Abstract
The present study aimed to design and evaluate controlled porosity osmotic pump (CPOP) tablets of enalapril maleate (ENP) being used for the treatment of hypertension. D-optimal response surface design was used, considering cellulose acetate and osmotic agents (lactose and fructose) as variables while physicochemical parameters of tablets were taken as responses. The asymmetric, leachable membrane of cellulose acetate on ENP tablets was applied and an increase in the thickness of core tablets from 5 ± 0.01 to 5.4 ± 0.17 mm was observed. The average weight of all CPOP formulations ranged from 376.7 ± 0.4 to 389.1 ± 0.3 mg and hardness was 6.2 ± 0.02 to 6.32 ± 0.06 Kg/cm2. The friability of all formulations was less than 1%. 89.53 ± 1.05% of ENP release was observed in phosphate buffer pH 6.8 after 12 h. Due to the smallest AIC (Akaike information criteria) and the greatest r2 values, zero-order release kinetics model with non-Fickian diffusion behavior was observed in all proposed formulations. f1 (difference factor) values were 1.28 ± 0.06 to 12.64 ± 0.41% and f2 (similarity factor) values were 59.75 ± 0.24 to 94.03 ± 1.36% in the same dissolution medium. pH-independent behavior was observed in pH-responsive study. Dissolution efficiency (DE) ranged from 51.49 ± 0.23 to 53.52 ± 0.52% and mean dissolution time (MDT) values ranged from 5.27 ± 0.05 to 5.59 ± 0.23 h. No interaction between the ingredients was found in FTIR analysis. The optimized formulation with improved drug release property was found stable in the accelerated stability study of six months. CPOP tablets of ENP can be considered as an effective substitute for immediate-release tablets to control hypertension in chronic conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional drug delivery systems lack control of drug release at the target site. Controlled drug delivery systems (CDDSs) provide special control over the drug delivery rate. For controlled drug delivery, osmotic pumps are the most encouraging systems. These can be used for oral administration as well as for implantation. For the release of drug(s), an osmotic pump involves the basic principle of osmosis. Elementary osmotic pump, Rose-Nelson pump, Higuchi-Leeper pump, and Higuchi-Theeuwes pump are some examples of osmotic drug delivery systems (ODDSs).1 These systems offer drug delivery to a great extent, not dependent on the physiological factors of the gastrointestinal tract. The drug delivery from osmotic pumps depends on a variety of factors including solubility of the drug, osmotic pressure in the core, nature of the rate-controlling membrane, and delivery aperture size.2
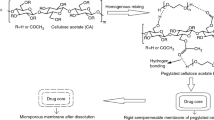
In a CPOP tablet, the semipermeable membrane (SPM) contains water-soluble pore-forming agent(s). Other components of CPOP include drug, osmotic agent(s), coating solvent(s), and plasticizer(s).3,4,5 CPOP system can be a single or a multicompartment dosage form and it consists of a core with the drug which is coated with a SPM having asymmetric structure supported by a porous substructure. The SPM allows water to permeate but prevents the passage of solute. Throughout the wall of the membrane, water-soluble pore-forming agents are dispersed. CPOP does not contain any drilled orifice for the drug delivery; instead, the drug is delivered through the pores formed by pore-forming agent in the SPM. When CPOP reaches an aqueous environment, some levels of water-soluble pore-forming ingredients are dissolved from the polymeric membrane. This results in a porous structure in the SPM walls hence drug is delivered through microporous semipermeable membrane. The pressure by water is created either by an osmogent or the drug after the entry of water across the SPM. Various factors that affect the drug release include water permeability of the SPM, osmotic pressure of inner core tablet, and thickness of the coating. By controlling these factors, drug release can be achieved at desired rates.6,7
ENP, an angiotensin converting enzyme inhibitor, is used as an antihypertensive drug. It is effective either alone or when combined with thiazide diuretics and other antihypertensive drugs.8 ENP is also used for the treatment of congestive heart failure and is mostly given with digitalis and diuretics.9,10
The current study was carried out to design and evaluate CPOP tablets (having asymmetric membrane) with varying concentrations of cellulose acetate, lactose, and fructose. For this purpose, a D-optimal response surface design was used. Preformulation studies of powder blends of starch, ENP, lactose, fructose, and magnesium stearate were performed. Core tablets were prepared by wet granulation method while CPOP tablets were prepared by using the spray coating technique. Core and CPOP tablets were evaluated by their weight variation, thickness, diameter, hardness, in vitro drug release studies, and FTIR analysis. AIC, k, and r2 values were calculated in the case of each model-dependent in vitro kinetic approach. Fit factors, dissolution efficiency, and mean dissolution time values were calculated as model-independent in vitro kinetic approaches. Effects of pH, agitational intensity, osmogent, and coating polymer concentrations on %ENP release were observed. An accelerated stability study was conducted to observe the stability of the optimized formulation.
Materials and methods
Materials
Starch (MW: 359.33 Da, Merck Darmstadt, Germany) was used as a binder. Magnesium stearate (MW: 591.27 Da, Merck Darmstadt, Germany) was used as a lubricant. Lactose monohydrate (MW: 360.31 Da, Duksan pure chemicals, Korea) and fructose (MW: 180.16 Da, Fisher scientific, UK) were used as osmotic agents. Cellulose acetate (MW: 30 KDa, Sigma-Aldrich, USA) was used as a semipermeable membrane forming agent. PEG-400 (MW: 400 Da, Applichem GmbH Darmstadt, Germany) was used as a channeling agent. Enalapril maleate (MW: 492.53 Da, Nabiqasim Industries Pvt. Ltd. Karachi, Pakistan) was used as a model drug. Dichloromethane (DCM) and methanol (Merck Darmstadt, Germany) were of analytical grade.
Experimental design
By using Design-Expert software (version 7.1 Stat–Ease Inc., Minneapolis USA), D-optimal factorial design was applied taking concentration of cellulose acetate and osmotic agents (lactose and fructose) as variables as shown in Table 1. Weight variation and hardness of tablets were selected as responses and six planned D-optimal design formulations were evaluated against them.11 Equation (1) was used for the prediction of responses in D-optimal design.
where β0 was intercept, B1 and B2 were regression coefficients, and X1 and X2 were the applied variables.
Preformulation studies
The powder blends of drug and excipients of all the planned formulations were analyzed for preformulation parameters by the same procedure as used by Akhtar et al.12 Bulk densities, tapped densities, angle of repose, Hausner’s ratios, and compressibility indices were calculated as preformulation parameters.
Preparation of core tablets
Starch paste-based wet granulation method was used for the preparation of core tablets. Accurately weighed amounts of ENP, starch, lactose, and fructose as shown in Table 2 were geometrically mixed with sigma mixer (Erweka AR 400 Apparatebau–GMBH Germany) and sieved through mesh no. 12 (TWP Inc., Berkeley, California). Starch paste was used for the preparation of wet granules, dried at room temperature and subsequently in an oven at 45°C, and again sieved through mesh no. 14. Resulted granules were mixed with magnesium stearate and compressed by using a single punch tablet machine (Minipress MII, Pharma Test, GMBH Hainburg/Germany) under controlled humidity conditions (less than 60% relative humidity).11
Preparation of CPOP tablets
By using HG-15D homogenizer (Daihan Scientific, USA), cellulose acetate was homogenized at 3000 rpm for 30 min in a mixture of DCM/methanol (1:1) having 15% PEG-400.13 The prepared coating solution was sprayed at a spray rate of 2–4 mL/min on the compressed ENP core tablets using a conventional coating pan having atomizing air pressure 1 Kg/cm2 and rotated with the speed of 18 rpm. The difference of inlet and outlet air temperature was adjusted to 100°C and CPOP tablets were dried at 50°C keeping the weight gain constant at 3 ± 0.5% w/w.14
Physicochemical evaluation of core and CPOP tablets
Physicochemical evaluation of core and CPOP tablets was performed by using the already reported procedure of Dandagi et al.15 In brief, by using the British Pharmacopeial procedure, weight variation test was applied on core and CPOP tablets. The thickness and diameter of core and CPOP tablets were measured with the help of a calibrated Vernier caliper (Anyi Instrument Co., Ltd. Guangxi, China). Hardness Tester (Erweka, GMBH Heusenstamm, Germany) was used to determine the hardness of all formulations before and after coating. By using the British Pharmacopeial procedure, friability of core tablets was carried out in a friabilator (Pharma Test D-63512 Hainburg, Germany) (Table 3).
In vitro ENP release studies
By using USP type II dissolution apparatus at 37 ± 0.5°C and 50 rpm, percentage release of ENP from core A and B tablets was assessed for 30 min while from CPOP tablets for the first 2 h in 900 mL 0.1 N HCl solution pH 1.2 and the remaining 10 h in phosphate buffer pH 6.8. Next, 5 mL aliquots were withdrawn after predetermined time intervals and were replaced with the same amount of fresh medium of pH 1.2 and 6.8. Percentage release of ENP was calculated by using UV&Visible spectrophotometer (PerkinElmer, Waltham, MA, USA) and the already prepared standard curve of increasing concentration of pure ENP at 215 nm.
In vitro model-dependent kinetic approaches like zero-order, first-order, Higuchi, Hixson Crowell, and Korsmeyer–Peppas models (equations 2, 3, 4, 5, and 6, respectively) were used for the confirmation of release behavior of ENP.16
where Ft was the fraction of drug released at time t. Ko, K1, K3, KH, and Kd were zero-order, first-order, Peppas, Higuchi, and dissolution rate constants, respectively. Q0 and Qt were the initial quantity of drug and the drug quantity at time t, respectively. Mt and \({M}_{\mathrm{\infty }}\) were the amounts of ENP released at time t and infinity, respectively, and n was diffusion constant.
Applied in vitro model-independent kinetic approaches were AIC, f1, f2, DE, and MDT. To check the relevance of the applied in vitro release models, the AIC was applied.17 Equation 7 was used for the calculation of AIC values.
where n was the number of dissolution data points, p was the number of parameters of the model, WSSR was the weight sum of square of residues.
By using DDSolver (an add-in program of MS Excel), f1 and f2 were applied on all formulations. Equations used for f1 and f2 are given below (equations 8 and 9).
where Ri and Ti were the % ENP release of reference and test products and n was the number of dissolution data points.18
DE and MDT values were calculated by the previously reported method of Simionato et al. while comparing dissolution profiles of nine meloxicam tablet brands.19 In brief, DE was calculated as the percentage ratio of the area under the dissolution curve up to time t (AUC0t) to that of the area of the rectangle described by 100% dissolution at the same time point (Q100.t), while mean dissolution time was determined from the accumulative curves of the dissolved active pharmaceutical ingredient as a function of time.
where ti was an intermediate time of the intervals of sampling time, ΔQi was the amount of active pharmaceutical ingredient dissolved in every interval of time t and Q∞ was the maximum of active pharmaceutical ingredient dissolved.
Effects of osmotic agent and cellulose acetate concentrations
Effect of osmotic agent (lactose and fructose) concentration was observed as reported by Choudhury, Gale, and Guthman in their studies.20,21,22 Briefly, %ENP release was observed as a function of osmotic agent concentration in such a way that CPOP1, CPOP3, CPOP5 contained 50% osmotic agent each while CPOP2, CPOP4, and CPOP6 contained 60% osmotic agent each. Effect of cellulose acetate concentration on %ENP release was observed as reported by Zentner and Appel in their studies of controlled porosity osmotic pumps.23,24. In short, three coating solutions having different concentrations of cellulose acetate (3.5, 4, and 4.4%) were used and their effects on the % ENP release were observed.
Effects of pH and agitational intensity
ENP release of the optimized formulation was studied in different dissolution media, i.e., water, 0.1 N HCl buffer, and phosphate buffer pH 6.8. Akhtar et al. also observed the effect of pH while studying controlled porosity osmotic pump tablets of Captopril.25 The effect of agitational intensity on %ENP release was observed by using the already reported method of Ahmad et al. for preparing CPOP tablets of Eperisone HCl.26 Briefly, dissolution of the optimized formulation was performed at different agitational intensities, i.e., 50, 100, and 150 rpm.
FTIR analysis and stability study
FTIR analysis of ENP, core, and CPOP tablets was done by using an FTIR spectrophotometer (Platinum-ATR, ALPHA, Bruker). Core and CPOP tablets were crushed into fine powder forms by mortar and pestle for FTIR analysis. An average of twenty-five scans was used to understand the characteristic peaks. For stability study, the optimized CPOP formulation of ENP was stored in an ICH-certified stability chamber maintained at 40°C and 75% RH for six months. The samples were withdrawn at different time intervals and evaluated for their appearance, weight variation, thickness, diameter, hardness, friability, and for in vitro ENP release.
Results and discussion
Preformulation properties of powder blends
All the micromeritic properties of powder blends of both the core formulations, i.e., core A and core B were found to be within acceptable limits as mentioned in Table 3. Habib et al. measured these properties while evaluating CPOP tablets of trimetazidine dihydrochloride and found similar results.27 Both core tablets A and B powder blends showed good flow properties as the values of angle of repose were less than 30°. In short, as per citation of Ma and Hadzija, values of angle of repose, Hausner’s ratio, and compressibility index indicated good flowability and compressibility of powders.28
Physical properties of core and CPOP tablets
Both core and CPOP tablets were round, biconvex in shape, and white in color. Core A and B showed thickness and diameter of 5 mm and 10 mm, respectively, while the thickness of all CPOP formulations ranged from 5.35 ± 0.07 to 5.4 ± 0.17 mm and the diameter of CPOP formulations was 10 mm. The average weight of core A, core B, and CPOP formulations was in the range given in USP. The hardness of core A and B was 6.25 ± 0.59 and 6.3 ± 0.40 Kg/cm2, respectively, while the hardness of all CPOP formulations was 6.2 ± 0.02 to 6.32 ± 0.06 Kg/cm2. All core formulations showed friability less than 1% which indicated good strength of all formulations to withstand any external force for breakage (Table 4). Karmakar et al. performed an in vitro evaluation of quality control parameters while comparing paracetamol and paracetamol/caffeine tablets and found similar results.29
D-optimal design responses
The interaction between factors and responses was studied and quadratic equations were solved (equations 13 and 14). The positive sign showed optimization while the negative sign showed an antagonistic effect of factors on the responses.
X1 (lactose and fructose) showed a positive effect while X2 (cellulose acetate) showed a negative effect. Interaction of X1 and X2 also showed a negative effect on the hardness of formulations.
X1 (lactose and fructose) and X2 (cellulose acetate) showed negative effects while the interaction of X1 and X2 showed a positive effect on weight variation of formulations.
In vitro ENP release studies
The target in vitro release time was 12 h. Firstly, core tablets were prepared. In the drug release study, core tablets released enalapril in 30 min (Fig. 1). ENP release from core B (94.07 ± 1.36%) was more than that of core A (88.65 ± 1.85%) possibly due to more osmotic agents in core B (60%) as compared to core A (50%). Choudhury, Gale, and Guthman described the effects of osmotic agents in their studies and observed that drug release was directly proportional to the content of osmotic agents.20,21,22 By the formulation of CPOP tablets, release of enalapril was retarded up to 12 h (Fig. 2). The optimized formulation (CPOP2 formulation) was selected on the basis of better physicochemical properties, better release pattern of enalapril up to 12 h, and better stability profile. Akhtar et al. selected an optimized formulation based on better release pattern of drug while evaluating controlled porosity osmotic pump tablets of captopril.25 Adnan et al. selected an optimized formulation based on better drug release retarding characteristics while developing isosorbide mononitrate sustained release matrix tablets.30 CPOP5 formulation having 4.4% cellulose acetate and 50% osmotic agent showed the least ENP release, i.e., 76.68 ± 1.22% while CPOP2 formulation having 3.5% cellulose acetate and 60% osmotic agent showed the maximum ENP release, i.e., 89.53 ± 1.05% (Fig. 2). Assumingly, because cellulose acetate decreases drug release by forming compact SPM and osmotic agents increase drug release due to their osmotic pressure gradient increasing property. Sakore et al. also prepared and evaluated ENP sustained release matrix tablets and found similar results.31 When comparing data sets, the model associated with the highest r2 values and the smallest AIC values was considered the best fit. r2 values of CPOP formulations for zero order were in the range of 0.993–0.998, for first order the values were in the range of 0.953–0.967, for Higuchi model the values were in the range of 0.862–0.893, for Korsmeyer–Peppas the values were in the range of 0.971–0.988, and values for Hixson–Crowel were in the range of 0.980–0.987. AIC was a measure of the best fit based on maximum probability. AIC values of CPOP formulations for zero order were in the range of 36.61–49.18, for first order, the values were in the range of 65.67–71.30, for Higuchi model the values were in the range of 80.49–83.10, for Korsmeyer–Peppas the values were in the range of 54.40–64.90 and values for Hixson–Crowel were in the range of 54.49–61.33. In zero-order model, r2 values were closer to 1 and AIC values were the least as compared to other models, hence all formulations followed zero-order release kinetics model. The values of exponent 'n' of drug release of CPOP formulations were above 0.45 hence all formulations followed non-Fickian diffusion (Table 4). The dominance of zero-order release behavior was also observed by Philip et al. while preparing asymmetric membrane capsules for achieving delayed and osmotic flow of cefadroxil.32
Due to the absence of reference product with same release pattern, the release profiles of the prepared formulations were compared by calculating the difference factor (f1) and similarity factor (f2). Results of difference factor (f1) and similarity factor (f2) of CPOP formulations are shown in Table 5. The similarity in dissolution profile was observed among all six CPOP formulations. f1 values ranged from 1.28 ± 0.06 to 12.64 ± 0.41% and f2 values ranged from 59.75 ± 0.24 to 94.03 ± 1.36%. As per citations of Stevens et al. and Liu et al. the parameter f1 values in the range from 0 to 15, and f2 values in the range from 50 to 100, are used to define the equivalence of two dissolution profiles.33, 34 CPOP2 (optimized formulation) was 93.22 ± 1.28% similar to CPOP1, 88.20 ± 1.59% similar to CPOP3, 65.69 ± 0.56% similar to CPOP4, 65.46 ± 0.48% similar to CPOP5 and 61.60 ± 0.39% similar to CPOP6. Asif et al. selected an optimized formulation based on the maximum similarity values while formulating febuxostat tablets by direct compression method.35 DE values of CPOP formulations ranged from 51.49 ± 0.23 to 53.52 ± 0.52% and MDT values ranged from 5.27 ± 0.05 to 5.59 ± 0.23 h. As the CPOP formulations were similar in composition, their DE and MDT results were closely related (Fig. 3).
Effects of osmotic agent and cellulose acetate concentrations on %ENP release
The effect of osmotic agent concentration is shown in Fig. 2. ENP release from CPOP2, CPOP4, and CPOP6 with a greater concentration of osmotic agent (60% osmotic agent) was more than that of other formulations indicating that the ENP release was directly proportional to the concentration of osmotic agents (lactose and fructose). CPOP2 formulation having the lowest cellulose acetate concentration and the highest osmotic agent concentration showed the highest ENP release while CPOP5 formulation having the highest cellulose acetate concentration and the lowest osmotic agent concentration showed the lowest ENP release. This might be because cellulose acetate decreases drug release by forming compact SPM while osmotic agents increase drug release due to their osmotic pressure gradient increasing property. This is also described by Zentner and Appel in their studies of controlled porosity osmotic pumps (Fig. 3). 23,24
Effects of pH and agitational intensity on %ENP release through CPOP tablets
The results of ENP release at different pH values are shown in Fig. 4. There was no evident change in the dissolution profile of CPOP2 (optimized formulation) at three different pH values which proved that change in pH did not affect the ENP release from CPOP tablets. Kanagale et al. observed the effect of pH while formulating and optimizing the osmotic pump-based controlled release system of oxybutynin and found that pH did not affect the drug release from the osmotic system.36 There was no noticeable effect of agitational intensity on ENP release through CPOP tablet as can be seen in Fig. 5. Similar behavior was reported by Ahmad et al. while evaluating CPOP tablets of Eperisone HCl.26
FTIR analysis and stability study
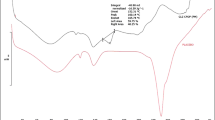
FTIR spectrum of ENP showed peaks at 666.55 cm−1 due to C=C bending in alkene, at 872.87 cm−1 due to strong C–H bending, and at 1644.36 cm−1 due to C=O in amides.37 In the FTIR spectrum of core tablet, peaks at 778.31; 899.91; 988.41, and 1092.49 cm−1 belonged to lactose and were due to strong C–H bending; strong C=C bending in alkene and strong C–O stretching in ether, respectively. Peaks at 977.34, 1052.60, 1142.55, and 1336.88 cm−1 were due to strong C=C bending in alkene, strong C–O stretching in primary alcohol, strong C–O stretching in tertiary alcohol and O–H bending in alcohol, respectively, and these peaks belonged to fructose. Peaks at 667.58 cm−1 and 701.22 cm−1 were due to C=C bending in alkene, at 874.30 cm−1 and 3329.84 cm−1 were due to strong C-H bending and N–H stretching in aliphatic primary amine, respectively, and they all belonged to the drug ENP. Peaks at 751.69 cm−1 and 1072.06 cm−1 were due to strong C–H bending and strong C–O bending in primary alcohol, respectively, belonging to starch. Peaks at 1230.61, 1072.48, 776.97, and 668.10 cm−1 in a CPOP formulation were due to the bending and stretching of different functional groups.38,39,40 The FTIR spectra of the formulations were compared with the FTIR spectrum of the pure drug. The results indicated that the characteristic absorption peaks of pure ENP also appeared in the formulated tablets, without any significant change in their position after successful formulation, indicating the absence of any chemical interaction between ENP and other ingredients of core and CPOP formulations (Fig. 6).
The optimized formulation (CPOP2) was found stable in terms of appearance, weight variation, thickness, diameter, hardness, friability, and in vitro release profile as shown in Table 6. The in vitro release profile of ENP initially and after 4 months of stability study was almost the same and there was not much difference observed. But after six months of stability, the release of ENP declined from 89.13 ± 1.05 to 86.56 ± 1.28%.
Conclusions
Controlled porosity osmotic pump tablets of ENP were successfully prepared and evaluated. Six formulations with varying concentrations of lactose, fructose, and cellulose acetate were designed with the help of a D-optimal response surface design. Core tablets were prepared by wet granulation method while CPOP tablets were prepared by spray coating on the core tablets. The average weight of all CPOP formulations ranged from 376.7 ± 0.4 to 389.1 ± 0.3 mg. The hardness of CPOP formulations was observed to be 6.2 ± 0.02 to 6.32 ± 0.06 Kg/cm2. The in vitro dissolution test was performed and CPOP6 formulation showed the maximum drug release, i.e., 89.53 ± 1.05% after 12 h. Based on the smallest AIC and the greatest r2 values, all formulations followed zero-order release kinetic model. f1 values of CPOP formulations ranged from 1.28 ± 0.06 to 12.64 ± 0.41% and f2 values ranged from 59.75 ± 0.24 to 94.03 ± 1.36% which showed similarity between all six formulations. DE values of CPOP formulations ranged from 51.49 ± 0.23 to 53.52 ± 0.52% and MDT values ranged from 5.27 ± 0.05 to 5.59 ± 0.23h which were closely related due to the similar composition of the formulations. The formulations showed pH-independent behavior in pH-responsive study. No change in ENP release was observed while evaluating the effect of agitational intensity. The ENP release from CPOP formulations was directly proportional to the concentrations of lactose, fructose, and cellulose acetate. In FTIR analysis, no incompatibility was found. The optimized formulation (CPOP2) was found stable in the accelerated stability study of six months. Hence, CPOP tablets of ENP can be considered as an effective substitute for immediate-release tablets to control hypertension in chronic conditions.
References
Singh, K, Walia, MK, Agarwal, G, Harikumar, S, “Osmotic Pump Drug Delivery System: A Noval Approach.” J. Drug Deliv. Therap., 3 (5) 156–162 (2013)
Verma, RK, Krishna, DM, Garg, S, “Formulation Aspects in the Development of Osmotically Controlled Oral Drug Delivery Systems.” J. Contr. Rel., 79 (1–3) 7–27 (2002)
Ajay, B, Prasada, R, Vijaya, RJ, “Controlled-Porosity Osmotic Pump Tablets—An Overview.” Int. J. Pharm. Pharm. Sci., 2 114–26 (2010)
Sahoo, CK, Sahoo, NK, Rao, SRM, Sudhakar, M, Satyanarayana, K, “A Review on Controlled Porosity Osmotic Pump Tablets and Its Evaluation.” Bull. Fac. Pharm., Cairo Uni., 53 (2) 195–205 (2015)
Gupta, BP, Thakur, N, Jain, NP, Banweer, J, Jain, S, “Osmotically Controlled Drug Delivery System with Associated Drugs.” J. Pharm. Pharm. Sci., 13 (4) 571–588 (2010)
Mene, HR, Mene, NR, Parakh, DR, Ingale, TB, Magar, DR, Mangale, MR, “Formulation Aspects in Development of Controlled Porosity Osmotic Pump Tablet.” Pharm. Biol. Eval., 3 (1) 1–18 (2016)
Kaushal, AM, Garg, S, “An Update on Osmotic Drug Delivery Patents.” Pharm. Technol., 27 (8) 38–44 (2003)
De Blasi, A, Cortellaro, M, Costantini, C, “Enalapril in Essential Hypertension: A Comparative Study with Propranolol. Enalapril in Hypertension Study Group (UK).” Br. J. Clin. Pharmacol., 18 (1) 51–56 (1984)
Gomez, HJ, Cirillo, VJ, Davies, RO, Bolognese, JA, Walker, JF, “Enalapril in Congestive Heart Failure: Acute and Chronic Invasive Hemodynamic Evaluation.” Int. J. Cardiol., 11 (1) 37–48 (1986)
Borer, JS, “Angiotensin-Converting Enzyme Inhibition: A Landmark Advance in Treatment for Cardiovascular Diseases.” Eur. Heart J. Suppl., 9 (Suppl_E) E2–E9 (2007)
Syed, SM, Lahoti, S, Syed, AA, “Controlled Porosity Osmotic Tablet of Atenolol: In-Vitro and In-Vivo Evaluation.” Marmara Pharm. J., 20 (3) 325–332 (2016)
Akhtar, MF, Hanif, M, Majeed, A, Shah, S, “Formulation Development and Optimization of Captopril Containing Tablets Through Box-Behnken Design.” Latin Am. J. Pharm., 37 (7) 1414–1423 (2018)
Zentner, GM, Rork, GS, Himmelstein, KJ, “The Controlled Porosity Osmotic Pump.” J. Controll. Release, 1 (4) 269–282 (1985)
Reddy, BV, Navaneetha, K, Reddy, BR, “Tablet Coating Industry Point View—A Comprehensive Review.” Int. J. Pharm. Biol. Sci., 3 (1) 248–61 (2013)
Dandagi, PM, Patel, CP, Sharma, R, Gadad, AP, Mastiholimath, V, “Studies on Formulation and Evaluation of Osmotically Controlled Drug Delivery System of Carbamazepine.” Int. J. Pharm. Pharm. Sci., 6 (2) 239–250 (2014)
Brindha, VS, Sathali, AAH, Arun, K, Priya, PS, “Swellable Osmotic Drug Delivery System of Amitriptyline Hydrochloride-Design and Evaluation.” J. Curr. Chem. Pharm. Sci., 2 55–68 (2012)
Aguilar Ros, A, Caamaño Somoza, M, Martín Martín, F and Montejo Rubio, M, "Parámetros Amodelísticos: Comparación De Perfiles." In: Biofarmacia y Farmacocinética, Ejercicios y Problemas Resueltos, 1st Ed. pp. 3-4. Elsevier España SL, Barcelona, España (2008)
Costa, P, Lobo, JMS, “Modeling and Comparison of Dissolution Profiles.” Eur. J. Pharm. Sci., 13 (2) 123–133 (2001)
Simionato, LD, Petrone, L, Baldut, M, Bonafede, SL, Segall, AI, “Comparison Between the Dissolution Profiles of Nine Meloxicam Tablet Brands Commercially Available in Buenos Aires, Argentina.” Saudi Pharm. J., 26 (4) 578–584 (2018)
Choudhury, PK, Ranawat, MS, Pillai, MK, Chauhan, CS, “Asymmetric Membrane Capsule for Osmotic Delivery of Flurbiprofen.” Acta Pharm., 57 (3) 343–350 (2007)
Gale, R, Chandrasekaran, SK, Swanson, D, Wright, J, “Use of Osmotically Active Therapeutic Agents in Monolithic Systems.” J. Membr. Sci., 7 (3) 319–331 (1980)
Guthmann, C, Lipp, R, Wagner, T, Kranz, H, “Development of a Novel Osmotically Driven Drug Delivery System for Weakly Basic Drugs.” Eur. J. Pharm. Biopharm., 69 (2) 667–674 (2008)
Zentner, GM, Rork, GS, Himmelstein, KJ, “Osmotic Flow Through Controlled Porosity Films: An Approach to Delivery of Water Soluble Compounds.” J. Controll. Release, 2 217–229 (1985)
Appel, LE, Zentner, GM, “Use of Modified Ethylcellulose Lattices for Microporous Coating of Osmotic Tablets.” Pharm. Res., 8 (5) 600–604 (1991)
Akhtar, MF, Hanif, M, “Leachable Pegylated Cellulose Acetate Complex: A Promising Approach for Controlled Porosity Osmotic Pump Tablets of Captopril.” J. Coat. Technol. Res., 17 (2) 439–446 (2020)
Ahmed, K, Shoaib, MH, Yousuf, RI, Qazi, F, Anwer, S, Nasiri, MI, Mahmood, ZA, “Use of Opadry® CA—A Cellulose Acetate/Polyethylene Glycol System for Rate-Controlled Osmotic Drug Delivery of Highly Soluble Antispastic Agent Eperisone HCl.” Adv. Polym. Technol., 37 (8) 2730–2742 (2018)
Habib, BA, Abd El Rehim, RT, Nour, SA, “Feasibility of Optimizing Trimetazidine Dihydrochloride Release from Controlled Porosity Osmotic Pump Tablets of Directly Compressed Cores.” J. Adv. Res., 5 (3) 347–356 (2014)
Ma, JK, Hadzija, B, Basic Physical Pharmacy. Jones & Bartlett Publishers (2012)
Karmakar, P, Kibria, MG, “In-Vitro Comparative Evaluation of Quality Control Parameters Between Paracetamol and Paracetamol/Caffeine Tablets Available in Bangladesh.” Int. Curr. Pharm. J., 1 (5) 103–109 (2012)
Adnan, S, Shehzadi, K, Farooq, M, Ranjha, NM, Hanif, M, Masood, Z, Zaman, M, “Application of Box-Behnken Design for the Development of Isosorbide Mononitrate Sustained Release Matrix Tablets.” Latin Am. J. Pharm., 35 (5) 1018–1025 (2016)
Sakore, S, Chakraborty, B, “Formulation and Evaluation of Enalapril Maleate Sustained Release Matrix Tablets.” Int. J. Pharm., 4 (1) 21–26 (2013)
Philip, AK, Pathak, K, Shakya, P, “Asymmetric Membrane in Membrane Capsules: A Means for Achieving Delayed and Osmotic Flow of Cefadroxil.” Eur. J. Pharm. Biopharm., 69 (2) 658–666 (2008)
Stevens, RE, Gray, V, Dorantes, A, Gold, L, Pham, L, “Scientific and Regulatory Standards for Assessing Product Performance Using the Similarity Factor, f2.” AAPS J., 17 (2) 301–306 (2015)
Liu, L, Wang, X, “Solubility-Modulated Monolithic Osmotic Pump Tablet for Atenolol Delivery.” Eur. J. Pharm. Biopharm., 68 (2) 298–302 (2008)
Asif, U, Sherwani, AK, Akhtar, N, Shoaib, MH, Hanif, M, Qadir, MI, Zaman, M, “Formulation Development and Optimization of Febuxostat Tablets by Direct Compression Method.” Adv. Polym. Technol., 35 (2) 129–135 (2016)
Kanagale, P, Lohray, BB, Misra, A, Davadra, P, Kini, R, “Formulation and Optimization of Porous Osmotic Pump-Based Controlled Release System of Oxybutynin.” AAPS PharmSciTech, 8 (3) E13–E19 (2007)
Swamy, NGN, Abbas, Z, “Design and Characterization of Oral Dispersible Tablets of Enalapril Maleate Using a Co-Processed Excipient.” J. Appl. Pharm. Sci., 2 (11) 40 (2012)
Ibrahim, M, Alaam, M, El-Haes, H, Jalbout, AF, de Leon, A, “Analysis of the Structure and Vibrational Spectra of Glucose and Fructose.” Ecletica quimica, 31 (3) 15–21 (2006)
Solís-Oba, M, Teniza-García, O, Rojas-López, M, Delgado-Macuil, R, Díaz-Reyes, J, Ruiz, R, “Application of Infrared Spectroscopy to the Monitoring of Lactose and Protein from Whey After Ultra and Nano Filtration Process.” J. Mex. Chem. Soc., 55 (3) 190–193 (2011)
Raut, DM, Allada, R, Pavan, K, Deshpande, G, Patil, D, Patil, A, Deshmukh, A, Sakharkar, D, Bodke, P, Mahajan, D, “Dehydration of Lactose Monohydrate: Analytical and Physical Characterization.” Der Pharm. Lett., 3 (5) 202–212 (2011)
Acknowledgments
The authors are very thankful to the research development of Bahauddin Zakariya University Multan, Pakistan, for providing the financial grant and to the Department of Pharmaceutics, Bahauddin Zakariya University Multan, Pakistan, for providing laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akhtar, M.F., Ashraf, H., Uzair, M. et al. Development of leachable enalapril tablets by controlled porosity osmotic pump technique; a unique approach to enhance its sustained release effect. J Coat Technol Res 19, 497–507 (2022). https://doi.org/10.1007/s11998-021-00536-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-021-00536-3