorous moiety and the by-products formed at higher temperatures.33, 34 Figure 3 shows phosphorus-based flame retardants' gas and condensed phase mechanism.35 Furthermore, it has been observed that phosphoramidates or compounds with both phosphorous and nitrogen show better flame-retardant properties than compounds with only phosphorous in the system. Also, compared with alkyl phosphates, phosphoramidates offer good thermal stability, are cheap, and are less volatile. Intumescence is another phenomenon observed in phosphorus-based FR systems that improve the overall system's flame retardancy. Such a phenomenon is generally observed in the systems containing a carbon source such as polyalcohols like glucose, starch, pentaerythritol, an acid source such as ammonium phosphates, or a gas source such as melamine or its derivatives. These systems lead to the formation of a foamed char layer which acts as a barrier for heat and mass transfer, hindering oxygen diffusion to the site and lowering the temperature of the substrate.36,37,38,39 Primarily, phosphorous-nitrogen-containing systems are widely used as intumescent flame retardants. The acid and gas sources are created by the thermal degradation of the phosphorous moieties in the flame-retardant system.
Phosphorous-containing FRs can be of the additive and reactive type, which means they can be added physically or incorporated into the polymer chain via a chemical reaction.40, 41 Additive-type FRs are generally physically added to thermoplastic resins before or during processing. On the other hand, reactive-type phosphorus-based FR systems are usually made with thermoset resins such as epoxies, polyurethanes (PU), acrylates, and phenolics.42 The source of these thermoset resins is acquired, making the system bio-based phosphorous-containing FRs. The following subsections discuss various extraction sources of such bio-based resins and their chemistries in detail.
Epoxy-based FR systems
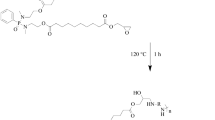
Epoxies are among the most widely used thermoset resins with applications in innumerable fields. They have been widely used as adhesives, coatings, and lamination materials in a wide range of industrial applications because of their excellent dielectric properties, low shrinkage, strong adhesion to both metallic and nonmetallic materials, good stability, high hardness, good flexibility, and excellent stability in many solvents and alkali solutions. However, most commercially available epoxy resins are synthesized from petroleum-based raw materials like bisphenol A, epichlorohydrin, etc.43, 44 This makes them a big contributor to pollution and highly flammable. Therefore, the synthesis of bio-based epoxies with FR properties is rising to lower the dependence on petrochemicals and reduce their harmful environmental impact. Epoxies from bio-based sources such as furan derivatives, rosin acid, itaconic acid, vegetable oil, have been reported earlier.45, 46 Table 1 represents the recent works in bio-based flame-retardant epoxy systems and elucidates the modifications, the improvements observed, and the reasons for improved FR performance. Figures 4a and 4b represent the chemical structures of the bio-based FR epoxy prepolymers and the stand-out properties of the crosslinked FR epoxy matrices. Phosphorous-containing bio-based FR epoxies are usually synthesized by incorporating phosphorus-containing groups into a molecule, followed by epoxidation to include oxirane rings.47 Xu et al. synthesized a phosphorus-based FR similarly using vanillin, wherein they reacted with diamino diphenyl methane (DDM) and diphenyl phosphite to obtain an intermediate which was further epoxidized to form a reactive phosphorus-based FR prepolymer.48 Ammonium polyphosphate (APP), also an environment-friendly FR, was used as an additive to increase the FR property of the overall system and impart intumescent property to the char formed.48 The latter part of this review discusses such systems where additive and reactive FRs are used in combination. Vanillin, combined with amines, diethyl phosphites, and epichlorohydrin, has also been used to obtain FR epoxy resin systems. An epoxy system was also reported to be made using glycidyl ether epoxy isoeugenol resin, derived from isoeugenol—a compound found naturally in the essential oils from plants, cured with camphoric anhydride.49 An intrinsic flame-retardant system was also synthesized successfully using daidzein, a naturally occurring compound found exclusively in soybeans, and this diglycidyl ether of daidzein (DGED) was then further cured with 4, 4-diaminodiphenylmethane (DDM).50
As mentioned earlier, structure and proper distribution play a significant role in the performance of the FR system. A low amount and uniform distribution of the FR helps to increase the flame retardancy without compromising the crosslinking density of resin and, thereby, the mechanical properties. The FR activity in the gas and condensed phases can be manipulated by carefully selecting the exemplary FR compounds and their quantity to be incorporated in the system, thus achieving higher efficiency. Too low an amount of FR compounds is also not recommended as it can reduce the flame-retardant property of the system. These bio-based raw materials like vanillin, cardanol, isoeugenol, etc., provide an excellent opportunity and platform for increasing the thermal stability of the FR system due to their rigid and aromatic structure. The variation in the amounts of certain elements can also affect the ability of an FR system to perform optimally in the gas or condensed phase. In some epoxy systems, it was observed that lowering the APP content reduced the nitrogen content, thereby reducing the ammonia formation in the gas phase, leading to reduced intumescence and lower dilution of the combustible gas phase. The residual content also decreased due to increased polymer degradation into fuel. This also increased heat transfer to the substrate and the subsequent flame propagation. The presence of phosphorous in an FR system contributes to the increased thermal stability of the system as well.46, 51 This is due to the phosphonate group promoting higher char formation, thereby protecting the layer below the charred layer from coming in contact with heat energy and gases. It was also observed that the rigidity of the aromatic rings and N–H intramolecular hydrogen bonding also improves the system's thermal stability and tensile strength of the polymer system.52 However, too many aromatic and bulky structures are also not favorable. A lower reactivity during the curing step and steric hindrance caused due to bulky phosphate groups affect the crosslinking density and the crosslinked system's glass transition temperature (Tg).49
Cardanol is one of the most widely tested bio-based phenols for epoxy resin synthesis. It has been reported to undergo dehydrochlorination, epoxidation, and ring-opening reaction to obtain phosphaphenanthrene groups containing triscardanyl phosphate (PTCP), which are further used as an FR in epoxy resin.53, 54 However, in this system, crosslinking is observed to be lower than that observed for the commercial epoxy system. This is due to bulky groups such as that of PTCP. But these phosphorus-based moieties promote high char formation. The high compatibility of PTCP with epoxy resin also increases tensile strength and improves the overall toughness of the system. From this, one can understand that one must carefully justify the chemistry of the compounds used in an FR system to obtain the perfect balance of properties needed for the suitable application. It is also observed that even the long alkyl chains of cardanol lead to toughening effect in the FR system.53, 54 A combination of cardanol and eugenol-based epoxy monomers has also been used as FRs.55 The flexibility to manipulate the toughness and rigidity based on the amounts of cardanol and eugenol in the system makes this system a perfect option for different application scenarios. Highly functional bio-based epoxy resin also impacts the crosslinked structure's overall property. The core polyols' structure and degree of substitution cause differences in properties among polyol esters. For example, unreacted hydroxyls can promote hydrogen bonding.56 The hydroxyl groups in polyols usually contribute to the network formation and enhance mechanical properties, thereby imparting rigidity to the structure.56 Castor oil-based epoxies have also been synthesized and are reported to be further used as a curing agent for forming FR systems.55 The castor oil-based curing agent showed that long chains of ricinoleic acid reduced the Tg and altered the mechanical properties.57 In some cases, the Tg decreases by increasing the phosphorous content, decreasing the crosslinking density. This can be attributed to the steric hindrance of the phosphorous moiety involved in the synthesis of the FR system.58, 59 On the contrary, enhancement in properties is sometimes observed due to specific compounds in the FR system. For example, improvement in thermomechanical property was observed in the epoxy obtained from diglycidyl ether of daidzein (DGED) compared to the epoxy from DGEBA because of its higher crosslink density due to the structure of diglycidyl ether of daidzein (DGED), which showcased the dimerization of the benzopyrone ring.50 The unsaturation in DGED provided additional crosslinking points, and the rigidity of molecular segments and density further contributed to improved mechanical properties.
As discussed earlier, the phosphorus-containing groups (P–O–C and P=O) work in the gas and condensed phases by char promotion.60 In addition, the aromaticity and functionality of monomers, their ability to crosslink, the phosphorous content of the monomers, etc., also influence the thermal stability, char formation ability, and the glass transition temperature (Tg) of the polymer system.26 However, it is to be noted that incomplete combustions caused by burning polycyclic aromatic hydrocarbons lead to smoke generation. The presence of only the gas phase mechanism for flame retardation lowers the total char yield % of the system as there is no promotion for char formation in the condensed phase.61 As mentioned before, bulky groups may cause hindrance in proper packing of the polymer chains while curing, which may lead to lower energy requirements to break the interchain linkage. This eventually leads to the reduced thermal stability of the system. The crosslinking density also gets affected, resulting in lower mechanical properties and flame-retardant system's flame retardant.62 Thus it is always essential to study the structure of the bio-based raw material before its application in FR systems.
Polyurethane (PU)-based FR systems
PUs are organic polymers in which the urethane group is the primary repeating unit. These repeating units are produced by the reaction between polyol (–OH) and diisocyanate (–NCO). However, PUs also contain other functional groups in their structure, such as ethers, esters, urea, and aromatic compounds.63, 64 Among organic coatings, PU coatings have excellent abrasion resistance, toughness, low-temperature flexibility, corrosion resistance, and chemical resistance, and thus are used in a wide range of applications from automobile finishing to industrial maintenance to chemical resistance.65 However, PUs also suffer from flammability issues due to their organic nature and VOC released during curing.63, 64 Thus, to avoid these drawbacks associated with PUs, many efforts are being put into synthesizing them using bio-based materials and imparting them with flame retardancy. Various routes to the synthesis of bio-based PUs are known to us.66 Table 2 includes materials used for developing FR-PU systems and their property enhancements compared to commercially available PUs. Figure 5 summarizes the structural representations of various reaction routes through which FR properties can be incorporated in PU. Lignin, one of the most widely available bio-based materials, has been used to make FRs by reacting 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) and hexamethylene diisocyanate (HDI).67 Cardanol is another bio-based material used to make FR-PU coating with exceptional flame retardancy performance by reaction with polyphosphonic dichloride (PPDC) and dichlorodimethylsilane (DCDMS).68 Gallic acid-based phosphorous-containing FRs have also been developed and used as an additive in commercial PUs.69 Gallic acid in another scheme was acetylated and then reacted with POCl3, followed by hydrolysis using sodium hydroxide. This product was then used as a crosslinking agent for PU coating.69 The effect of intumescence in PUs based on linseed oil-phosphate-ester-polyol has been studied where melamine, expandable graphite, and APP were used as intumescent FRs. The melamine synergized with phosphate groups, while expandable graphite proved to be the most effective additive.70 The concept of synergism is discussed later in some detail.
A recurring issue that has been seen is that incorporating different moieties into the polymer leads to reduced crosslinking. However, this issue has been resolved using highly functional bio-based compounds. For example, in lignin-based PU systems mentioned before, the incorporation of lignin provided improved crosslinking density due to its high functionality and biodegradability due to its inherent bio-degradable nature. It increased ultraviolet (UV) stability and antioxidant properties due to its natural UV protection.67 Also, it was observed that, as the FR content increased, the char residues also increased due to the char-promoting phosphate compounds being produced during degradation. However, the crosslinking did not reduce with increased phosphate content due to the phosphate groups. This was because the hydroxyl groups present in PU structures were found to improve the adhesion to substrates and the crosslinking owing to the hydrogen bonding.67 Using lignin also enhanced the system's mechanical properties and decomposition temperature. It also generated stable char due to its aromatic structure.67, 71,72,73 The synergistic effects between phosphorous and nitrogen have been used in some PU systems, leading to improved flammability and higher char formation.64, 75 In another synergistic approach, silicon has been used to promote char formation in the condensed phase along with phosphorous. It has also been observed that Si affects the crosslinking density of the film. Also, as the crosslinking density increased with silicon content, water absorption has been found to decrease due to the water repellency of the film.68 In bio-based FR systems, the aromaticity significantly affects the coating's crosslinking density and thermal stability. Modification beyond a certain point without considering the accumulated impacts of conversion affects the crosslinked structure's properties. For example, the bulky structures like long side chains and aromatic groups in the side chains in quite a few instances have caused improper packing and increased the permeability of the crosslinked system due to an increase in the number of voids. Thus, the study of chemical structure and identification of balance between aromaticity and aliphatic is essential in determining bio-based materials' applicability in synthesizing bio-based FR systems.
In the case of bio-based PU systems, phosphorous compounds have shown a tendency to release phosphoric acid and water at higher temperatures providing a cooling effect. The presence of phosphorous in these bio-based PU systems has also led to forming a protective char layer on the substrate when in contact with flames.67, 68 The presence of polar groups has improved the adhesion to substrate via the secondary forces of attraction.69 In some PU systems, melamine and APP have decreased the smoke formation due to their ability to work in the gas phase of flame retardation by releasing ammonia gas and radical quenching. In some cases, graphite has led to increased char formation due to its ability to withstand very high temperatures.70 Various additives such as feldspar, kaolinite, calcium carbonate (nanosized), zirconia, alumina, and expandable graphite, have also been tested as potential additives for castor oil-based and rosin-based PU foams.76,77,78
Acrylate-based FR systems
Acrylates are high-value daily-life polymers commonly prepared from chemicals like acrylic acid (AA), methacrylic acid (MAA), and their corresponding esters using nonsustainable strategies like propylene oxidation and the acetone cyanohydrin process (ACH).79,80,81 However, due to the current nonsustainable pathways used to produce (meth)acrylate monomers and the resulting polymers, this industry largely contributes to the growing greenhouse gas emissions and fossil resource depletion.82 Due to these reasons, the focus has shifted to producing acrylates using bio-based materials. However, due to the flammable nature of these materials and their intensive use in interior coatings, it was necessary to incorporate FR property into them. Table 3 expresses the raw materials used, the comparative performance of flame-retardant and nonflame-retardant acrylate systems, and the reasons for their marked improvements. Figure 6 represents the different acrylate flame-retardant monomers and their stand-out properties. Due to phosphorous’ excellent flame-retardant properties and its compatibility with acrylates, phosphorus-containing bio-based acrylates are being designed and synthesized in good numbers. Bio-based compounds like cardanol have recently gained widespread importance in synthesizing bio-based phosphorous-containing flame-retardant acrylates due to their aromatic content, which provides thermal stability and aliphatic content that regulates the necessary flexibility and toughness of the final film. A cardanol-based FR system was developed by epoxidizing the cardanol, followed by the acrylation using HEMA. Lastly, the phosphorylation of this cardanol-based intermediate using diphenyl phosphoryl chloride.83, 84 This acrylated cardanol diphenyl phosphate (ACP) was used as a reactive diluent with a urethane di-acrylate (UA) oligomer. Oligomers from jatropha oil, due to their long aliphatic chains providing the required flexibility, have also been reported wherein jatropha oil was epoxidized and then acrylated using acrylic acid.85, 86 Isosorbide obtained from starch has also been tested for its use as a potential material for use in making acrylate-based FR systems. In one such case, isosorbide was converted into acrylate ester by a reaction between isosorbide and poly(styrene-acrylate) co-polymer and then phosphite incorporation by Michael addition to generating FR compounds.87,88,89 These compounds were then incorporated into polymers to impart flame-retardant characteristics. Acrylic acid made from bio-based resources has also been converted to hydroxyethyl acrylate (HEA) in the past.80 HEA was phosphorylated with phosphoryl chloride, and Michael's addition with DOPO gave acrylated FR monomers.80
In the case of bio-based acrylic systems, crosslinking density has improved with the incorporation of the aromatic rigid structure due to reduced chain mobility. The double-bond utilization during the curing process further added to the improvement of crosslinking density. In turn, these all cause the system's Tg to increase. Aromatic compounds add to the system's higher thermal stability and char-forming ability, thus providing improved flame retardancy via forming a better protective layer over the substrate. The generation of outer dense and compact char layers formed by decomposition products in some acrylated systems provided a better barrier to flame, with the inner char layer of holes (honeycomb structure) providing heat insulation.90 The system's viscosity also gets affected due to the high crosslinking density found in some acrylates, which affects the diffusion of molecules, thereby adding to improved flame retardancy due to lower oxygen diffusion.91 As observed earlier in other systems, even in acrylate systems, the long aliphatic chains like in cardanol improve the elongation of the final crosslinked structure.84
The adhesive property is sometimes enhanced due to increased hydroxyl functionality. For example, Jatropha-based oligomers have shown good adhesive properties due to the epoxy groups that form hydroxyl groups on opening, which get replaced by acrylic acid, thereby giving good adhesion with low viscosity.85, 86 Level of oxygenation of phosphorous in acrylates has been found to affect the mode of action of phosphorous compounds. The compounds with a higher degree of oxygen are active in the solid-state mechanism, while those with a lower degree are engaged in the gas phase.88 Influential FRs in the case of acrylates are significantly active in the gas phase.88 Phosphate esters release phosphorous-based free radicals into the gas phase to provide flame retardancy by curbing the free radicals that are otherwise responsible for polymer chain breakdown generating more fuel for the fire.92 FRs and their interaction with the polymer matrix also significantly affect the behavior of the flames and their outcomes, as this interaction determines the activation of the FR compounds at elevated temperatures. Also, the phosphorous concentration, type of phosphorous groups, and the polymer matrix affect the release rate of phosphorous compounds during burning.
Phenolic FR systems
Phenolic resin synthesized from phenol and formaldehyde has been part of the resin industry for over a century after its first development. Phenolic resins possess versatile properties like excellent mechanical properties, flame retardancy, flexibility, low cost, high thermal stability, and water and chemical resistance. This versatility in properties and performance made it useful in various applications. It made it the material of choice in the aerospace industry, as adhesives in particleboard manufacturing units, paints and coatings, insulating foams, and the electrical and lighting industry.93,94,95 Phenolic resins contain an ether (–O–) and a methylene (–CH2–) bridge connecting the aromatic rings. These molecules in the phenolic resin structure make them susceptible to decomposition at higher temperatures and lead to the emission of volatile hydrocarbon compounds. This significantly affects the phenolic system's thermal stability and flame retardancy properties. It also involves using phenolic resins in areas exposed to prolonged high temperatures. However, phenolic resins have one characteristic which can make them suitable for high-temperature operation, albeit, after necessary modifications, they exhibit good charring performance.96,97,98 This, coupled with phosphorous compounds in their matrix, can lead to excellent high-performing FR systems. The flame retardancy and high-temperature performances of some phenolic resins with and without the flame retardants incorporated in them are expressed in Table 4.
The reasons for their marked performance improvement are explained as well. Figure 7 represents some of the phosphorous-containing bio-based phenolic prepolymers and the stand-out properties of their crosslinked structures. From the table, we can also conclude that generally, phosphorous-containing phenolics show lower thermal stability when compared to nonphosphorous systems. This is due to the lower thermal strength of the P–C bond in the phosphorous moieties in the system, which in turn help to promote char formation, thereby improving the flame retardancy from a lower temperature. This is also evident from the higher char yield in the modified resin compared to the unmodified systems.
One of the bio-based compounds, tannin, has been identified as a potential replacement for phenols in phenolic resins due to the many phenolic rings in their structure. It has been modified in the past by acetylation, hydrolysis, condensation, polymerization, etc., and further reacted with other compounds to form FR phenolic resins. The extract from green tea was another phenolic compound, which can be epoxidized using epichlorohydrin.99 Phenolic prepolymers were synthesized using green tea tannic acid that was further employed to synthesize bio-based epoxy resins. Phosphorous may also be incorporated into these by using the free hydroxyl functional groups in their structure. Benzoxazines are another class of phenols that can be synthesized using a phenol, an amine, and formaldehyde. Benzoxazines synthesized from bio-based phenols like eugenol, cardanol, guaiacol, rosin, and vanillin have been recorded in the past.100,101,102 Traditionally, bio-based benzoxazines have low thermal stability, crosslinking density, and char yield.100 Due to this reason, many researchers have shown interest in the modification of bio-based benzoxazine to improve its properties. Diphenolic acid obtained from levulinic acid is another bio-based raw material with great potential for making phenolic resins. Diphenolic acid and phenyl phosphonic dichloride have been combined to obtain phosphorous-containing oligomer with flame-retardant properties.103, 104 They have been used with APP and EG and act as a carbon and acid source in the system, increasing the char yield and improving the FR performance in the condensed phase.103, 104 Nanocoatings based on phenolic systems have also been employed to improve the FR performance in some cases, like the poly(diphenolic acid-phenyl phosphate) (poly[-DPA-PDCP]), and polyethylenimine (PEI) on ramie fabrics.105, 106 The presence of Si on the ramie fabrics reduced its flammability. The intumescent char layer considerably increased flame retardancy by preventing the matrix's heat transfer and thermal degradation. The nanocoating applied generated phosphoric acid on decomposition, which played an essential role in catalyzed dehydration of cellulose to initiate char formation.106
Other FR systems
There are a few instances where it is not economically wise to utilize reactive-type FRs for getting the desired FR effects. Sometimes, a large-scale fire outbreak risk is not high, or high performance is not expected. So, during such instances, a combination of reactive-type and additive-type FR can be employed to achieve the desired flame-retardant properties. In some intumescent phosphorous and nitrogen-based FR systems, titanium oxide (TiO2) is used as an FR additive. Generally, TiO2 is used as a pigment, but TiO2 is helpful as a critical reactant in many phosphorus-based FR systems. When exposed to flames, titanium and phosphorus-based FR systems form an intumescent coating that, when fully foamed, shows a white exterior. At the same time, the inner part near the substrate remains dark due to the carbonized material. The reaction between APP and TiO2 leads to a white-colored substance called titanium pyrophosphate. This white layer can reflect heat waves and reduce energy flow to the unreacted layers below. It has also been proposed that the reaction of P2O5 and TiO2 leads to the formation of titanium pyrophosphate.107 Thus, the desired flame-retardant property is obtained without using a complete reactive-type FR system but a combination of reactive and additive-type FR system, which is relatively cheaper. Another example is a complex epoxy mastic containing boric acid, APP, a triaryl phosphate, tris (2-hydroxyethyl) isocyanurate (THEIC), silica, perlite, and ceramic fibers that have been developed and commercialized as a coating for the protection of steel from hydrocarbon fires. Using boric acid in phosphorus-based FR systems has improved thermal and mechanical resistance and adhesion.107, 108 The boron in the system forms a glassy layer that blocks oxygen supply to the layer below, thereby curbing the flames. The use of multilayer coatings such as one intumescent layer above the other has also been deployed wherein the outer layer provides immediate fire protection. In case of a breakthrough of the outer layer, the inner layer acts as a second layer of defense.107
Synergistic effects
In all the types of phosphorus-based FR systems discussed so far, we have observed that along with phosphorous, different elements like nitrogen, sulfur, silicon, etc., and additives like boric acid, TiO2, etc., have also been part of the FR systems. The presence of these elements/compounds has proven the improvements in the overall FR properties of the system. This marked improvement in the FR performance falls in the category of synergistic effects. At the surface level, synergism might appear to be just the result of a favorable combination of different FRs or elements. However, this is far from what synergism is. We cannot say synergistic effects have been achieved because different compounds produced a favorable result when added together. Synergism involves different FR effects like quenching, cooling, dilution, barrier formation, oxygen inhibition, etc., working in unison to produce results that far surpass the results obtained when different FR works individually. For example, in a phosphorous-nitrogen-based FR system, phosphorous compounds act in the condensed phase by promoting char formation and forming a protective layer that inhibits heat and oxygen flow to the system underneath, while phosphorous and nitrogen act in the gas phase. Nitrogen releases gases such as ammonia, nitrogen dioxide, and nitrogen oxide, that dilute the flammable gas mixture. On the other hand, the phosphorous compound releases charged radicals which kill the free radicals present in the gas phase that otherwise contribute to the degradation of the polymeric system, forming more fuel for the flames. This system shows that not just the mixing of compounds leads them to exhibit synergism. Hence, the FR system can utilize multiple FR effects to produce exceptional flame retardancy results, and synergism does not require mixing different compounds. An FR system containing only phosphorous can show synergism as the phosphorous moieties formed on thermal decomposition work in the gas and condensed phase together to curb the flames. Not only that, there are other compounds too that show such results. The inherent tendency of Si–O-Si bonds to provide thermal stability and fire retardancy is also well known and documented. Phosphinamides, phosphoramides, phosphoramidates, phosphorodiamidates, phosphoramidites, etc., are some widely used phosphorous and nitrogen-based compounds for synergistic effects in FRs.109 Synergism between boron, nitrogen, and phosphorous has also been observed in the past, wherein boron moieties form an impermeable glass-like layer over the substrate in the condensed phase.110 Table 5 shows some FR systems in addition to the others mentioned earlier in other tables of epoxy, PU, and polylactic acid-based systems where synergistic effects have been observed. This table further substantiates the fact that synergism helps to improve the flame-retardant property of a system when used correctly. Random mixing of FR compounds does not produce synergism. The potential fire risks must be understood before finalizing the FR system to be adopted. The fire type (size, speed of spread, etc.), the structure to be protected (material study), the different types of objects that will be present in the location (potential fuel source for flames), etc., need to be studied to identify the correct mixture of FR compounds which is to be utilized to obtain the synergistic effect. This is because using synergistic effects in an FR system helps reduce the total FR content of the system, thereby providing the opportunity for saving money and limiting the incorporation of compounds that are not part of the polymeric system.
Of all the synergisms we have come across, phosphorous-nitrogen synergism has been one of the most widely used. Phosphorous and nitrogen-based FR systems show improved FR properties, higher thermal stability, and increased crosslinking density. As discussed before, the flame retardancy of the overall system depends on the types of synergistic flame retardants used. For example, using DOPO, DEA, and diphenolic acid, an FR epoxy system was synthesized. The phosphorous groups improved the char-forming ability and limited the release of combustible gases, while the nitrogen-containing groups increased the number of nonflammable gases and diluted the concentration of combustible gases.111, 112 In the past, castor oil-based polyols containing phosphorous and nitrogen have been synthesized. The flame retardancy tests of these systems showed that the higher char content was attributed to the system's synergistic effects of phosphorus and nitrogen moieties.74, 75, 113
In one of the studies, FR-PU prepolymer containing phosphorous and nitrogen synthesized from diethanolamine, formaldehyde, diethyl phosphite, and methylene diisocyanate was used as a curing agent and reactive-type FR for PU sealants. The increased char formation in the system was mainly due to the P-N synergism. In some cases, it has been observed that the formation of polyphosphate and phosphoric derivatives promotes char formation, with nitrogen acting as a synergistic agent.75 As curing agents, PU sealants have also been reported with ricinoleic acid-based polyols and methyl diphenyl diisocyanate. Flame retardancy in those systems was due to the phosphorous and nitrogen acting synergistically and working together in the gas and condensed phases simultaneously. Limiting oxygen index (LOI) and cone calorimetry test results showed the synergistic effect observed in nitrogen and phosphorous.74 FR polyols from castor oil used for PU foams were studied, where the flame retardancy and increased crosslinking density due to the FR compounds in the system were functioning in tandem via different FR effects to curb the fire.114, 115 Flame-retardant polylactic acid (PLA) composites have also been synthesized for packaging, coating, and textile applications by incorporating phosphorous and silicone-based filler into the PLA matrix.116 The filler compound showed synergistic effects in the gas and condensed phase to impart FR property to the PLA composite.
Conclusions
FRs are gradually becoming integral to fire safety systems due to their efficiency and dependability. Their ability to provide additional time for escape in times of a fire outbreak has proven to be a boon for millions around the globe. Various phosphorous-based FRs are widely gaining prominence and are used in different coating systems to meet flammability standards. Even though halogenated FRs are cheap, these systems release toxic gases and cause harm to the environment. Hence, it is inevitable that alternatives to the halogenated FR systems will gain prominence shortly. Because of their promising and dependable results, other FR systems based on phosphorous, nitrogen, silicon, etc., will soon take over the commercial sector. Also, bio-based resources have been gaining attention due to the depleting petroleum reserves and increasing concern for the environment and sustainability. The age of petro-chemicals is soon ending, but society's needs must be met. With the knowledge that we have accumulated, we can create fire-safe systems by using resources that are available in nature. Also, with the performance of phosphorus-based FR systems, it is impossible to overlook them. With continuous research and development, the future is only bright for P-based FR systems. Also, synergistic effects of nitrogen, silicon, boron, sulfur when used, and phosphorous are a bonus that favors P-based FR systems. Recent developments and advances in nanotechnology can excite biobased phosphorus-containing FR systems.
References
Betts, KS, “New Thinking on Flame Retardants.” Environ. Health Perspect. https://doi.org/10.1289/ehp.116-a210 (2008)
Beard, A, Angeler, D, “Flame Retardants: Chemistry, Applications, and Environmental Impacts.” Handb. Combust., 1 415–440. https://doi.org/10.1002/9783527628148.hoc017 (2010)
Dasari, A, Yu, Z, Cai, G, Mai, Y, “Progress in Polymer Science Recent Developments in the Fire Retardancy of Polymeric Materials.” Prog. Polym. Sci., 38 1357–1387. https://doi.org/10.1016/j.progpolymsci.2013.06.006 (2013)
Speight, JG, “Sources and Types of Organic Pollutants.” In: Environmental Organic Chemistry for Engineers, pp. 153–201, 1st edn. Butterworth-Heinemann, Oxford (2017)
Purser, DA, “Fire Safety Performance of Flame Retardants Compared with Toxic and Environmental Hazards.” In: Polymer Green Flame Retardants, pp. 45–86. Elsevier (2014). https://doi.org/10.1016/C2010-0-66406-6
Li, J, Zeng, X, “Recycling Printed Circuit Boards.” In: Waste Electrical and Electronic Equipment (WEEE) Handbook, 2nd edn., pp. 287–311. Woodhead Publishing, Cambridge (2012)
Georgette, P, “Applications of Halogen Flame Retardants.” In: Fire Retardant Materials, pp. 264–292, 1st edn. Woodhead Publishing, Cambridge (2001)
Shaw, SD, Blum, A, Weber, R, “Halogenated Flame Retardants: Do the Fire Safety Benefits Justify the Risks?” Rev. Environ. Health, 25 261–305. https://doi.org/10.1515/REVEH.2010.25.4.261 (2010)
De Wit, CA, “An Overview of Brominated Flame Retardants in the Environment.” Chemosphere, 46 583–624. https://doi.org/10.1016/S0045-6535(01)00225-9 (2002)
Darnerud, PO, “Toxic Effects of Brominated Flame Retardants in Man and in Wildlife.” Environ. Int., 29 841–853. https://doi.org/10.1016/S0160-4120(03)00107-7 (2003)
Birnbaum, LS, Staskal, DF, “Brominated Flame Retardants: Cause for Concern?” Environ. Health Perspect., 112 9–17. https://doi.org/10.1289/ehp.6559 (2004)
Hull, TR, Witkowski, A, Hollingbery, L, “Fire Retardant Action of Mineral Fillers.” Polym. Degrad. Stab., 96 1462–1469. https://doi.org/10.1016/j.polymdegradstab.2011.05.006 (2011)
Rothon, R, Fillers for Polymer Applications. Springer, New York. https://doi.org/10.1007/978-3-319-28117-9 (2017)
Horacek, H, Grabner, R, "Advantages of Flame Retardants Based on Nitrogen Compounds." 3910 (1996)
Vothi, H, Nguyen, C, Pham, LH, “Novel Nitrogen-Phosphorus Flame Retardant Based on Phosphonamidate: Thermal Stability and Flame Retardancy.” ACS Omega, 4 17791–17797. https://doi.org/10.1021/acsomega.9b02371 (2019)
Ganachaud, F, Hamdani, S, Longuet, C, Perrin, D, “Flame Retardancy of Silicone Based Materials.” Polym. Degrad. Stab., 94 465–495. https://doi.org/10.1016/j.polymdegradstab.2008.11.019 (2009)
Ai, L, Chen, S, Zeng, J, “Synergistic Flame Retardant Effect of an Intumescent Flame Retardant Containing Boron and Magnesium Hydroxide.” ACS Omega, 4 3314–3321. https://doi.org/10.1021/acsomega.8b03333 (2019)
He, W, Yuan, Z, Liu, Y, et al. “Synergistic Flame Retardancy of Aluminium Dipropyl-Phosphinate and Melamine Cyanurate in Polyamide-6.” Asian J. Chem., 26 8319–8324. https://doi.org/10.14233/ajchem.2014.16917 (2014)
Xu, ZS, Yan, L, Chen, L, “Synergistic Flame Retardant Effects between Aluminium Hydroxide and Halogen-free Flame Retardants in High Density Polyethylene Composites.” Procedia Eng., 135 631–636. https://doi.org/10.1016/j.proeng.2016.01.130 (2016)
Nolan, DP, “Methods of Fire Suppression.” In: Andrew, William (ed.) Handbook of Fire and Explosion Protection Engineering Principles for Oil, Gas, Chemical and Related Facilities, pp. 211–241, 2nd edn. Elsevier, Oxford (2011)
Ruff, GA, Urban, DL, Pedley, MD, Johnson, PT, Fire Safety Journal, 1st edn. Elsevier, Amsterdam (2009)
Kim, YO, Cho, J, Yeo, H, “Flame Retardant Epoxy Derived from Tannic Acid as Biobased Hardener.” ACS Sustain. Chem. Eng., 7 3858–3865. https://doi.org/10.1021/acssuschemeng.8b04851 (2019)
Gao, YY, Deng, C, Du, YY, “A Novel Bio-based Flame Retardant for Polypropylene from Phytic Acid.” Polym. Degrad. Stab., 161 298–308. https://doi.org/10.1016/j.polymdegradstab.2019.02.005 (2019)
Yang, H, Yu, B, Xu, X, “Lignin-Derived Bio-based Flame Retardants Toward High Performance Sustainable Polymeric Materials.” Green Chem., 22 2129–2161. https://doi.org/10.1039/d0gc00449a (2020)
Hobbs, CE, “Recent Advances in Bio-based Flame Retardant Additives for Synthetic Polymeric Materials.” Polymers (Basel), 11 224. https://doi.org/10.3390/polym11020224 (2019)
Ménard, R, Negrell, C, Fache, M, “From a Bio-based Phosphorus-Containing Epoxy Monomer to Fully Bio-based Flame-Retardant Thermosets.” RSC Adv., 5 70856–70867. https://doi.org/10.1039/c5ra12859e (2015)
Costes, L, Laoutid, F, Brohez, S, Dubois, P, “Bio-based Flame Retardants: When Nature Meets Fire Protection.” Mater. Sci. Eng. R Rep., 117 1–25. https://doi.org/10.1016/j.mser.2017.04.001 (2017)
Alongi, J, Carletto, RA, Di Blasio, A, “DNA: A Novel, Green, Natural Flame Retardant and Suppressant for Cotton.” J. Mater. Chem. A, 1 4779–4785. https://doi.org/10.1039/c3ta00107e (2013)
Li, YC, Yang, YH, Kim, YS, “DNA-Based Nanocomposite Biocoatings for Fire Retarding Polyurethane Foam.” Green Mater., 2 144–152. https://doi.org/10.1680/gmat.14.00003 (2014)
Velencoso, MM, Battig, A, Markwart, JC, Schartel, B, Wurm, FR, “Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy.” Angew. Chem. Int. Ed., 57 10450–10467. https://doi.org/10.1002/anie.201711735 (2018)
Van der Veen, I, de Boer, J, “Phosphorus Flame Retardants: Properties, Production, Environmental Occurrence, Toxicity and Analysis.” Chemosphere, 88 1119–1153. https://doi.org/10.1016/j.chemosphere.2012.03.067 (2012)
Wei, ED, “Phosphorus-Based Flame Retardants.” In: Lewin, M, Atlas, SM, Pearce, EM (eds.) Flame—Retardant Polymeric Materials. Springer, Boston. https://doi.org/10.1007/978-1-4684-6973-8_4 (1978)
Salmeia, KA, Jovic, M, Ragaisiene, A, “Flammability of Cellulose-Based Fibers and the Effect of Structure of Phosphorus Compounds on Their Flame Retardancy.” Polymers (Basel), 8 293. https://doi.org/10.3390/polym8080293 (2016)
Gaan, S, Mauclaire, L, Rupper, P, “Thermal Degradation of Cellulose Acetate in Presence of Bis-Phosphoramidates.” J. Anal. Appl. Pyrolysis, 90 33–41. https://doi.org/10.1016/j.jaap.2101.10.005 (2011)
Schartel, B, “Phosphorus-Based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development?” Materials, 3 4710–4745. https://doi.org/10.3390/ma3104710 (2010)
Hörold, S, “Phosphorus-based and Intumescent Flame Retardants.” In: Polymer Green Flame Retardants, pp. 221–254 (2014)
Hao, J, Chow, WK, “A Brief Review of Intumescent Fire Retardant Coatings.” Archit. Sci. Rev., 46 89–95. https://doi.org/10.1080/00038628.2003.9696967 (2003)
Mariappan, T, “Recent Developments of Intumescent Fire Protection Coatings for Structural Steel: A Review.” J. Fire Sci., 34 120–163. https://doi.org/10.1177/0734904115626720 (2016)
Levchik, SV, Levchik, GF, Camino, G, Costa, L, “Mechanism of Action of Phosphorus-Based Flame Retardants in Nylon 6 II. Ammonium Polyphosphate/Talc.” J. Fire Sci., 13 43–58. https://doi.org/10.1177/073490419501300103 (1995)
Hörold, S, “Phosphorus Flame Retardants in Thermoset Resins.” Polym. Degrad. Stab., 64 427–431. https://doi.org/10.1016/S0141-3910(98)00163-3 (1999)
Szolnoki, B, Toldy, A, Konrád, P, Szebényi, G, Marosi, G, “Comparison of Additive and Reactive Phosphorus-Based Flame Retardants in Epoxy Resins.” Period. Polytech. Chem. Eng., 57 85–91. https://doi.org/10.3311/PPch.2175 (2013)
Levchik, SV, Weil, ED, “A Review of Recent Progress in Phosphorus-Based Flame Retardants.” J. Fire Sci., 24 345–364. https://doi.org/10.1177/0734904106068426 (2006)
Edward, MP, Epoxy Adhesive Formulations. McGraw Hill, New York (2005)
Liu, JQ, Bai, C, Jia, DD, Liu, WL, He, FY, Liu, QZ, Wu, YZ, RSC Adv., 4 8025–18032 (2014)
Kumar, S, Samal, SK, Mohanty, S, Nayak, SK, “Recent Development of Biobased Epoxy Resins: A Review.” Polym. Plast. Technol. Eng., 57 133–155. https://doi.org/10.1080/03602559.2016.1253742 (2016)
Zhang, J, Mi, X, Chen, S, “A Bio-Based Hyperbranched Flame Retardant for Epoxy Resins.” Chem. Eng. J., 381 122719. https://doi.org/10.1016/j.cej.2019.122719 (2020)
Sag, J, Goedderz, D, Kukla, P, “Phosphorus-Containing Flame Retardants from Biobased Chemicals and Their Application in Polyesters and Epoxy Resins.” Molecules, 24 376. https://doi.org/10.3390/molecules24203746 (2019)
Xu, X, Wang, S, Ma, S, “Vanillin-Derived Phosphorus-Containing Compounds and Ammonium Polyphosphate as Green Fire-Resistant Systems for Epoxy Resins with Balanced Properties.” Polym. Adv. Technol., 30 264–278. https://doi.org/10.1002/pat.4461 (2019)
Pourchet, S, Sonnier, R, Ben-Abdelkader, M, “New Reactive Isoeugenol Based Phosphate Flame Retardant: Toward Green Epoxy Resins.” ACS Sustain. Chem. Eng., 7 14074–14088. https://doi.org/10.1021/acssuschemeng.9b02629 (2019)
Dai, J, Peng, Y, Teng, N, “High-Performing and Fire-Resistant Biobased Epoxy Resin from Renewable Sources.” ACS Sustain. Chem. Eng., 6 7589–7599. https://doi.org/10.1021/acssuschemeng.8b00439 (2018)
Xu, B, Wu, X, Qian, L, “Intumescent Flame-Retardant Poly(1, 4-butylene terephthalate) with Ammonium Polyphosphate and a Hyperbranched Triazine Charring-Foaming Agent: Flame Retardancy Performance and Mechanisms.” J. Fire Sci., 35 317–340. https://doi.org/10.1177/0734904117708529 (2017)
Wang, S, Xu, C, Liu, Y, "Vanillin-Derived High-Performance Flame Retardant Epoxy Resins: Facile Synthesis and Properties." https://doi.org/10.1021/acs.macromol.7b00097 (2017)
Wang, X, Zhou, S, Guo, WW, “Renewable Cardanol-Based Phosphate as a Flame Retardant Toughening Agent for Epoxy Resins.” ACS Sustain. Chem. Eng., 5 3409–3416. https://doi.org/10.1021/acssuschemeng.7b00062 (2017)
Chen, J, Li, X, Wang, Y, “Synthesis and Application of a Novel Environmental Plasticizer Based on Cardanol for Poly(vinyl chloride).” J. Taiwan Inst. Chem. Eng., 65 488–497. https://doi.org/10.1016/j.jtice.2016.05.025 (2016)
Ecochard, Y, Decostanzi, M, Negrell, C, “Cardanol and Eugenol-Based Flame Retardant Epoxy Monomers for Thermostable Networks.” Molecules, 24 1–21. https://doi.org/10.3390/molecules24091818 (2019)
Pan, X, Webster, DC, “Impact of Structure and Functionality of Core Polyol in Highly Functional Biobased Epoxy Resinsa.” Macromol. Rapid Commun., 32 1324–1330. https://doi.org/10.1002/marc.201100215 (2011)
Yang, X, Wang, C, Xia, J, “Study on Synthesis of Novel Phosphorus-Containing Flame Retardant Epoxy Curing Agents from Renewable Resources and the Comprehensive Properties of Their Combined Cured Products.” Prog. Org. Coat., 110 195–203. https://doi.org/10.1016/j.porgcoat.2017.01.012 (2017)
Wang, XL, Chen, L, Wu, JN, “Flame-Retardant Pressure-Sensitive Adhesives Derived from Epoxidized Soybean Oil and Phosphorus-Containing Dicarboxylic Acids.” ACS Sustain. Chem. Eng., 5 3353–3361. https://doi.org/10.1021/acssuschemeng.6b03201 (2017)
Ma, S, Liu, X, Jiang, Y, “Synthesis and Properties of Phosphorus-Containing Biobased Epoxy Resin from Itaconic Acid.” Sci. China Chem., 57 379–388. https://doi.org/10.1007/s11426-013-5025-3 (2014)
Xie, W, Tang, D, Liu, S, Zhao, J, “Facile Synthesis of Bio-based Phosphoruscontaining Epoxy Resins with Excellent Flame Resistance.” Polym. Test., 86 1066. https://doi.org/10.1016/j.polymertesting.2020.106466 (2020)
Jian, R, Wang, P, Xia, L, Zheng, X, “Effect of a Novel P/N/S-Containing Reactive Flame Retardant on Curing Behavior, Thermal and Flame-Retardant Properties of Epoxy Resin.” J. Anal. Appl. Pyrolysis, 127 360–368. https://doi.org/10.1016/j.jaap.2017.07.014 (2017)
Sheth, P, Mestry, S, Dave, D, Mhaske, S, “Isosorbide-Derived Boron- and Phosphorus-Containing Precursors for Flame-Retardant Epoxy Coating.” J. Coat. Technol. Res., 17 231–241. https://doi.org/10.1007/s11998-019-00262-x (2020)
Zia, KM, Anjum, S, Zuber, M, Mujahid, M, Jamil, T, “Synthesis and Molecular Characterization of Chitosanbased Polyurethane Elastomers Using Aromatic Diisocyanate.” Int. J. Biol. Macromol., 66 26–32 (2014)
Chattopadhyay, D, Webster, DC, “Thermal Stability and Flame Retardancy of Polyurethanes.” Prog. Polym. Sci., 34 1068–1133 (2009)
Wicks, ZW, Jones, FN, Pappas, SP, Wicks, DA, Organic Coatings: Science and Technology, 3rd edn. Wiley, Hoboken (2007)
Noreen, A, Zia, KM, Zuber, M, “Bio-based Polyurethane: An Efficient and Environment Friendly Coating Systems: A Review.” Prog. Org. Coat., 91 25–32. https://doi.org/10.1016/j.porgcoat.2015.11.018 (2016)
Zhang, YM, Zhao, Q, Li, L, “Synthesis of a Lignin-Based Phosphorus-Containing Flame Retardant and its Application In Polyurethane.” RSC Adv., 8 32252–32261. https://doi.org/10.1039/c8ra05598j (2018)
Mestry, S, Kakatkar, R, Mhaske, ST, “Cardanol Derived P and Si Based Precursors to Develop Flame Retardant PU Coating.” Prog. Org. Coat., 129 59–68. https://doi.org/10.1016/j.porgcoat.2018.12.016 (2019)
Patel, M, Mestry, S, Khuntia, SP, Mhaske, S, “Gallic Acid-Derived Phosphorus Based Flame-Retardant Multifunctional Crosslinking Agent for PU Coating.” J. Coat. Technol. Res., 17 293–303. https://doi.org/10.1007/s11998-019-00273-8 (2020)
Yakushin, V, Abolins, A, Vilsone, D, Sevastyanova, I, “Polyurethane Coatings Based on Linseed Oil Phosphate Ester Polyols with Intumescent Flame Retardants.” Fire Mater., 43 92–100. https://doi.org/10.1002/fam.2672 (2019)
Alinejad, M, Nikafshar, S, Gondaliya, A, “Lignin-Based Polyurethanes: Opportunities for and Adhesives.” Polymers (Basel), 11 1202 (2019)
Mahmood, N, Yuan, Z, Schmidt, J, Xu, C, “Depolymerization of Lignins and Their Applications for the Preparation of Polyols and Rigid Polyurethane Foams: A Review.” Renew. Sustain. Energy Rev., 60 317–329. https://doi.org/10.1016/j.rser.2016.01.037 (2016)
Huang, X, De Hoop, CF, Xie, J, “Characterization of Biobased Polyurethane Foams Employing Lignin Fractionated from Microwave Liquefied Switchgrass.” Int. J. Polym. Sci., 2017 1–8. https://doi.org/10.1155/2017/4207367 (2017)
Ding, H, Wang, J, Wang, C, Chu, F, “Synthesis of a Novel Phosphorus and Nitrogen-Containing Bio-based Polyols and its Application in Flame Retardant Polyurethane Sealant.” Polym. Degrad. Stab., 124 43–50. https://doi.org/10.1016/j.polymdegradstab.2015 (2016)
Ding, H, Xia, C, Wang, J, Wang, C, “Inherently Flame-Retardant Flexible Bio-based Polyurethane Sealant with Phosphorus and Nitrogen-Containing Polyurethane Prepolymer.” J. Mater. Sci., 51 5008–5018. https://doi.org/10.1007/s10853-016-9805-y (2016)
Agrawal, A, Kaur, R, “Effect of Nano Filler on the Flammability of Bio-Based RPUF.” Integr. Ferroelectr., 202 20–28. https://doi.org/10.1080/10584587.2019.1674820 (2019)
Agrawal, A, Kaur, R, Walia, RS, “Investigation on Flammability of Rigid Polyurethane Foam-Mineral Fillers Composite.” Fire Mater., 43 917–927. https://doi.org/10.1002/fam.2751 (2019)
Agrawal, A, Kaur, R, Singh Walia, R, “Flame Retardancy of Ceramic-Based Rigid Polyurethane Foam Composites.” J. Appl. Polym. Sci., 136 1–10. https://doi.org/10.1002/app.48250 (2019)
Makshina, EV, Canadell, J, Van Krieken, J, Peeters, E, Dusselier, M, Sels, BF, “Bio-Acrylates Production: Recent Catalytic Advances and Perspectives of the Use of Lactic Acid and Their Derivates.” ChemCatChem, 11 180–201 (2019)
Beerthuis, R, Rothenberg, G, Shiju, NR, “Catalytic Routes Towards Acrylic Acid, Adipic Acid and ε-Caprolactam Starting from Biorenewables.” Green Chem., 17 1341–1361 (2015)
Darabi Mahboub, MJ, Dubois, JL, Cavani, F, Rostamizadeh, M, Patience, GS, “Catalysis for the Synthesis of Methacrylic Acid and Methyl Methacrylate.” Chem. Soc. Rev., 47 7703–7738 (2018)
Gattuso, JP, Magnan, A, Billé, R, Cheung, WWL, Howes, EL, Joos, F, Allemand, D, Bopp, L, Cooley, SR, Eakin, CM, Hoegh-Guldberg, O, Kelly, RP, Pörtner, HO, Rogers, AD, Baxter, JM, Laffoley, D, Osborn, D, Rankovic, A, Rochette, J, Sumaila, UR, Treyer, S, Turley, C, “Contrasting Futures for Ocean and Society from Different Anthropogenic CO2 Emissions Scenarios.” Science, 349 1655–1734 (2015)
Hu, Y, Wang, C, Zhou, Y, "Renewable Epoxidized Cardanol Based Acrylate as a Reactive Diluent for UV Curable Resins." pp. 2–4 (2018). https://doi.org/10.1002/pat.4294
Phalak, G, Patil, D, Patil, A, Mhaske, S, “Synthesis of Acrylated Cardanol Diphenyl Phosphate for UV Curable Flame-Retardant Coating Application.” Eur. Polym. J, 121 109320. https://doi.org/10.1016/j.eurpolymj.2019.109320 (2019)
Wong, JL, Aung, MM, Lim, HN, Md Jamil, SNA, “Spectroscopic Analysis of Epoxidised Jatropha Oil (EJO) and Acrylated Epoxidised Jatropha Oil (AEJO).” Pertanika J. Trop Agric. Sci., 40 435–447 (2017)
Aung, MM, Yaakob, Z, Abdullah, LC, “A Comparative Study of Acrylate Oligomer on Jatropha and Palm Oil-based UV-Curable Surface Coating.” Ind. Crops Prod., 77 1047–1052. https://doi.org/10.1016/j.indcrop.2015.09.065 (2015)
Howell, BA, Daniel YG, "Isosorbide as a Platform for the Generation of New Biobased Organophosphorus Flame Retardants." Insights Chem. Biochem. ICBC, (2020). https://doi.org/10.33552/ICBC.2020.01.000509
Daniel, YG, Howell, BA, “Phosphorus Flame Retardants from Isosorbide Bis-Acrylate.” Polym. Degrad. Stab., 156 14–21. https://doi.org/10.1016/j.polymdegradstab.2018.0 (2018)
Howell, BA, Daniel, YG, “Synthesis and Characterization of Isomeric Diphenylphosphatoisosorbide Acrylates.” Phosphorus Sulfur Silicon Relat. Elem., 195 638–643. https://doi.org/10.1080/10426507.2019.1708361 (2020)
Bai, Z, Song, L, Hu, Y, Yuen, RKK, “Preparation, Flame Retardancy, and Thermal Degradation of Unsaturated Polyester Resin Modified with a Novel Phosphorus Containing Acrylate.” Ind. Eng. Chem. Res., 52 12855–12864. https://doi.org/10.1021/ie401662x (2013)
Shen, L, Wang, Y, Zhao, Q, “Influence of a Long-Side-Chain-Containing Reactive Diluent on the Structure and Mechanical Properties of UV-Cured Films.” Polym. Int., 65 1150–1156. https://doi.org/10.1002/pi.516 (2016)
Sag, J, Kukla, P, Goedderz, D, “Synthesis of Novel Polymeric Acrylate-Based Flame Retardants Containing Two Phosphorus Groups in Different Chemical Environments and Their Influence on the Flammability of Poly (Lactic Acid).” Polymers (Basel), https://doi.org/10.3390/POLYM12040778 (2020)
Xu, Y, Guo, L, Zhang, H, Zhai, H, Ren, H, “Research Status, Industrial Application Demand and Prospects of Phenolic Resin.” RSC Adv., 9 28924–28935 (2019)
Xue, B, Zhang, XL, “Application and Development Trend of Phenolic Resin.” Thermosetting Resin, 22 47–50 (2007)
Hirano, K, Asami, M, “Phenolic Resins—100 Years of Progress and Their Future.” React. Funct. Polym., 73 256–269 (2013)
Yang, Z, Peng, H, Wang, W, Liu, T, “Crystallization Behavior of Poly(ε-caprolactone)/Layered Double Hydroxide Nanocomposites.” J. Appl. Polym. Sci., 116 2658–2667. https://doi.org/10.1002/app.31787 (2010)
Deng, P, Liu, Y, Liu, Y, “Preparation of Phosphorus-Containing Phenolic Resin and its Application in Epoxy Resin as a Curing Agent and Flame Retardant.” Polym. Adv. Technol., 29 1294–1302. https://doi.org/10.1002/pat.4241 (2018)
Wang, DC, Chang, GW, Chen, Y, “Preparation and Thermal Stability of Boroncontaining Phenolic Resin/Clay Nanocomposites.” Polym. Degrad. Stab., 93 125–133. https://doi.org/10.1016/j.polymdegradstab.2007.10.021 (2008)
Benyahya, S, Aouf, C, Caillol, S, “Functionalized Green Tea Tannins as Phenolic Prepolymers for Bio-Based Epoxy Resins.” Ind. Crops Prod., 53 296–307. https://doi.org/10.1016/j.indcrop.2013.12.045 (2014)
Thirukumaran, P, Shakila, A, Muthusamy, S, “Synthesis and Characterization of Novel Bio-Based Benzoxazines from Eugenol.” RSC Adv., 4 7959–7966. https://doi.org/10.1039/c3ra46582a (2014)
Sharma, P, Dutta, P, Nebhani, L, “Sustainable Approach Towards Enhancing Thermal Stability of Bio-based Polybenzoxazines.” Polymer (Guildf), 184 121905. https://doi.org/10.1016/j.polymer.2019.121905 (2019)
Liu, X, Zhang, R, Li, T, "Novel Fully Biobased Benzoxazines from Rosin: Synthesis and Properties." pp. 10682–10692 (2017). https://doi.org/10.1021/acssuschemeng.7b02650
Zhang, Y, Chen, X, Fang, Z, “Synergistic Effects of Expandable Graphite and Ammonium Polyphosphate with a New Carbon Source Derived from Biomass in Flame Retardant ABS.” J. Appl. Polym. Sci., 128 2424–2432. https://doi.org/10.1002/app.38382 (2013)
Liu, Y, Zhang, Y, Fang, Z, “Design, Synthesis, and Application of Novel Flame Retardants Derived from Biomass.” BioResources, 7 4914–4925. https://doi.org/10.15376/biores.7.4.4914-4925 (2012)
Yan, H, Li, N, Cheng, J, Fabrication of Flame Retardant Benzoxazine Semi-Biocomposites Reinforced by Ramie Fabrics with Bio-Based Flame Retardant Coating. 1–9 (2017). https://doi.org/10.1002/pc.24617
Yan, H, Li, N, Fang, Z, Wang, H, Application of Poly(Diphenolic Acid-Phenyl Phosphate)-Based Layer by Layer Nano-Coating in Flame Retardant Ramie Fabrics 44795 1–13 (2017). https://doi.org/10.1002/app.44795
Weil, ED, “Fire-Protective and Flame-Retardant Coatings—A State-of-the-Art Review.” J. Fire Sci., 29 259–296. https://doi.org/10.1177/0734904110395469 (2011)
Jimenez, M, Duquesne, S, Bourbigot, S, “Characterization of the Performance of an Intumescent Fire Protective Coating.” Surf. Coat. Technol., 201 979–987. https://doi.org/10.1016/j.surfcoat.2006.01.026 (2006)
Nazir, R, Gaan, S, “Recent Developments in P(O/S)–N Containing Flame Retardants.” J. Appl. Polym. Sci., 137 1–27. https://doi.org/10.1002/app.47910 (2020)
Zhu, W, Yang, M, Huang, H, Dai, Z, Chang, B, Hao, S, “A Phytic Acid-Based Chelating Coordination Embedding Structure of Phosphorus–Boron–Nitride Synergistic Flame Retardant to Enhance Durability and Flame Retardancy of Cotton.” Cellulose, 27 4817–4829. https://doi.org/10.1007/s10570-020-03063-3 (2020)
Chi, Z, Guo, Z, Xu, Z, “A DOPO-Based Phosphorus-Nitrogen Flame Retardant Biobased Epoxy Resin from Diphenolic Acid: Synthesis, Flame-Retardant Behaviour and Mechanism.” Polym. Degrad. Stab., 176 109151. https://doi.org/10.1016/j.polymdegradstab.2020.109151 (2020)
Chen, L, Song, L, Lv, P, “A New Intumescent Flame Retardant Containing Phosphorus and Nitrogen: Preparation, Thermal Properties and Application to UV Curable Coating.” Prog. Org. Coat., 70 59–66. https://doi.org/10.1016/j.porgcoat.2010.10.002 (2011)
Vahabi, H, Rastin, H, Movahedifar, E, “Flame Retardancy of Bio-Based Polyurethanes: Opportunities and Challenges.” Polymers (Basel), 12 1234. https://doi.org/10.3390/POLYM12061234 (2020)
Ding, H, Huang, K, Li, S, “Synthesis of a Novel Phosphorus and Nitrogen-Containing Bio-Based Polyol and its Application in Flame Retardant Polyurethane Foam.” J. Anal. Appl. Pyrolysis, 128 102–113. https://doi.org/10.1016/j.jaap.2017.10.020 (2017)
Wang, T, Li, L, Cao, Y, Wang, Q, Guo, C, “Preparation and Flame Retardancy of Castor Oil Based UV-Cured Flame Retardant Coating Containing P/Si/S on Wood Surface.” Ind. Crops Prod., 130 562–570. https://doi.org/10.1016/j.indcrop.2019.01.017 (2019)
Liao, F, Zhou, L, Ju, Y, Yang, Y, Wang, X, “Synthesis of A Novel Phosphorus–Nitrogen-Silicon Polymeric Flame Retardant and Its Application in Poly(lactic acid).” Ind. Eng. Chem. Res., 53 10015–10023 (2014)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they do not have any conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naiker, V.E., Mestry, S., Nirgude, T. et al. Recent developments in phosphorous-containing bio-based flame-retardant (FR) materials for coatings: an attentive review. J Coat Technol Res 20, 113–139 (2023). https://doi.org/10.1007/s11998-022-00685-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-022-00685-z