Abstract
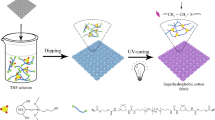
A one-pot sonochemical irradiation method was developed for the fabrication of superhydrophobic and superoleophilic cotton fabric from a solution consisting of branched silica nanoparticles and tetraethoxysilane-dodecyltrimethoxysilane sol. The silica/sol-coated cotton fabric could be wetted by liquids of low surface tension, but was water repellent with a water contact angle of 159 ± 1.2° and water shedding angle of 6 ± 0.8°. The as-prepared cotton fabric could be used as effective materials for the separation of oil from water with separation efficiency as high as 98.2% and maintained separation efficiency above 94% after 30 separation cycles for the kerosene-water mixture. Moreover, the superhydrophobic and superoleophilic cotton fabric could maintain stable superhydrophobicity after treatment with strong acidic and alkali solutions, and harsh mechanical damage. Therefore, this reported robust superhydrophobic cotton fabric exhibits encouraging practical application for oil-water separation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the development of industrial productions, as well as frequent offshore oil exploration, production, and transportation, the leakage of oil and fuel has become one of the most serious environmental problems.1,2 Oil-polluted water includes numerous toxic compounds, which result in a disastrous threat to the marine and aquatic ecosystems. Therefore, cleaning up oil spills from water is attracting worldwide attention. In past, several conventional methods involving oil skimmers, direct burning, oil containment booms, and chemical degradation were applied.3,4,5,6 However, these techniques generally showed some common drawbacks such as large floor space, time-consuming procedures, and low separation efficiency.
Recently, solid materials with special wettabilities and microscale-nanoscale binary structures have received considerable interest for oil-water separation.7,8,9 To overcome the drawbacks of the general oil-water separation methods, materials that simultaneously display reverse wettability such as superhydrophobic-superoleophilic or superhydrophilic-superoleophobic properties have been investigated as a means to separate oil and water.10,11,12 These materials can selectively absorb either oil or water while simultaneously repelling another liquid, thus facilitating separation of the two immiscible liquids with high efficiency.13,14 Quite a few of such functional materials were developed for the separation of oil from water, including sponges and foam-based materials,15,16,17 carbon-based materials,18,19,20 mesh-membrane materials,21,22,23,24 and filter paper.25 However, challenges still existed for the large-scale fabrication of such functional materials because of their high cost, low commercial availability of raw materials, time-consuming procedures, harsh practical conditions, as well as poor mechanical stability, and weak flexibility. Therefore, it is necessary to explore facile, low-cost methods for preparing such materials.
Cotton fabric, a kind of soft and flexible organic material, exhibits excellent comprehensive characteristics like nontoxicity, porous surface, high absorption ability, good elasticity, and easily scalable fabrication. Therefore, cotton fabric is considered to be a good candidate to realize the separation of oils from water after constructing a rough microstructure on fabric surfaces with both superhydrophobic and superoleophilic properties. Recently, a variety of methods were developed to fabricate superhydrophobic fabrics, such as drop coating,26,27 grafting polymerization,28 hydrothermal process,29 sol-gel method,30,31 chemical vapor deposition,32 and so forth.33,34 For practical applications, harsh environments like strong light, high temperature, mechanical abrasion or corrosive solution may damage the surface coatings of cotton fabric easily; thus, the stability of the as-prepared superhydrophobic cotton fabrics is critically important. Hence, polymer binders and silica bridges through sol-gel reactions were utilized in order to improve the environmental durability of superhydrophobic fabrics.30,35,36,37 Nevertheless, the fabrication of superhydrophobic fabrics was often complicated and not cost-efficient.
Sonochemical irradiation has been proven as an effective technique for the deposition and insertion of nanoparticles on fabrics.38,39 In this work, we report on a facile and simple one-pot sonochemical irradiation method to fabricate the silica/sol-coating on the cotton fabrics. The as-prepared cotton fabric exhibited durable superhydrophobic and superoleophilic properties with rough hierarchical structure and low surface energy, which were derived from the assembly of branched silica nanoparticles and the hydrolysis and condensation of tetraethoxysilane-dodecyltrimethoxysilane (TEOS/DTMS) sol. Due to its reverse wettability, the superhydrophobic and superoleophilic cotton fabric could capture oils from water and be used as effective materials for the separation of oil from water with high separation efficiency and good reusability. In addition, the as-prepared robust cotton fabric could remain superhydrophobic after various harsh treatments such as immersion in corrosive solutions (acidic, basic liquids), and harsh mechanical damage (adhesive tape and sandpaper abrasion tests). These excellent properties make the superhydrophobic fabric an ideal material for oil-water separation even under harsh conditions.
Experimental
Materials
Branched silica nanoparticles (30 wt%) dispersed in ethanol were supplied by Nanjing Dongjian Biological Technology Co., LTD. XRD results indicate that the branched silica nanoparticle had amorphous structure (Fig. S1, Supporting Information). Dodecyltrimethoxysilane (DTMS, 99.5%) was obtained from Alfa Aesar. Kerosene and soybean oil were commercial products. Tetraethoxysilane (TEOS), sodium hydroxide (NaOH), ammonia solution (25%), ethanol, n-hexadecane, chloroform, hexane, and toluene were purchased from Sinopharm Chemical Reagents and used without further purification. Desized, scoured, bleached, and mercerized woven pure cotton fabrics, weight per unit area 245.6 gm−2, were purchased from a local market, and were ultrasonically cleaned before usage with ethanol and ultrapure water to remove possible impurities.
Fabrication of superhydrophobic cotton fabric
In a typical procedure to prepare the superhydrophobic cotton fabric, the cotton fabrics were first immersed in the 5 wt% aqueous NaOH solution for 10 min at room temperature followed by rinsing with ultrapure water. Then, ethanol (60 mL), ultrapure water (6 mL), ammonia solution (3 mL), and 0.75 g of branched silica nanoparticles ethanol solution were mixed by magnetic stirring at room temperature for 15 min. TEOS (0.3 mL) and DTMS (0.15, 0.3, and 0.45 mL) were slowly added into the mixture and stirred at room temperature for 15 min. Subsequently, the pretreated cotton fabric was immersed into the mixture and treated with a KQ-200KDE ultrasound instrument from Kunshan ultrasonic instruments Co. LTD (China) for 2 h. After reaction, the sample was removed from the solution and thoroughly washed with ethanol and then annealed at 60°C for 1 h to remove the residual solvent and cure the particulate films. For comparison, one reference cotton fabric sample was fabricated by reaction of the cotton fabric with branched silica nanoparticles and TEOS/DTMS under magnetic stirring without sonication, to highlight the effects of the reaction conditions on the wetting properties of the cotton fabric.
Stability tests of the superhydrophobic fabrics
To investigate the durability of a resultant superhydrophobic fabric, continuous immersion into corrosive solutions, adhesive tape tear and sandpaper abrasion tests were carried out. The superhydrophobic cotton fabric was fixed on the surface of a glass slide and then immersed in a solution with different pH values. After immersion for 6 h, the cotton fabric was dried in an oven and the water contact angles and water shedding angles of the cotton fabric were recorded accordingly. Tear test was carried out by pasting an adhesive tape on the fabric surface at 2.5 kPa and then peeling off. This process was repeated 30 times, and the corresponding water contact angle and water shedding angles after each five cycles were recorded. Sandpaper abrasion test was carried out according to a previously reported method.35,40 The superhydrophobic cotton fabric (2 × 2 cm2) was fixed onto the bottom of a stainless steel column and moved back and forth on the 2000 mesh sandpaper at 2.5 kPa. The move speed and abrasion length were about 3 cm s−1 and 20 cm in each cycle, respectively.
Characterizations
The morphology of branched silica nanoparticles was investigated using a Jeol-7500F field-emission scanning electron microscope (FE-SEM) operated at 20.0 kV. The surface morphology and chemical composition of cotton fabrics were investigated by a 3400-I scanning electron microscope (SEM) operated at 15 kV. The powder X-ray diffraction (XRD) pattern of branched silica nanoparticles was characterized by a Bruker AXS D8-FOCUS X-ray diffractometer. FTIR spectra were recorded on a Thermo Nicolet iS10 spectrophotometer with a scanning range of 500–4000 cm−1. Elemental information on the modified silica nanoparticles was obtained using Thermo VG ESCALab-250 X-ray photoelectron spectroscopy (XPS). Water and oil contact angles were characterized using a KRÜSS DSA100 drop shape analysis instrument at ambient temperature by sessile drop method. Each liquid droplet was 10 μL. Each contact angle value was a statistic average of five measurements on different positions of the fabric surface. Water shedding angle was measured based on the literature41 using a 10 μL water droplet. The testing was repeated five times for each sample, and the average water shedding angle was obtained.
Results and discussion
Fabrication of superhydrophobic cotton fabrics
The schematic illustration of the fabrication of superhydrophobic cotton fabrics via a one-pot sonochemical irradiation process is illustrated in Fig. 1a. In this study, a kind of branched silica nanoparticle was utilized as a building block to construct the porous nanostructure on the cotton fabrics. The branched silica nanoparticles appeared as the aggregation of primary spherical nanoparticles (diameter of 40–50 nm) and thus possessed several arm-like branches (Fig. 1b). A loosely packed structure with nanopores could be formed after solvent evaporation of the branched nanoparticles ethanol solution (Fig. 1c), which is beneficial for building the rough structure on the fabric surface. A number of hydroxyl groups are present on the branched SiO2 nanoparticles surface. It is necessary to modify the branched SiO2 nanoparticles with hydrophobic material to obtain superhydrophobicity. In addition, to improve the durability of superhydrophobic coatings, connection of the branched SiO2 nanoparticles and the cotton fabric, and the neighboring nanoparticles with functional groups is equally important. In this study, TEOS and DTMS were mixed with the branched SiO2 nanoparticles and NaOH pretreated cotton fabric in an ammonia-ethanol solution. The NaOH solution pretreatment improved the accessibility of hydroxyl groups of cotton fiber due to the disruption of the interchain hydrogen bonds of cellulose.42 With the catalysis of ammonia, TEOS and DTMS formed polymer sol after the hydrolysis and partial condensation and reacted with the hydroxyl groups on the silica nanoparticles and fiber surfaces under ultrasonic irradiation.
Surface morphology and structure of superhydrophobic cotton fabrics
Figure 2 shows the SEM images of the cotton fabrics before and after being treated with branched silica nanoparticles and TEOS/DTMS sol solution under a sonochemical irradiation process. As shown in Figs. 2a and b, the pristine cotton fabric before the treatment showed a highly textured microscale fiber with a relatively smooth surface and evident grooves along the fiber. However, the fibers after being treated with silica nanoparticles-sol solution demonstrated a rough structure (Figs. 2c and d). The low-magnification image (Fig. 2c) indicates that the SiO2 nanoparticles covered all the fibers and aggregated around the grooves between neighboring fibers of fabrics due to the assembly of branched silica nanoparticles. A high-magnification SEM image (Fig. 2d) shows that the branched SiO2 nanoparticles with TEOS/DTMS sol were aggregated on the fiber surface, leading to the microscale-nanoscale roughness. Such hierarchical microscaled-nanoscaled roughness is essential for the water repellency of the cotton fabrics. For the branched SiO2 nanoparticles and TEOS/DTMS-treated fabric in the absence of sonication irradiation, the SEM image was also obtained, as shown in Fig. S2 (Supporting Information). It was found that the particles were agglomerated and randomly distributed in the spaces of interfibers of fabrics, while the fiber surface was uncovered with particles. This result demonstrated that sonication treatment played an effective role in the formation of homogeneous coating on the fibers. In ultrasonic irradiation the reaction mixture caused two primary effects, which involved heating and acoustic cavitation (bubble formation, growth, and collapse). When the acoustic cavitation bubbles collapsed near the surface of the cotton fiber, the strong shock waves and the microjets formed and caused effective stirring-mixing of the mixture. In our case, the ultrasonic waves promoted fast migration of the silica nanoparticles to the fiber surface. In addition, the powerful ultrasonic waves might have caused local melting of the fibers at the contact sites, which might be the reason why the particles strongly adhered to the fabric.43
The chemical structure of the branched SiO2 nanoparticles with and without TEOS/DTMS sol was characterized by FTIR and XPS (Fig. 3). Figure 3a shows FTIR analysis of particles. For the TEOS/DTMS sol-modified SiO2 nanoparticles, the peaks at 2921, 2851, and 1465 cm−1 corresponding to the stretching and bending of CH2 groups appeared, while they were absent in the spectrum of the pure branched SiO2 nanoparticles. Figure 3b shows the XPS spectrum of the modified SiO2 nanoparticles. The peaks corresponding to O1s, C1s, and Si2p appeared, indicating that the modified SiO2 nanoparticles were mainly composed of oxygen, carbon, and silicon. This was in accordance with the FTIR results and demonstrated that the hydrolyzed DTMS reacted with the SiO2 nanoparticles.
Wettability of superhydrophobic cotton fabrics
Generally, the wetting behavior of a solid surface is governed by its surface chemistry and surface roughness, represented by dual-scale structures. Cotton fabrics used in this work are porous and rough substrate, which have interwoven bundles of fibers. Each bundle contains several layers of individual micrometer-sized fibers. Therefore, microscale roughness is naturally provided by cellulose fiber and further nanoscale roughness is necessary for the formation superhydrophobic surface. In addition, low surface energy material is needed for its conversion into the superhydrophobic surface. Here, as shown in SEM photographs, FTIR, and XPS, the nanoscaled roughness and low surface energy coating were formed through the deposition of branched SiO2 nanoparticles on the surface of cellulose fiber along with the hydrolysis and condensation of DTMS.
In order to characterize the superhydrophobic and superoleophilic properties of the coated cotton fabric (TEOS 0.3 mL and DTMS 0.3 mL in the presence of the brunched silica nanoparticles), we measured the contact angles at room temperature from 10 μL liquids of different surface tension, including water (γ = 72.8 mN m−1) and n-hexadecane (γ = 27.5 mN m−1). However, the contact angle measurement of the superhydrophobic fabric is often not accurate and straightforward. It is usually difficult to determine the outline of the water droplet because the fabric surfaces are macroscopically rough. Therefore, the water shedding angle technique41 was employed to evaluate the wetting properties of superhydrophobic fabrics in a relatively accurate way besides the contact angle measurement in this study.
Due to the presence of abundant hydroxyl groups on the cellulose fibers, the pristine cotton fabric was superamphiphilic. As seen in Fig. 4a, water and n-hexadecane spread quickly on the fabric surface and were absorbed totally. After coating the fabric with the nanoparticles and sol, the water droplet stood on the cotton fabric surface like a ball and was easy to slip off. However, n-hexadecane was immediately absorbed by the coated cotton fabric (Fig. 4b). The cotton fabric surface exhibited superhydrophobicity with a water contact angle of 159 ± 1.2° and a water shedding angle of 6 ± 0.8° (Fig. 4c). For n-hexadecane, the contact angle was nearly 0°, indicating the good superoleophilicity of the coated cotton fabric (Fig. 4d). For comparison, the wetting properties of the cotton fabric fabricated without sonication treatment were investigated. In this case, the water droplets on the fabric surface were adsorbed and disappeared in seconds, indicating the weak hydrophobicity, which demonstrated the substantial effect of sonication in obtaining superhydrophobicity. In addition, altering the amount of DTMS introduced in the reaction system while maintaining the amount of TEOS constant, it was found that the volume ratio of DTMS/TEOS had a significant effect on the water contact angle and water shedding angle of the fabric (Fig. S3, Supporting Information). When DTMS and TEOS were mixed in volume ratio of 1:2 (DTMS 0.15 mL and TEOS 0.3 mL) with the branched SiO2 nanoparticles, the average water contact angle was about 142 ± 1° and water shedding angle was about 23 ± 0.9°, showing high hydrophobicity, but not superhydrophobicity. When the ratio was increased to 1:1 (DTMS 0.3 mL and TEOS 0.3 mL), the fabric exhibited superhydrophobicity. A further increase in the amount of DTMS had little effect on the water contact angle and water shedding angle. This might be because the surface of the rough hierarchical-structured fabric was nearly saturated by the induced long-chain hydrophobic alkyls of DTMS. Therefore, we considered the optimized volume ratio of TEOS to DTMS to be 1:1 for the fabrication of superhydrophobic cotton fabric.
Oil-water separation of the superhydrophobic cotton fabric
The phenomena of water repellency and oil absorption are very important to the oil-water separation of superhydrophobic cotton fabric. The superhydrophobic cotton fabric could selectively absorb oil as shown in Fig. 5. We poured some water into a Petri dish and added soybean oil (dyed with Oil Red O) to form a thin layer, and then floated a piece of treated cotton fabric on the liquid surface. The oil was completely removed within a few seconds, as shown in Fig. 5a. The soybean oil sorption capacity of the treated cotton fabric was 1.16 g/g. Besides, the capture and transportation of organic chemicals that had higher densities than water (in this case chloroform, dyed with Red oil O) were also performed using the superhydrophobic cotton fabric. When the superhydrophobic cotton fabric was immersed underwater to contact with chloroform, the chloroform droplet was immediately sucked in by the fabric (Fig. 5b).
To investigate the separation capability of the superhydrophobic cotton fabric, an oil-water separation experiment was performed by fixing the superhydrophobic cotton fabric between two glass tubes as a filter membrane, as shown in Fig. 6a and Movie S1 (Supporting Information). When the oil-water mixture (kerosene-water mixture) was poured into the simple device continuously, oil passed through the cotton fabric and dropped into the beaker underneath, while water was retained above the surface. Thus, the oil-water mixture was separated successfully by a simple filtering method with an oil (kerosene) penetration flux of 3.54 L s−1 m−2. The oil penetration flux was obtained by calculating the volume of the oil through a certain size of the coated fabric within a certain period of time for several times.11 In order to understand the oil-water mixture separation mechanism of the superhydrophobic and superoleophilic cotton fabric, the schematic diagram of the oil-water mixture separation process is shown in Fig. 6b. Before separation, when a water droplet was put onto the superhydrophobic fabric, a small amount of air was trapped in the microstructures and formed an air bubble on the fabric. Thus, the water droplet could easily move away from the fabric, while the oil droplet could quickly penetrate the as-prepared superoleophilic fabric, and there was no air bubbles on the fabric, because oil was trapped in the microstructures of the fabric. When the oil-water mixture was poured onto the fabric surface, the superoleophilic fabric could absorb oil and be quickly saturated by the oil in all the contact areas, resulting in a stable water-oil-solid tri-phase interface.11 As a result, oil permeated through the coated fabrics owing to the rough hierarchical-structured fabric fibers and their spacing, whereas the water stays on the fabric surface because of the silica nanoparticles and TEOS/DTMS sol-induced superhydrophobicity. Therefore, the as-prepared superhydrophobic and superoleophilic fabrics could be used effectively for the separation of oil and water.
Separation efficiency was calculated by the weight ratio of oil after and before the separation process. Five kinds of oil-water mixtures (kerosene-water, soybean oil-water, chloroform-water, hexane-water, and toluene-water) were used in the separation experiment. As provided in Fig. 6c, the fabricated superhydrophobic cotton fabric demonstrated separation efficiency higher than 95% for all the five kinds of oil-water mixtures. The reusability of the superhydrophobic cotton fabric is an important criterion in practical application. In this paper, the oil-contaminated cotton fabrics were cleaned with ethanol and dried for reuse after each oil-water separation cycle. The relationship between separation efficiency and cycle numbers of kerosene-water separation is given in Fig. 6d. The separation efficiency decreased with the increase in separation cycles. However, after 30 separation cycles, the superhydrophobic cotton fabric still possessed high separation efficiency up to 94% for the kerosene-water mixture, revealing the reusability of the modified cotton fabric was excellent.
In addition, intrusion pressure of water was introduced to study the separation stability of the as-prepared cotton fabric. The intrusion pressure of water is determined by the maximum height of water that the as-prepared superhydrophobic cotton fabric can support and the weight of water is also involved. Therefore, the intrusion pressure (P) value was calculated by the following equation:
where ρ is the density of water (that is, 1 × 103 g m−3), g is acceleration of gravity (that is, 9.8 m s−2), and h max is the maximum height of water that the modified fabric can support. The h max of the as-prepared superhydrophobic cotton fabric was 207 mm (Fig. S4, Supporting Information), and the intrusion pressure of water was about 2029 Pa. Water does not flow through the modified fabric under the pressure. The intrusion pressures of oils were 0 due to the superoleophilic property. Consequently, the as-prepared fabric had a high capability for the separation of a large amount of oil-water mixtures.
Durability tests of the superhydrophobic cotton fabric
The chemical stability and mechanical robustness of the coating are important in real applications. In general, most of the artificial superhydrophobic and superoleophilic surfaces have weak chemical stability and mechanical wear resistance toward corrosive liquid and external forces treatment, due to the vulnerable surface texture of superhydrophobic coatings. The chemical stability of the fabricated superhydrophobic cotton fabrics was evaluated by exposing them in ambient air for 3 months and immersing them into corrosive liquids. After the exposure tests, the superhydrophobic cotton fabric did not show any visible change, indicating good stability of the superhydrophobic cotton fabric. The superhydrophobic cotton fabric was also immersed in corrosive liquids in the entire pH range from 1 to 14 for 6 h. The variation of the water contact angle and the water shedding angle of the superhydrophobic cotton fabric after being immersed into corrosive solutions with different pH values was recorded (Fig. 7). The water contact angle and the water shedding angle changed little, and there was no evident change in the surface morphologies of the treated fabric after immersing them in strong acid and base liquid (Fig. S5, Supporting Information), indicating the excellent chemical stability of the silica nanoparticle-sol coatings.
The mechanical durability of the superhydrophobic and superoleophilic cotton fabric was assessed by adhesive tape tear and sandpaper abrasion tests. Figure 8a depicts the tear process with adhesive tape. Tear test was completed by peeling off the adhesive tape, which was pasted on the superhydrophobic cotton fabric at 2.5 kpa. Figure 8b shows the variation of the water contact angles and the water shedding angles of superhydrophobic fabrics with the number of peeling. Only a slight decline of the water contact angle (>154°) and an increase in the water shedding angle (<10°) were observed after 30 tear test cycles. The surface morphology of the fabrics after the tear test still exhibited the rough hierarchical structure (Fig. S6, Supporting Information), suggesting the strong adhesion of the silica nanoparticle-sol coating on the surface of the fibers.
(a) Process of adhesive tape test. (b) Water contact angles and water shedding angles of superhydrophobic fabric repeatedly torn by adhesive tape. (c) Variation of water contact angles and water shedding angles of superhydrophobic fabrics scratched by the sandpaper and (d) the corresponding separation efficiency of the oil-water mixture
To further demonstrate this, the sandpaper abrasion test was performed (Fig. S3, Supporting Information). The cotton fabric was fixed onto the stainless steel column and moved back and forth on sandpaper (2000 meshes) at 2.5 Kpa with a speed and abrasion length of about 3 cm s−1 and 20 cm in each cycle, respectively. The change in the water contact angles and the water shedding angles of the superhydrophobic cotton fabric with abrasion cycles is shown in Fig. 8c. The water contact angle decreased, and the water shedding angle increased gradually with the increase in the abrasion cycles. After 800 abrasion cycles, the cotton fabric was still superhydrophobic with water contact angle close to 150° and the water shedding angle less than 20°. The rough hierarchical structure of the superhydrophobic fabric was reserved besides the removal of a small fraction of SiO2 nanoparticles on the fiber surface after the abrasion test (Fig. S7, Supporting Information). The separation efficiency of the oil-water mixture was decreased during the abrasion test (Fig. 8d), which might be caused by the residual oil adhering on the fabric fiber. However, the separation efficiency still reached 94.5% after 800 abrasion cycles using the kerosene-water mixture as an example, indicating superior separation efficiencies for the oil-water mixture after the abrasion test.
Conclusions
In summary, robust and stable superhydrophobic and superoleophilic cotton fabric with rough hierarchical structure and low surface energy was prepared using branched silica nanoparticles dispersed in TEOS/DTMS sol solution via a versatile and one-pot sonochemical irradiation approach. The as-prepared cotton fabric could selectively remove oils from water with high separation efficiency. The separation process could be repeated for at least 30 cycles with separation efficiency above 94% (kerosene-water mixture), demonstrating the good reusability. Moreover, the as-prepared cotton fabric exhibited stable superhydrophobicity toward corrosive solutions (acidic and basic liquids), and harsh mechanical damage (adhesive tape tear and sandpaper abrasion). Due to the high separation efficiency, good reusability, and remarkable durability, the fabricated functionalized fabrics showed great potential for the separation of oil spills and other organic chemicals from water.
References
Wang, B, Liang, W, Guo, Z, Liu, W, “Biomimetic Super-Lyophobic and Super-Lyophilic Materials Applied for Oil/Water Separation: A New Strategy Beyond Nature.” Chem. Soc. Rev., 44 336–361 (2015)
Xue, Z, Wang, S, Lin, L, Chen, L, Liu, M, Feng, L, Jiang, L, “A Novel Superhydrophilic and Underwater Superoleophobic Hydrogel-Coated Mesh for Oil/Water Separation.” Adv. Mater., 23 4270–4273 (2011)
Al-Majed, AA, Adebayo, AR, Hossain, ME, “A Sustainable Approach to Controlling Oil Spills.” J. Environ. Manag., 113 213–227 (2012)
Lessard, RR, DeMarco, G, “The Significance of Oil Spill Dispersants.” Spill. Sci. Technol. Bull., 6 59–68 (2000)
Jadhav, SR, Vemula, PK, Kumar, R, Raghavan, SR, John, G, “Sugar-Derived Phase-Selective Molecular Gelators as Model Solidifiers for Oil Spills.” Angew. Chem., 49 7695–7698 (2010)
Wang, FJ, Lei, S, Li, CQ, Qu, JF, Xue, MS, Li, W, “Superhydrophobic Cu Mesh Combined with a Superoleophilic Polyurethane Sponge for Oil Spill Adsorption and Collection.” Ind. Eng. Chem. Res., 53 7141–7148 (2014)
Feng, L, Zhang, Z, Mai, Z, Ma, Y, Liu, B, Jiang, L, Zhu, D, “A Super-Hydrophobic and Super-Oleophilic Coating Mesh Film for the Separation of Oil and Water.” Angew. Chem. Int. Ed., 43 2012–2014 (2004)
Jing, B, Wang, H, Lin, KY, McGinn, PJ, Na, C, Zhu, Y, “A Facile Method to Functionalize Engineering Solid Membrane Supports for Rapid and Efficient Oil–Water Separation.” Polymer, 54 5771–5778 (2013)
Cao, Y, Liu, N, Fu, C, Li, K, Tao, L, Feng, L, Wei, Y, “Thermo and pH Dual-Responsive Materials for Controllable Oil/Water Separation.” ACS Appl. Mater. Interfaces, 6 2026–2030 (2014)
Ouyang, SS, Wang, T, Jia, XY, Chen, Y, Yao, JM, Wang, S, “Self-Indicating and Recyclable Superhydrophobic Membranes for Effective Oil/Water Separation in Harsh Conditions.” Mater. Des., 96 357–363 (2016)
Zheng, X, Guo, ZY, Tian, DL, Zhang, XF, Li, WX, Jiang, L, “Underwater Self-Cleaning Scaly Fabric Membrane for Oily Water Separation.” ACS Appl. Mater. Interfaces, 7 (7) 4336–4343 (2015)
Tian, DL, Zhang, XF, Wang, X, Zhai, J, Jiang, L, “Micro/nanoscale Hierarchical Structured ZnO Mesh Film for Separation of Water and Oil.” Phys. Chem. Chem. Phys., 13 14606–14610 (2011)
Wu, L, Zhang, JP, Li, BC, Wang, AQ, “Mechanical- and Oil-Durable Superhydrophobic Polyester Materials for Selective Oil Absorption and Oil/Water Separation.” J. Colloid. Interface Sci, 413 112–117 (2014)
Li, BC, Wu, L, Li, LX, Seeger, S, Zhang, JP, Wang, AQ, “Superwetting Double-Layer Polyester Materials for Effective Removal of Both Insoluble Oils and Soluble Dyes in Water.” ACS Appl. Mater. Interfaces, 6 (14) 11581–11588 (2014)
Pham, VH, Dickerson, JH, “Superhydrophobic Silanized Melamine Sponges as High Efficiency Oil Absorbent Materials.” ACS Appl. Mater. Interfaces, 6 14181–14188 (2014)
Chen, N, Pan, QM, “Versatile Fabrication of Ultralight Magnetic Foams and Application for Oil–Water Separation.” ACS Nano., 7 6875–6883 (2013)
Calcagnile, P, Fragouli, D, Bayer, IS, Anyfantis, GC, Martiradonna, L, Cozzoli, PD, Cingolani, R, Athanassiou, A, “Magnetically Driven Floating Foams for the Removal of Oil Contaminants from Water.” ACS Nano., 6 5413–5419 (2012)
Sun, HY, Xu, Z, Gao, C, “Multifunctional, Ultra-Flyweight, Synergistically Assembled Carbon Aerogels.” Adv. Mater., 25 2554–2560 (2013)
Liang, HW, Guan, QF, Chen, LF, Zhu, Z, Zhang, WJ, Yu, SH, “Macroscopic-Scale Template Synthesis of Robust Carbonaceous Nanofiber Hydrogels and Aerogels and Their Applications.” Angew. Chem. Int. Ed., 51 5101–5105 (2012)
Wang, J, Shang, LR, Cheng, Y, Ding, HB, Zhao, YJ, Gu, ZZ, “Microfluidic Generation of Porous Particles Encapsulating Spongy Graphene for Oil Absorption.” Small, 11 3890–3895 (2015)
Kwon, G, Post, E, Tuteja, A, “Membranes with Selective Wettability for the Separation of Oil–Water Mixtures.” MRS Commun., 5 475–494 (2015)
Liu, MM, Li, J, Shi, L, Guo, ZG, “Stable Underwater Superoleophobic Conductive Polymer Coated Meshes for High-Efficiency Oil–Water Separation.” RSC Adv., 5 (42) 33077–33082 (2015)
Tian, DL, Guo, ZY, Wang, YL, Li, WX, Zhang, X, Zhai, J, Jiang, L, “Photo-Tunable Underwater Oil-Adhesion of the Micro/Nanoscale Hierarchical Structured ZnO Mesh Films with Switchable Contact Mode.” Adv. Funct. Mater., 24 536–542 (2014)
Tian, DL, Zhang, XF, Tian, Y, Wu, Y, Wang, X, Zhai, J, Song, YL, Jiang, L, “Photo-Induced Water-Oil Separation Based on Switchable Superhydrophobicity-Superhydrophilicity and Underwater Superoleophobicity of the Aligned ZnO Nanorod Array-Coated Mesh Films.” J. Mater. Chem., 22 19652–19657 (2012)
Feng, XJ, Shi, YL, Liu, J, Yang, W, “Fabrication of Filter Paper with Tunable Wettability and Its Application in Oil–Water Separation.” J. Sol–Gel Sci. Technol., 76 129–137 (2015)
Zhang, M, Wang, CY, Wang, SL, Li, J, “Fabrication of Superhydrophobic Cotton Textiles for Water–Oil Separation Based on Drop-Coating Route.” Carbohydr. Polym., 97 59–64 (2013)
Wu, L, Zhang, JP, Li, BC, Wang, AQ, “Mimic Nature, Beyond Nature: Facile Synthesis of Durable Superhydrophobic Textiles Using Organosilanes.” J. Mater. Chem. B, 1 4756–4763 (2013)
Li, SH, Huang, JY, Ge, MZ, Li, SW, Xing, TL, Chen, GQ, Liu, YQ, Zhang, KQ, Al-Deyab, SS, Lai, YK, “Controlled Grafting Superhydrophobic Cellulose Surface with Environmentally–friendly Short Fluoroalkyl Chains by ATRP.” Mater. Des., 85 815–822 (2015)
Li, SH, Huang, JY, Ge, MZ, Cao, CY, Deng, S, Zhang, SN, Chen, GQ, Zhang, KQ, Al-Deyab, SS, Lai, YK, “Robust Flower-Like TiO2@cotton Fabrics with Special Wettability for Effective Self-Cleaning and Versatile Oil/Water Separation.” Adv. Mater. Interfaces, 2 1500220 (2015)
Guo, P, Zhai, SR, Xiao, ZY, An, QD, “One-Step Fabrication of Highly Stable, Superhydrophobic Composites from Controllable and Low-cost PMHS/TEOS Sols for Efficient Oil Cleanup.” J. Colloid. Interface Sci., 446 155–162 (2015)
Wu, XH, Fu, QT, Kumar, D, Ho, JWC, Kanhere, P, Zhou, HF, Chen, Z, “Mechanically Robust Superhydrophobic and Superoleophobic Coatings Derived by Sol–Gel Method.” Mater. Des., 89 1302–1309 (2016)
Cortese, B, Caschera, D, Federici, F, Ingo, GM, Gigli, G, “Superhydrophobic Fabrics for Oil–Water Separation Through a Diamond like Carbon (DLC) Coating.” J. Mater. Chem. A, 2 6781–6789 (2014)
Huang, JY, Li, SH, Ge, MZ, Wang, LN, Xing, TL, Chen, GQ, Liu, XF, Al-Deyab, SS, Zhang, KQ, Chen, T, Lai, YK, “Robust Superhydrophobic TiO2@fabrics for UV Shielding, Self-Cleaning and Oil–Water Separation.” J. Mater Chem. A, 3 2825–2832 (2015)
Wang, JF, Tsuzuki, T, Tang, B, Sun, L, Dai, XJ, Rajmohan, GD, Li, JL, Wang, XG, “Recyclable Textiles Functionalized with Reduced Graphene Oxide@ZnO for Removal of oil Spills and Dye Pollutants.” Aust. J Chem., 67 71–77 (2013)
Zhou, XY, Zhang, ZZ, Xu, XH, Guo, F, Zhu, XT, Men, XH, Ge, B, “Robust and Durable Superhydrophobic Cotton Fabrics for Oil/Water Separation.” ACS Appl. Mater. Interfaces, 5 7208–7214 (2013)
Zeng, C, Wang, HX, Zhou, H, Lin, T, “Self-Cleaning, Superhydrophobic Cotton Fabrics with Excellent Washing Durability, Solvent Resistance and Chemical Stability Prepared from an SU-8 Derived Surface Coating.” RSC Adv., 5 61044–61050 (2015)
Zhou, H, Wang, HX, Niu, HT, Gestos, A, Lin, T, “Robust, Self-Healing Superamphiphobic Fabrics Prepared by Two-step Coating of Fluoro-containing Polymer, Fluoroalkyl Silane, and Modified Silica Nanoparticles.” Adv. Funct. Mater., 23 1664–1670 (2013)
Perelshtein, I, Applerot, G, Perkas, N, Wehrschetz-Sigl, E, Hasmann, A, Guebitz, GM, Gedanken, A, “Antibacterial Properties of an In Situ Generated and Simultaneously Deposited Nanocrystalline ZnO on Fabrics.” ACS Appl. Mater. Interfaces, 1 (2) 363–366 (2009)
Enis, KH, Birer, Ö, Karakus, K, Yıldırım, C, “Shadow-Casted Ultrathin Surface Coatings of Titanium and Titanium/Silicon Oxide Sol Particles via Ultrasound-Assisted Deposition.” Ultrason. Sonochem., 31 481–489 (2016)
Lu, Y, Sathasivam, S, Song, JL, Crick, CR, Carmalt, CJ, Parkin, IP, “Robust Self-Cleaning Surfaces that Function when Exposed to Either Air or Oil.” Science, 347 1132–1136 (2015)
Zhang, JP, Seeger, S, “Polyester Materials with Superwetting Silicone Nanofilaments for Oil/Water Separation and Selective Oil Absorption.” Adv. Funct. Mater., 21 4699–4704 (2011)
Okano, T, Sarko, A, “Mercerization of Cellulose. I. X-ray Diffraction Evidence for Intermediate Structures.” J. Appl. Polym. Sci., 29 4175–4182 (1984)
Perelshtein, I, Applerot, G, Perkas, N, Wehrschetz-Sigl, E, Hasmann, A, Guebitz, G, Gedanken, A, “Antibacterial Properties of an In Situ Generated and Simultaneously Deposited Nanocrystalline ZnO on Fabrics.” ACS Appl. Mater. Interfaces, 1 361–366 (2008)
Acknowledgments
The authors are thankful for the financial support of Natural Science Foundation of Jiangsu Province of China (Grants BK20150072).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MP4 18586 kb)
Rights and permissions
About this article
Cite this article
Shang, Q., Liu, C. & Zhou, Y. One-pot fabrication of robust hydrophobia and superoleophilic cotton fabrics for effective oil-water separation. J Coat Technol Res 15, 65–75 (2018). https://doi.org/10.1007/s11998-017-9947-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-017-9947-0