Abstract
The aim of the present study was to develop pegylated cellulose acetate coating layer having leachable membrane created by controlled porosity osmotic pump (CPOP) technique. Already optimized core tablets of Captopril were used for the application of CPOP. In Box–Behnken design, cellulose acetate, polyethylene glycol, and sorbitol were used as variables and %Captopril release as a response for the coating process. Formulations were characterized by weight gain, thickness of leachable membrane, content uniformity, drug release, pH, dissolution medium osmolality effect, FTIR, and stability analysis for the optimization purpose. Optimized formulation was within the pharmacopoeial limits. Observed weight gain was 4.38–6.97%, and thickness of leachable membrane was 1.24–1.47 mm. Content uniformity was found to be 91.65–99.61%. Phosphate buffer of pH 6.8 showed 86.22–92.79% drug release and the first-order release pattern. pH-independent but osmolality-dependent drug release was observed. No incompatibility between the ingredients of prepared dosage form was observed in FTIR analysis. Accelerated stability study for 6 months showed no significant change in the prepared dosage form. Conclusively, prepared CPOP tablets can be used for the controlled release of Captopril in hypertensive patients to maintain the desired drug concentration within the body for required time period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional oral dosage forms have many drawbacks; among them, abrupt release of drug and low accessibility of effective concentration at the target site are considered the most common. Different alterations in the conventional dosage forms are described in the form of their sustained, controlled, and delayed release characteristics, but the abrupt release of drug from the surface of the modified dosage form made them inapplicable. These kinds of dosage regimes may result in irregular minimum effective concentration and ultimately poor bioavailability. Therefore, pharmaceutical scientists have an urge to develop such dosage forms which are independent of many physicochemical properties of the drug, excipients, and patient physiological factors like the presence of food and pH of the gastrointestinal tract. Coating techniques may provide the solution for the above-mentioned problems.1
Rate of drug release from oral dosage form can be controlled by creating an osmosis-like process in the coated formulation. Two types of osmotic controlled drug delivery systems are reported in the previous studies. Elementary osmotic pump (EOP) drug delivery system has the drug in the core with or without an osmotic agent surrounded by a semipermeable membrane possessing an orifice for drug release. EOP systems have multiple advantages of controlled release, but their complex preparation methods, creation of orifice either by drilling or by laser techniques, and the increase in orifice size during the drug release process which ultimately creates the burst release effect of the drug made them less acceptable. Another approach is the CPOP having a leachable component in the coating layer.1 Imbibition of water inside the device increases hydrostatic pressure and volume of the device which releases drug through the orifice either in solution or in suspension form.2 Rate of drug release from CPOP formulation depends upon type, thickness, surface area, and leachability of the semipermeable membrane, level of components, and solubility of drug in the tablet core and on the difference of osmotic pressure across the membrane.3,4,5 CPOP follows zeroth-order kinetics, but in certain cases where the drug is highly aqueous soluble, the first-order release kinetic shows dominancy. Drug release from CPOP is independent of gastric pH, presence of food, and other physiological factors.3,4,6,7
Keeping the view of above comments, it is therefore the aim of the present study to prepare CPOP tablets of Captopril by applying Box–Behnken design (BBD). To obtain our goal, core tablets were prepared and evaluated by method already described in our previous study.8 Pegylated cellulose acetate was used for the preparation of a leachable membrane having sorbitol as a pore-forming substance. Influences of different concentrations of cellulose acetate (CA), polyethylene glycol (PEG), and sorbitol on %drug release were evaluated. Effects of pH and osmolality of the dissolution medium on percentage release of Captopril were evaluated under controlled conditions of temperature. Compatibility of the pegylated cellulose acetate complex, drug, and excipients was evaluated by FTIR. International Council for Harmonisation of Technical Requirements for Human Use (ICH) guidelines were followed for accelerated stability studies.
Materials and methods
Materials
Captopril was generously gifted by Mediceena Pharma (Pvt.) Ltd., Pakistan. D-Sorbitol (MW: 182.17 Da) was used as an osmogenic substance; cellulose acetate (MW: 30 kDa) was used as a semipermeable membrane forming polymer, and both were purchased from Sigma-Aldrich, USA. PEG 400 (MW: 400 Da) served as a channeling agent and was obtained from AppliChem GmbH Darmstadt, Germany. Dichloromethane and methanol (Merck Darmstadt, Germany) were used as solvents. Double distilled water was used in complete study, and all the chemicals were of analytical grade.
Methods
Experimental design
Three levels of each independent variable were placed at one of three equal-spaced values by BBD using Design-Expert® version 7.1 Stat-Ease Inc., Minneapolis, CA, PEG-400 and sorbitol were used as variables, and twelve CPOP formulations were planned as shown in Tables 1 and 2. Percentage drug release at different time intervals was selected as a response, and equation 1 was applied for the optimization of planned formulations.
where Yi is the measured response of the dependent variables, b0 is the intercept, and b1 to b33 are the regression coefficients computed from the observed experimental values of Y. X1, X2, and X3 are the coded values of the independent variables. XaXb (a, b = 1, 2, 3) and \(X_{i}^{2}\) (i = 1, 2, 3) represent the interaction and quadratic terms, respectively.9
Formulation of CPOP tablets
Previously, optimized Captopril core tablets of our research group8 were used for the formation of CPOP tablets. For coating purpose, CA was dissolved in a mixture of DCM:methanol (1:1) by homogenizer (Wisd HG-15D Homogenizer, Daihan Scientific, USA) at 2700 rpm. PEG-400 was added to make the pegylated cellulose acetate blend (Table 2). Aqueous solution of D-sorbitol and blend of pegylated cellulose acetate were mixed by homogenizer at 3000 rpm for 30 min. Pegylation of CA and its application in CPOP technique are represented in Fig. 1.
The coating operation was performed in a laboratory model stainless steel spray coating pan with hot air gun. The speed of the pan was adjusted to 50 rpm. The coating was done manually by intermittent spraying (4–6 ml/min) and drying technique with 5-inch gun-to-bed distance. Coated tablets were allowed to dry completely in a hot air oven at 50°C for 12 h. Weight of the tablet and thickness were determined before and after coating.10
Physical evaluation of CPOP Tablets
Thickness and diameter of CPOP tablets were measured using a calibrated Vernier caliper (Anyi Instrument Co., Ltd., Guangxi, China). Weight variation test was applied on CPOP tablets according to B.P. specifications. For percentage weight gain calculation, twenty tablets (before and after coating) from each formulation were selected randomly and weighed individually. Formula used for percentage weight gain calculation is shown in equation 2.10
Content uniformity
To study the uniformity of drug in prepared CPOP formulations, twenty tablets from each formulation were weighed and finely powdered. An accurately weighed portion of powder equivalent to 12.89 mg of Captopril was transferred to 100-ml volumetric flask containing 0.01 M HCl. Contents of the volumetric flask were manually mixed for a minute and then sonicated in a sonicator (Elmasonic–E30H–Elma-Hans, Germany) for a further 15 min. Solutions were filtered. Diluted samples were analyzed using spectrophotometer (UV-1900, BMS, Canada) at a wavelength of 205 nm. Drug content present in sample of each formulation was determined by already plotted linearity curve of Captopril.8
Drug release kinetics
To access the release mechanism, dissolution was performed using USP type-II paddle apparatus (Erweka, DT 600 Heusenstamm, Germany) at 100 rpm in 900 ml of 0.1 M hydrochloric acid pH 1.2 for first 2 h. followed by phosphate buffer pH 6.8 up to 24 h at 37 ± 0.50°C. Next, 5 ml test specimens were withdrawn after predetermined time intervals. Volume of each specimen withdrawn was replaced by equal volume of fresh dissolution medium. Specimens after filtration and suitable dilution were subjected to the spectrophotometric determination at a wavelength of 205 nm.11 Data obtained from in vitro dissolution studies were analyzed using various kinetic models such as first-order, zeroth-order, Higuchi, and Korsmeyer–Peppas.12,13
Effect of dissolution medium osmolality on %Captopril release
To confirm that osmotic pressure gradient is the main driving force for drug release from the developed osmotic device, in vitro drug release of optimized formulation was carried out in phosphate buffer pH 6.8 and phosphate buffer pH 6.8 containing 1 and 2 M NaCl.14
Effect of pH on %Captopril release
To study the effect of pH on drug release, dissolution of the optimized formulation was carried out in different media: 0.1 M HCl pH 1.2, acetate buffer solution pH 4.5, and phosphate buffer solution pH 6.8.15
FTIR spectroscopic analysis
To study the compatibility of drug with excipients, Fourier transform infrared (FTIR) spectroscopic analysis was performed by using ATR-FTIR spectrophotometer (Platinum-ATR, ALPHA, Bruker) over the wavelength range 400–4000 cm−1. CPOP tablet was crushed and converted into powder form for the analysis purpose. Average of the ten scans was used to understand the characteristic peaks of CA, PEG, and sorbitol.
Stability study
To study the stability of the developed product, the optimized formulation was stored in a stability chamber TH-1000G (Electrolab) maintained at 40°C and 75% RH for 6 months. The samples were withdrawn at different time intervals and evaluated for their appearance, average weight, thickness, diameter, and %drug release.
Statistical analysis
Mean and standard deviations were calculated by using Microsoft Excel.
Results and discussion
Physicochemical process of CPOP tablets
Physicochemical parameters of coated tablets were assessed, and the results are shown in Table 3. Weight variation of ± 5% was found in all formulations (524 ± 0.672–537 ± 0.919 mg) which were within the pharmacopoeial limits.11 %weight gain was 4.38–6.97%. The results of thickness (5.56 ± 0.01–5.79 ± 0.07 mm) and diameter (12.09 ± 0.12–12.23 ± 0.17 mm) as shown in Table 3 indicated a negligible variation from the average value, and Ahmed et al. also found insignificant variation from the mean value while manufacturing osmotic system of Eperisone HCl.14 These two parameters play an important role in determining the volume of a tablet. The major variation in thickness or diameter may influence the surface area of tablets, which in turn alter drug release characteristics.11,14 Thickness of leachable membrane was found to be 1.24–1.47 mm.
Content uniformity of CPOP tablets
The results of the content uniformity (assay) were within the prescribed limits (90–110%) for all formulations as shown in Table 3.11 It proved that all the contents are uniformly distributed throughout the formulation. Ahmed et al. also observed the similar results while manufacturing osmotic system of Eperisone HCl.14
Drug release kinetics for CPOP tablets
Drug release profiles of different CPOP tablets were fitted into various mathematical models to describe the mechanism of drug release. Table 4 shows the coefficient of correlation values obtained by analyzing in vitro release data of different formulations in various kinetic models. It is clearly evident from the results that drug release follows first-order kinetics. McClelland et al. and Zentner et al.16,17 also reported the release profile of diltiazem hydrochloride from controlled porosity osmotic pumps and found similar results. As shown in equation 3, CA showed negative effect but PEG-400 and sorbitol showed positive effect on %Captopril release. The interaction of CA and PEG-400 and of CA and sorbitol showed a negative effect on %Captopril release, while the interaction of PEG-400 and sorbitol showed a positive effect (Fig. 2). A 86.22–92.79% drug release was observed. Optimized formulation CPOP11 was selected on the basis of better release pattern of drug up to 24 h. Rapolu et al. studied the effect of different polymer concentrations on release profile of gastroretentive floating drug delivery system of metronidazole by using BBD.18 It was seen that the developed formulation draws water through the semipermeable membrane and water-soluble material leaches away, but the semipermeable membrane remains intact. Rathbone et al. also reported similar behavior of semipermeable membrane.2
Effect of dissolution medium osmolality on %Captopril release
As the amount of NaCl in dissolution medium increased, the rate of dissolution of the drug decreased (Fig. 3) due to a reduction in osmotic pressure gradient. This indicated osmosis-dependent drug release from the developed controlled porosity osmotic pump tablets. Thakkar et al. also reported that release of tapentadol hydrochloride from osmotic pumps was dependent on osmotic pressure difference.19
Effect of pH on %Captopril release
It is obvious from Fig. 4 that the release rate was unaffected by pH of the medium. A semipermeable membrane produced by CA does not readily allow the exchange of ions across it; thus, the drug present within the core can be delivered at a rate which is not altered by external environment pH. The delivery rate from the system is governed by osmotic pressure of the formulation and water permeability of the membrane and is therefore independent of the environmental pH. Verma et al., Theeuwes, and Santus and Baker also reported pH-independent drug release behavior of osmotic systems.3,20,21
FTIR spectroscopic analysis
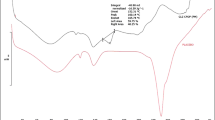
FTIR analysis of core tablet (Fig. 5a) has already been discussed in our previous article.8 The FTIR spectrum of final coating solution (Fig. 5b) contains strong peaks of –OH and –C–O stretching vibrations at 3373 and 1081 cm−1, respectively, and the peak of C–H stretching vibration appeared at 2937 cm−1. Several peaks of –C–H bending vibrations are observed between 1250 and 1418 cm−1. The above-mentioned peaks represent sorbitol.22 PEG showed the following characteristic peaks: O–H stretching 3432, C–H stretch of CH2 2889, C–H bending 1467, 1342, 1281, C–O stretching 1242, 1100, C–O deformation 963, and C–H deformation 842 cm−1.23 For CA, a broad absorbance peak at 3380 cm−1 is attributed to –OH stretching. Peaks at 2910 cm−1 and 2860 cm−1 refer to C–H stretching vibration. The band at 1750 cm−1 corresponds to the C–O stretching of ester group (carbonyl groups). The peak at 1435 cm−1 is due to O–C–OR stretching vibration. Peaks located at 1235 cm−1 and 1050 cm−1 refer to C–C–O and C–O stretching vibration.24 Figure 5c proved that no interaction was found among the ingredients of CPOP tablet.
Stability study
The formulation CPOP11 was found stable in terms of appearance, average weight, thickness, diameter, and in vitro release profile as shown in Table 5. The in vitro release profile of CPOP11, initially and after 6 months, was almost the same. Thus, the developed formulation was found to be stable for 6 months in accelerated stability studies. Asif et al. also performed stability study on Febuxostat tablets and found the similar results.25
Conclusions
Based on CPOP technique, oral CR formulation of Captopril was realistically prepared. Release kinetics were first-order while using in vitro kinetic models. Optimized formulation CPOP11 was selected on the basis of better release pattern of drug up to 24 h. The drug release was significantly altered by changing osmolality but not the pH of dissolution medium. FTIR was conducted, and no interaction was found. The product was found stable for 6 months in accelerated stability study. In future, it can be used to establish a system for CR of a variety of drugs.
References
Makhija, SN, Vavia, PR, “Controlled Porosity Osmotic Pump-Based Controlled Release Systems of Pseudoephedrine: I. Cellulose Acetate as a Semipermeable Membrane.” J. Control. Release, 89 (1) 5–18 (2003)
Rathbone, MJ, Hadgraft, J, Roberts, MS, Lane, ME, Modified-Release Drug Delivery Technology. CRC Press, Boca Raton (2008)
Verma, RK, Krishna, DM, Garg, S, “Formulation Aspects in the Development of Osmotically Controlled Oral Drug Delivery Systems.” J. Control. Release, 79 (1–3) 7–27 (2002)
Gupta, S, Singh, RP, Sharma, R, Kalyanwat, R, Lokwani, P, “Osmotic Pumps: A Review.” Int. J. Compr. Pharm., 2 (6) 1–8 (2011)
Keraliya, RA, Patel, C, Patel, P, Keraliya, V, Soni, TG, Patel, RC, Patel, M, “Osmotic Drug Delivery System as a Part of Modified Release Dosage Form.” ISRN pharmaceutics, 2012 (2012)
Aulton, ME, Pharmaceutics: The Science of Dosage Form Design. Churchill Livingstone, London (2000)
Aulton, ME, Taylor, KM, Aulton’s Pharmaceutics E-Book: The Design and Manufacture of Medicines. Elsevier, Amsterdam (2017)
Akhtar, MF, Hanif, M, Majeed, A, Shah, S, “Formulation Development and Optimization of Captopril Containing Tablets Through Box-Behnken Design.” Latin Am. J. Pharm., 37 (7) 1414–1423 (2018)
Abbas, G, Hanif, M, Khan, MA, “pH Responsive Alginate Polymeric Rafts for Controlled Drug Release by Using Box Behnken Response Surface Design.” Des. Monomers Polym., 20 (1) 1–9 (2017)
Anju, CL, Palanichamy, S, Rajesh, M, Ramasubramaniyan, P, Solairaj, P, “Formulation and Evaluation of Osmotic Drug Delivery System of Ibuprofen.” Ars Pharm., 55 (4) 44–51 (2014)
Pharmacopoeia, B, “Specific Monograph: British Pharmacopoeia Commission.” London (2015)
Qazi, F, Shoaib, MH, Yousuf, RI, Qazi, TM, Mehmood, ZA, Hasan, SMF, “Formulation Development and Evaluation of Diltiazem HCl Sustained Release Matrix Tablets Using HPMC K4 M and K100M.” Pak. J. Pharm. Sci., 26 (4) 653–663 (2013)
Costa, P, Lobo, JMS, “Modeling and Comparison of Dissolution Profiles.” Eur. J. Pharm. Sci., 13 (2) 123–133 (2001)
Ahmed, K, Shoaib, MH, Yousuf, RI, Qazi, F, Anwer, S, Nasiri, MI and Mahmood, ZA, “Use of Opadry® CA—A Cellulose Acetate/Polyethylene Glycol System for Rate‐Controlled Osmotic Drug Delivery of Highly Soluble Antispastic Agent Eperisone HC l.” Adv. Polym. Tech., 37 (8) 2730–2742 (2018)
Pharmacopeia, U, “USP 39 NF 34.” (2015)
McClelland, GA, Sutton, SC, Engle, K, Zentner, GM, “The Solubility-Modulated Osmotic Pump: In Vitro/In Vivo Release of Diltiazem Hydrochloride.” Pharm. Res., 8 (1) 88–92 (1991)
Zentner, GM, McClelland, GA, Sutton, SC, “Controlled Porosity Solubility- and Resin-Modulated Osmotic Drug Delivery Systems for Release of Diltiazem Hydrochloride.” J. Control. Release, 16 (1–2) 237–243 (1991)
Rapolu, K, Sanka, K, Vemula, PK, Aatipamula, V, Mohd, AB, Diwan, PV, “Optimization and Characterization of Gastroretentive Floating Drug Delivery System Using Box-Behnken Design.” Drug Dev. Ind. Pharm., 39 (12) 1928–1935 (2013)
Thakkar, HP, Pancholi, N, Patel, CV, “Development and Evaluation of a Once-Daily Controlled Porosity Osmotic Pump of Tapentadol Hydrochloride.” AAPS PharmSciTech, 17 (5) 1248–1260 (2016)
Theeuwes, F, “Elementary Osmotic Pump.” J. Pharm. Sci., 64 (12) 1987–1991 (1975)
Santus, G, Baker, RW, “Osmotic Drug Delivery: A Review of the Patent Literature.” J. Control. Release, 35 (1) 1–21 (1995)
Shaikh, SF, Mane, RS, Min, BK, Hwang, YJ, Joo, O, “D-Sorbitol-Induced Phase Control of TiO2 Nanoparticles and Its Application for Dye-Sensitized Solar Cells.” Sci. Rep., 6 20103 (2016)
Chen, C, Liu, W, Wang, Z, Peng, K, Pan, W, Xie, Q, “Novel Form Stable Phase Change Materials Based on the Composites of Polyethylene Glycol/Polymeric Solid-Solid Phase Change Material.” Sol. Energy Mater. Sol. Cells, 134 80–88 (2015)
Zhijiang, C, Yi, X, Haizheng, Y, Jia, J, Liu, Y, “Poly (Hydroxybutyrate)/Cellulose Acetate Blend Nanofiber Scaffolds: Preparation, Characterization and Cytocompatibility.” Mater. Sci. Eng., C, 58 757–767 (2016)
Asif, U, Sherwani, AK, Akhtar, N, Shoaib, MH, Hanif, M, Qadir, MI, Zaman, M, “Formulation Development and Optimization of Febuxostat Tablets by Direct Compression Method.” Adv. Polym. Technol., 35 (2) 129–135 (2016)
Acknowledgment
Authors are very much thankful to the department of research and development of Bahauddin Zakariya University Multan, Pakistan, and Higher Education Commission (HEC), Pakistan, for providing the financial grant and Department of Pharmaceutics, Bahauddin Zakariya University Multan, Pakistan, for providing laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akhtar, M.F., Hanif, M. Leachable pegylated cellulose acetate complex: a promising approach for controlled porosity osmotic pump tablets of Captopril. J Coat Technol Res 17, 439–446 (2020). https://doi.org/10.1007/s11998-019-00290-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-019-00290-7