Abstract
With the increasing occurrences of industrial oily wastewater emissions and oil spills, considerable efforts have been made to develop superhydrophobic materials for oil–water separation. Herein, we report a facile dipping-UV curing approach to fabricate superhydrophobic organosilicon/silica hybrid coating with crosslinked network structure on cotton fabric via thiol-ene reaction between thiol-functionalized silica nanoparticles (SH-SiO2 NPs) and acryloyloxy-terminated polydimethylsiloxane (A-PDMS-A). With the optimized mass ratio of SH-SiO2 NPs to A-PDMS-A at 0.2, the water contact angle of the fabric reached 155° and the water sliding angle was 8°, exhibiting excellent water repellency. Furthermore, the superhydrophobic cotton fabric possessed self-cleaning ability and good surface stability. In addition, the fabric was successfully applied for effective oil–water separation, and the separation efficiency reached up to 99.06%. Even after 15 cycles, the separation efficiency still maintained 98.93%, demonstrating excellent reusability. Our findings stand out as a new tool to fabricate UV-curable superhydrophobic coating on cotton fabric for efficient oil–water separation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inspired by the special plants and insects in nature such as lotus leaf,1,2,–3 red rose petal,4,5 water strider6,7and butterfly wing,8,9 superhydrophobic materials and surfaces with a water contact angle (WCA) above 150° have been developed and applied in the fields of self-cleaning,10,11 antiicing,12,13 and oil–water separation.14,15,–16 Particularly, with the increasing occurrences of industrial oily wastewater emissions and oil spills, the deteriorated environment has attracted a great deal of attention worldwide, and the superhydrophobic materials based on porous substrates such as fabrics,17,18,–19 metal meshes,20,21,–22 and sponges23,24 for efficient oil–water separation are considered to be of great significance.
To endow the porous materials with the superhydrophobic characteristic, it is necessary to not only construct micro/nano-hierarchical structure on the surface, but also utilize nonpolar chemicals to provide low surface energy.25,26,–27 For example, Wu et al.28 fabricated superhydrophobic polyurethane sponges for oil absorption by chemical vapor deposition of tetraethoxysilane to bind Fe3O4 nanoparticles tightly on the sponge and then dip-coating was carried out in a fluoropolymer aqueous solution. The sponge showed a high WCA of 157° and was able to absorb 44.5 times of tetrachloromethane from water. By virtue of reduction and strong adhesion roles of polydopamine (PDA), Xu et al.29 deposited Ag particles on the PDA-coated surface of polyester fabric to form a lotus leaf-like rough structure, and further modified it by 1H,1H,2H,2H-perfluorodecanethiol to achieve superhydrophobicity with a WCA of 155°. The obtained superhydrophobic fabric could efficiently separate oil from oil/water mixtures and also showed excellent self-cleaning property. Cai et al.30 prepared a superhydrophobic coating with fluorinated alkyl silane (FAS)-modified electroless Ni–P plating on the stainless steel (SS) mesh surface. The oil–water separation efficiency of the superhydrophobic mesh reached up to 96.8%. However, in the fabrication processes of the superhydrophobic materials, fluorine-containing chemicals were used and recognized to be harmful to natural ecology, thus leading to the limitation of their practical applications. Compared with fluorine-containing substances, the low-surface-energy organosilicon is environment friendly.31,32 Cao et al.33 first deposited an organically modified silica aerogel (ormosil) thin-film onto cotton fabric and then dip-coated with a layer of polydimethylsiloxane (PDMS, Sylgard 184) after curing at 80°C for 2 h. The PDMS-ormosil composite coating displayed a uniform 3D fractal-like structure with numerous microscale pores, and the WCA reached above 160°. Additionally, the fabricated superhydrophobic cotton fabric exhibited high oil–water separation efficiency of 98.5% even after 10 cycles. Pan et al.34 also prepared a stable PDMS-copper stearate (CuSA2) superhydrophobic coating on cotton fabric by in situ growth of CuSA2 and subsequent dip-coating and thermal curing of Sylgard 184. The obtained fabric maintained superhydrophobicity with WCA above 150° after ultraviolet (UV) irradiation, mechanical abrasion, and ultrasonication treatments and was applied for the separation of oil/water mixture with separation efficiency of 96%. Nevertheless, to form a crosslinked coating layer for the PDMS-based superhydrophobic materials, the high-temperature curing procedure was necessary, and the curing time was generally at least 2 h, leading to the consumption of energy and the reduction of production efficiency. Therefore, it is imperative to develop a new efficient approach to fabricate superhydrophobic materials for oil–water separation.
Herein, we propose a facile approach to fabricate a UV-curable superhydrophobic organosilicon/silica hybrid coating on cotton fabric for oil–water separation. Firstly, silica nanoparticles (SiO2 NPs) were modified with (3-mercaptopropyl) trimethoxysilane (MPTMS) to generate thiol-functionalized silica nanoparticles (SH-SiO2 NPs). Then, acryloyloxy-terminated polydimethylsiloxane (A-PDMS-A) was synthesized via a one-pot two-step reaction with isophorone diisocyanate (IPDI), α,ω-aminopropyl-terminated polydimethylsiloxane (NH2-PDMS-NH2), and 2-hydroxyethyl acrylate (HEA) as raw materials. After dipping into the solution containing SH-SiO2 NPs, A-PDMS-A, and photoinitiator Darocur 1173, the cotton fabric was irradiated under UV light to form crosslinked organosilicon/silica hybrid superhydrophobic coating. The as-fabricated superhydrophobic cotton fabric possessed not only self-cleaning ability, but also good surface stability. Importantly, the fabric was able to effectively separate oil–water mixture and the separation efficiency reached up to 99.06%. By means of UV light, the fabrication process of the superhydrophobic cotton fabric only needs several minutes, and no fluorine-containing chemical is used. Our approach is time-saving and cost-effective and shows the great potential in the treatment of industrial oily wastewater emissions and crude oil spills.
Experimental
Materials
α,ω-Aminopropyl-terminated polydimethylsiloxane (NH2-PDMS-NH2, Mn = 3000 g/mol) was supplied by Meryer (Shanghai) Chemical Technology Co., Ltd. (China). Tetraethyl orthosilicate (TEOS, 98%), (3-mercaptopropyl) trimethoxysilane (MPTMS), isophorone diisocyanate (IPDI, 99%), 2-hydroxyethyl acrylate (HEA, 96%), 2-hydroxy-2-methylproplophenone (Darocur 1173), anhydrous ethanol (AR), hexane (AR), and oil red O were all purchased from Aladdin Reagent Co., Ltd. (China). Dibulytin didodecylate (DBTDL, ≥ 90%) was obtained from Shanghai Chemical Reagent Co., Ltd. (China). Ammonia (25–28 wt%), tetrahydrofuran (THF, ≥ 99%), toluene (AR), dichloromethane (AR), and trichloromethane (AR) were bought from Guangzhou Chemical Reagent Factory (China). Methylene blue was provided by Tianjin Tianxin Fine Chemical Development Center (China). Cotton fabric (plain weave fabric, 375 g/m2) was bought from local store. All chemicals were used as received without further purification, and deionized water was used for all the experiments and tests.
Synthesis of thiol-functionalized silica NPs (SH-SiO2 NPs)
First, SiO2 NPs were synthesized according to the Stöber procedure.35 Two hundred milliliters of anhydrous ethanol and 24 mL of ammonia were first mixed to form a homogenous solution; then, 20 mL of TEOS was slowly dropped into the solution. After magnetic stirring at room temperature for 12 h, a milky suspension was generated and purified by centrifuging at 8000 rpm for 15 min. After washing with plenty of ethanol and drying at 60°C for 12 h, SiO2 NPs were obtained. Subsequently, 5 g of SiO2 NPs was dispersed in 100 mL of toluene under sonication for 30 min and 2.5 mL of MPTMS was added. After stirring at 120°C for 24 h, the suspension was post-treated as described above, and the SH-SiO2 NPs were obtained.
Synthesis of acryloyloxy-terminated polydimethylsiloxane (A-PDMS-A)
Typically, 0.7104 g (0.0032 mol) of IPDI was added into 4 mL of THF in a 50-mL three-neck round-bottom flask under nitrogen atmosphere. Then, 16 mL of THF solution containing 4.8 g (0.0016 mol) of NH2-PDMS-NH2 was added dropwise into the above solution within 40 min at 40°C. After stirring for 3 h, 10 mL of THF solution containing 0.3716 g (0.0032 mol) of HEA and 3.7 mg of DBTDL was added dropwise into the flask within 25 min, and the reaction mixture was stirred at 40°C for another 12 h. The product was purified by precipitation in plenty of water and dried under vacuum at 40°C to obtain A-PDMS-A.
Fabrication of superhydrophobic organosilicon/silica hybrid coating on cotton fabric under UV irradiation
The fabrication process of superhydrophobic organosilicon/silica hybrid coating on cotton fabric under UV irradiation is illustrated in Fig. 1. Typically, the pristine cotton fabric with suitable size (20 mm × 20 mm) was cleaned by ethanol under sonication and dried at 60°C for 1 h. Synchronously, a variable amount of SH-SiO2 NPs (the mass ratios of SH-SiO2 to A-PDMS-A were 0, 0.05, 0.2, 0.3, and 0.4, respectively) was added into 8.5 g of THF solution containing 0.68 g of A-PDMS-A and 6.8 mg of Darocur 1173, and sonicated for 20 min to form a homogeneous solution. After that, the cleaned cotton fabric was dipped into the above solution for 2 min. Subsequently, the removed cotton fabric was irradiated under UV light (320 nm, 100 mW/cm2) for 2 min with a distance of 10 cm from the center of UV light lamp (INTELLI-RAY 400, Uvitron International, Inc., USA), and the superhydrophobic organosilicon/silica hybrid coating on cotton fabric was obtained.
Self-cleaning test
A piece of as-fabricated superhydrophobic cotton fabric was fixed on a glass slide at a suitable tilt angle and covered with blue CuSO4·5H2O particles. Then, self-cleaning ability was evaluated by using water drops to remove the blue CuSO4·5H2O particles.
Thermal and chemical stabilities
The thermal stability of the superhydrophobic cotton fabrics was evaluated by measuring the WCAs after being heated separately at 40, 70, 100, 130, and 170°C for 6 h. To evaluate the chemical stability, the superhydrophobic cotton fabrics were separately immersed into deionized water, toluene, and hexane for 96 h, NaCl solution (1 mol/L) and HCl solution (pH = 1) for 24 h, and NaOH solution (pH = 12) for 12 h, and the removed fabrics were rinsed with ethanol and dried at 60°C for WCA measurement.
Oil–water separation test
The device for oil–water separation was made up of a piece of the fabricated superhydrophobic cotton fabric, two glass tubes, and two metal clips. Specifically, the superhydrophobic cotton fabric acting as a filter membrane was sandwiched between the two glass tubes and firmly fixed by metal clips. When the oil–water mixture containing oil colored with oil red O and deionized water colored with methylene blue was poured into the upper tube, oil easily penetrated through the fabric while water was impeded. The oil–water separation efficiency was calculated according to the following equation (1):
where m0 and m1 represented the masses of the initial oil and collected oil, respectively.
In addition, to assess the reusability of the superhydrophobic cotton fabric, the oil–water separation process with the same cotton fabric was repeated for 15 cycles. After each separation cycle, the fabric was cleaned with ethanol and dried at 60°C for 1 h before the next cycle.
Characterizations
The surface morphologies of the SH-SiO2 NPs and superhydrophobic cotton fabric were observed on an EVO18 scanning electron microscope (SEM, Carl Zeiss Jena, Germany) at an acceleration voltage of 10.0 kV. Fourier transform infrared spectroscopy (FTIR) was carried out on a Bruker Tensor 27 spectrometer (Bruker Optics, Germany) from 4000 to 600 cm−1 with a resolution of 4 cm−1 and scanning times of 32. The surface wettability of the superhydrophobic cotton fabrics was measured by a DSA100 contact angle meter (Kruss, Germany). The water droplet with a volume of 5 μL was used as a probe liquid, and at least five different locations were measured to calculate the average value at room temperature.
Results and discussion
Synthesis and characterization of acryloyloxy-terminated PDMS (A-PDMS-A)
In the last decade, the two classic organosilicon products including Sylgard 184 and Sylgard 186 by Dow Corning Corporation in USA were widely used for the fabrication of superhydrophobic coatings and surfaces.36,37 However, to form a crosslinking structure, the thermal curing generally needs to be above 80°C for at least 2 h. Compared with thermal curing, UV curing technology has the obvious advantage of fast reaction speed and the curing process can be completed within several minutes or even seconds.38,39,–40 At present, vinyl-terminated PDMS for sale has reactive vinyl groups and seems to possibly react under UV irradiation. However, the vinyl groups adjacent to Si are very inactive and the UV-initiated radical reaction is difficult to proceed.41,42,–43 In our work, to accelerate the fabrication process of superhydrophobic cotton fabric, PDMS terminated with reactive acryloyloxy groups was synthesized via a one-pot two-step reaction as shown in Fig. 2a. The amino groups (–NH2) of NH2–PDMS–NH2 first reacted with the primary isocyanate groups (–NCO) of IPDI to synthesize isocyanate-terminated PDMS (NCO–PDMS–NCO), and then, the terminated secondary –NCO further reacted with the hydroxyl groups (–OH) of HEA to achieve A-PDMS-A. Figure 2b shows the FTIR spectra of the A-PDMS-A synthesized at the initial time and the ends of the first and second reaction steps. At the initial reaction time, the absorption band at 2264 cm−1 was attributed to the stretching vibration of –NCO, while the absorption bands at 1645 and 1560 cm−1 were assigned to the stretching vibrations of carbonyl groups (–CO–) and the bending vibration of imino groups (–NH–) in urea groups (–NH–CO–NH–), respectively.44,45 After the first reaction step, the intensity of the absorption band of –NCO was reduced by half. Furthermore, after the second reaction step, the absorption band of –NCO disappeared, and a new absorption band at 1735 cm−1 corresponding to the ester groups (–COO–) vibration of HEA appeared. Additionally, the absorption band of vinyl groups (–C=CH2) of HEA was supposed to be covered by the absorption band at 1645 cm−1. In the 1H NMR spectrum of A-PDMS-A, the peaks at 5.8 and 6.1 ppm were related to the protons of vinyl groups (–CH=CH2) (Fig. S1), further confirming the successful synthesis of A-PDMS-A.46
Fabrication of superhydrophobic coating on cotton fabric
It is well known that micro/nano-hierarchical structure and low surface energy are the two essential factors for superhydrophobic materials and surfaces.24,47,48 Here, cheap SiO2 NPs functionalized with thiol groups were synthesized with TEOS as precursor and MPTMS as modifier for the construction of roughness. The successful grafting of MPTMS was confirmed by FTIR spectrum and a test of oil affinity. Moreover, the mean diameter of SH-SiO2 NPs from SEM image was about 200 nm (Fig. S2). To provide low surface energy, the A-PDMS-A with flexibility was utilized to react with SH-SiO2 NPs. The superhydrophobic coating on cotton fabric was fabricated by a simple dipping procedure and subsequent UV irradiation. In the UV curing process, the thiol-ene addition reaction between the terminated acryloyloxy groups of A-PDMS-A and the thiol groups of SH-SiO2 NPs easily occurred to form a crosslinked coating layer in the presence of photoinitiator Darocur 1173 even under the existence of oxygen in air.49,50 Therefore, the SH-SiO2 NPs also played the role of crosslinking points, which was beneficial for the improvement of the stability of the superhydrophobic coating on the cotton fabric. From the FTIR spectra of the A-PDMS-A/silica hybrid coating on cotton fabric before and after UV curing (Fig. S3), it was clear that the intensity of the absorption band at 1645 cm−1 obviously decreased after UV curing, demonstrating the occurrence of the thiol-ene reaction between –SH of SH-SiO2 NPs and –CH=CH2 of A-PDMS-A.
The effect of mass ratios of SH-SiO2 NPs to A-PDMS-A on the microstructure and wettability of the cotton fabric was investigated. Notably, the pristine fiber surface was quite smooth, the cotton fabric was hydrophilic with a WCA of 0°, and water droplet was quickly absorbed (Fig. S4a). With the coverage of pure A-PDMS-A coating layer, the WCA of the cotton fabric was remarkably increased to 126° (Fig. S4b). However, due to the limited roughness, it was still unable to reach superhydrophobicity. With the incorporation of SH-SiO2 NPs, the WCA obviously increased. When the mass ratio of SH-SiO2 NPs to A-PDMS-A was 0.05, the fiber surface became rough and the WCA increased to 143° (Fig. 3a). With the mass ratio further increasing to 0.2, the fiber surface was rougher, and the WCA and WSA of the fabric, respectively, reached 155° and 8°, exhibiting excellent water repellency (Fig. 3b). This revealed the important role of SH-SiO2 NPs for the construction of roughness and the improvement of hydrophobicity. Furthermore, when the mass ratio increased to 0.3 and 0.4, the roughness was more evident and some big protrusions by the agglomeration of SH-SiO2 NPs appeared. However, the WCAs of the fabrics changed little and both remained at 154°, and the WSAs were 6.5° and 6°, respectively (Figs. 3c and 3d). It should be pointed out that with the content of SH-SiO2 NPs increasing, the softness of the cotton fabric obviously decreased. Taking hydrophobicity and softness into consideration, the cotton fabric fabricated with the mass ratio of 0.2 was chosen for the subsequent studies.
To confirm the hydrophobicity, various liquids including water, coffee, black tea, and soy milk were separately dropped onto the as-fabricated cotton fabric. It was clear that the different kinds of droplets all stably stood on the surface and kept a nearly spherical shape, showing the superhydrophobicity (Figs. 4a–4d). In addition, a droplet of water colored by methylene blue and a droplet of hexane colored by oil red O were both dropped on the pristine cotton fabric and the fabricated fabric, respectively. The water and oil droplets were absorbed by the pristine cotton fabric immediately. Differently, on the fabricated cotton fabric, the water droplets kept their spherical shape while the oil droplet quickly permeated, exhibiting the obvious superoleophilicity (Figs. 4e, 4f). A jet of water easily bounced off the surface of the fabricated cotton fabric without residue (Fig. 4g). Moreover, when the fabricated cotton fabric was immersed into water, a mirror-like bright surface appeared (Fig. 4h). It was a result of a uniform air layer existing between the coating surface on the fabric and the surrounding water.51 Besides, when the surface of fabric contacted the water droplet hanging on a syringe needle, it could separate from the water droplet even if the water droplet was out of shape by extrusion (Fig. 4i), demonstrating the nonadhesion property of the superhydrophobic cotton fabric.
Photographs of (a) water, (b) coffee, (c) black tea, and (d) soy milk on the as-fabricated cotton fabric. Photographs of water and hexane droplets on the (e) pristine cotton fabric and (f) the as-fabricated cotton fabric. Photographs of (g) a jet of water bouncing off the surface, and (h) the as-fabricated cotton fabric fixed on a glass slide in water. (i) Photographs of contact, deformation, and departure processes of a 4 μL water droplet with respect to the as-fabricated cotton fabric
Self-cleaning ability and stability of the superhydrophobic coating on cotton fabric
To assess the self-cleaning ability of the superhydrophobic coating, the pristine and the fabricated superhydrophobic cotton fabrics were both fixed on a piece of glass slide with the same tilt angle and contaminated with blue CuSO4·5H2O powders (Fig. 5). With water droplets continuously dropping onto the pristine cotton fabric, the blue powders were difficult to be taken away by water, and almost all the powders still remained on the surface. Contrarily, the powders were easily carried away by water droplets from the superhydrophobic cotton fabric, showing excellent antifouling property (Video S1 and S2).
Surface stability is very important for the fabricated superhydrophobic cotton fabric in practical applications. As shown in Fig. 6a, the WCAs of the superhydrophobic cotton fabrics changed little after being placed under various temperatures of 0, 40, 70, 100, 130, and 170°C for 6 h. This indicated that the cotton fabric was insensitive to temperature and could work in a wide temperature range. Additionally, after being immersed into deionized water, toluene, and hexane for 96 h, NaCl solution (1 mol/L) and HCl solution (pH = 1) for 24 h, and NaOH solution (pH = 12) for 12 h, the WCAs of the fabricated cotton fabrics still remained above 150°, showing the strong stability of superhydrophobicity (Fig. 6b). Moreover, the stability of the cotton fabric was also evaluated through abrasion test. After 30 abrasion cycles, the WCA maintained 146.8°, demonstrating the good abrasion resistance (Fig. S6). Interestingly, although the fabric became hydrophilic with a WCA of 45° after plasma etching (0.2 mbar, 300 W, air) for 2 min, it was able to recover its superhydrophobicity by heating at 100°C for 2.5 h, which was mainly attributed to the migration of the hydrophobic organosilicon chains (Fig. S5).52,53
Oil–water separation behavior of the superhydrophobic cotton fabric
Owing to superhydrophobicity and superoleophilicity, the fabricated cotton fabric has great potential in the treatment of industrial wastewater emissions and oil spill accidents.54,55 At first, the oil absorption ability of the superhydrophobic cotton fabric was studied. As shown in Fig. 7a, several droplets of colored hexane as the representative of light oil were dropped into the beaker with water; they spread and merged on the water surface. It was clear that the red oil was easily absorbed by the cotton fabric within several seconds and no residual oil was observed (Video S3). Similarly, the trichloromethane as the representative of heavy oil at the bottom of the water was also completely absorbed by the fabric (Fig. 7b and Video S4).
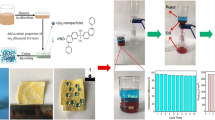
To further explore the feasibility of the superhydrophobic cotton fabric for the continuous separation of oil from water, a simple set of devices was set up with the fabric as a filter membrane, as shown in Fig. 8a. Clearly, when the trichloromethane-water mixture was poured into the upper glass tube, the red trichloromethane easily penetrated through the fabric and was collected by the beaker, while the blue water was impeded by the fabric and remained in the upper glass tube (Video S5). The separation efficiency of the cotton fabric for the trichloromethane–water mixture reached 99.06% (Fig. 8b). Meanwhile, the fabric was also used to continuously separate hexane, toluene, and dichloromethane from water, and the separation efficiencies were 96.57%, 95.45%, and 98.99%, respectively. Comparatively, the separation efficiencies of the fabric for hexane–water and toluene–water mixtures were lower, which were mainly due to the lower densities of the oils. Furthermore, to evaluate the reusability of the fabric, the separation of trichloromethane from water was carried out for 15 cycles, and the separation efficiency still maintained 98.93% (Fig. 8c).
Conclusions
In summary, we successfully fabricated a superhydrophobic organosilicon/silica hybrid coating on cotton fabric via a dipping-UV curing method. When the mass ratio of SH-SiO2 NPs to A-PDMS-A was 0.2, the as-fabricated cotton fabric appeared to have micro/nano-hierarchical morphology and the WCA and WSA, respectively, reached 155° and 8°. The superhydrophobic cotton fabric had self-cleaning ability, which could easily remove the contaminants on the surface by water. Owing to the formation of the crosslinked structure with SH-SiO2 NPs as crosslinking points, the superhydrophobic cotton fabric possessed good surface stability. In addition, the superhydrophobic cotton fabric could effectively separate organic solvents from water, and the separation efficiency reached up to 99.06%. Furthermore, the separation efficiency maintained 98.93% even after 15 separation cycles, exhibiting the excellent reusability. Our fabrication method is time-saving and cost-effective, and no fluorine-containing chemical is involved. The as-fabricated superhydrophobic cotton fabric is highly promising for application in the treatment of oil spill accidents and industrial sewage emission.
References
Cassie, ABD, Baxter, S, “Wettability of Porous Surfaces.” Trans. Faraday Soc., 40 546–551 (1944)
Neinhuis, C, Barthlott, W, “Characterization and Distribution of Water-Repellent, Self-Cleaning Plant Surfaces.” Ann. Bot., 79 667–677 (1997)
Latthe, SS, Terashima, C, Nakata, K, Fujishima, A, “Superhydrophobic Surfaces Developed by Mimicking Hierarchical Surface Morphology of Lotus Leaf.” Molecules, 19 4256–4283 (2014)
Bhushan, B, Her, EK, “Fabrication of Superhydrophobic Surfaces with High and Low Adhesion Inspired from Rose Petal.” Langmuir, 26 8207–8217 (2010)
Karaman, M, Çabuk, N, Özyurt, D, Köysüren, Ö, “Self-supporting Superhydrophobic Thin Polymer Sheets that Mimic the Nature’s Petal Effect.” Appl. Surf. Sci., 259 542–546 (2012)
Hu, DL, Chan, B, Bush, JWM, “The Hydrodynamics of Water Strider Locomotion.” Nature, 424 663 (2003)
Shi, F, Niu, J, Liu, J, Liu, F, Wang, Z, Feng, X, Zhang, X, “Towards Understanding Why a Superhydrophobic Coating is Needed by Water Striders.” Adv. Mater., 19 2257–2261 (2007)
Zheng, Y, Gao, X, Jiang, L, “Directional Adhesion of Superhydrophobic Butterfly Wings.” Soft Matter, 3 178–182 (2007)
Bixler, GD, Bhushan, B, “Bioinspired Rice Leaf and Butterfly Wing Surface Structures Combining Shark Skin and Lotus Effects.” Soft Matter, 44 11271–11284 (2012)
Fürstner, R, Barthlott, W, Neinhuis, C, Walzel, P, “Wetting and Self-cleaning Properties of Artificial Superhydrophobic Surfaces.” Langmuir, 21 956–961 (2005)
Selim, MS, Yang, H, Wang, FQ, Fatthallah, NA, Li, X, Li, Y, Huang, Y, “Superhydrophobic Silicone/SiC Nanowire Composite as a Fouling Release Coating Material.” J. Coat. Technol. Res., 16 1165–1180 (2019)
Cao, L, Jones, AK, Sikka, VK, Wu, J, Gao, D, “Anti-icing Superhydrophobic Coatings.” Langmuir, 25 12444–12448 (2009)
Zhan, YL, Ruan, M, Li, W, Li, H, Hu, LY, Ma, FM, Yu, ZL, Feng, W, “Fabrication of Anisotropic PTFE Superhydrophobic Surfaces Using Laser Microprocessing and Their Self-cleaning and Anti-icing Behavior.” Colloid Surf. A, 535 8–15 (2017)
Wang, B, Lei, B, Tang, Y, Xiang, D, Li, H, Ma, Q, Zhao, C, Li, Y, “Facile Fabrication of Robust Superhydrophobic Cotton Fabrics Modified by Polysiloxane Nanowires for Oil/Water Separation.” J. Coat. Technol. Res., 15 611–621 (2018)
Zhang, W, Shi, Z, Zhang, F, Liu, X, Jin, J, Jiang, L, “Superhydrophobic and Superoleophilic PVDF Membranes for Effective Separation of Water-in-Oil Emulsions with High Flux.” Adv. Mater., 25 2071–2076 (2013)
Su, X, Li, H, Lai, X, Zhang, L, Wang, J, Liao, X, Zeng, X, “Vapor–Liquid Sol–Gel Approach to Fabricating Highly Durable and Robust Superhydrophobic Polydimethylsiloxane@Silica Surface on Polyester Textile for Oil–Water Separation.” ACS Appl. Mater. Interfaces, 33 28089–28099 (2017)
Zhou, C, Chen, Z, Yang, H, Hou, K, Zeng, X, Zheng, Y, Cheng, J, “A Nature-Inspired Strategy Toward Superhydrophobic Fabrics for Versatile Oil/Water Separation.” ACS Appl. Mater. Interfaces, 9 9184–9194 (2017)
Gu, J, Xiao, P, Chen, P, Zhang, L, Wang, H, Dai, L, Song, L, Huang, Y, Zhang, J, Chen, T, “Functionalization of Biodegradable PLA Nonwoven Fabric as Superoleophilic and Superhydrophobic Material for Efficient Oil Absorption and Oil/Water Separation.” ACS Appl. Mater. Interfaces, 9 5968–5973 (2017)
Shang, Q, Liu, C, Zhou, Y, “One-Pot Fabrication of Robust Hydrophobia and Superoleophilic Cotton Fabrics for Effective Oil–Water Separation.” J. Coat. Technol. Res., 15 65–75 (2018)
Crick, CR, Gibbins, JA, Parkin, IP, “Superhydrophobic Polymer-Coated Copper-Mesh; Membranes for Highly Efficient Oil–Water Separation.” J. Mater. Chem. A, 1 5943–5948 (2013)
Su, X, Li, H, Lai, X, Zhang, L, Liang, T, Feng, Y, Zeng, X, “Polydimethylsiloxane-Based Superhydrophobic Surfaces on Steel Substrate: Fabrication, Reversibly Extreme Wettability and Oil-Water Separation.” ACS Appl. Mater. Interfaces, 9 3131–3141 (2017)
Tudu, BK, Kumar, A, “Robust and Durable Superhydrophobic Steel and Copper Meshes for Separation of Oil-Water Emulsions.” Prog. Org. Coat., 133 316–324 (2019)
Zhang, L, Li, H, Lai, X, Su, X, Liang, T, Zeng, X, “Thiolated Graphene-Based Superhydrophobic Sponges for Oil-Water Separation.” Chem. Eng. J., 316 736–743 (2017)
Li, J, Yan, L, Tang, X, Feng, H, Hu, D, Zha, F, “Robust Superhydrophobic Fabric Bag Filled with Polyurethane Sponges Used for Vacuum-Assisted Continuous and Ultrafast Absorption and Collection of Oils from Water.” Adv. Mater. Interfaces, 3 1500770 (2016)
Chen, L, Guo, Z, Liu, W, “Outmatching Superhydrophobicity: Bio-inspired Re-entrant Curvature for Mighty Superamphiphobicity in Air.” J. Mater. Chem. A, 5 14480–14507 (2017)
Bhushan, B, Jung, YC, Koch, K, “Micro-, Nano- and Hierarchical Structures for Superhydrophobicity, Self-cleaning and Low Adhesion.” Philos. Trans. R. Soc. A, 367 1631–1672 (2019)
Su, X, Li, H, Lai, X, Chen, Z, Zeng, X, “3D Porous Superhydrophobic CNT/EVA Composites for Recoverable Shape Reconfiguration and Underwater Vibration Detection.” Adv. Funct. Mater., 29 1900554 (2019)
Wu, L, Li, L, Li, B, Zhang, J, Wang, A, “Magnetic, Durable, and Superhydrophobic Polyurethane@Fe3O4@SiO2@Fluoropolymer Sponges for Selective Oil Absorption and Oil/Water Separation.” ACS Appl. Mater. Interfaces, 7 4936–4946 (2015)
Xu, Z, Miyazaki, K, Hori, T, “Fabrication of Polydopamine-Coated Superhydrophobic Fabrics for Oil/Water Separation and Self-cleaning.” Appl. Surf. Sci., 370 243–251 (2016)
Cai, Y, Li, S, Cheng, Z, Xu, G, Quan, X, Zhou, Y, “Facile Fabrication of Super-Hydrophobic FAS Modified Electroless Ni-P Coating Meshes for Rapid Water-Oil Separation.” Colloid Surf. A, 540 224–232 (2018)
Zhang, J, Li, B, Wu, L, Wang, A, “Facile Preparation of Durable and Robust Superhydrophobic Textiles by Dip Coating in Nanocomposite Solution of Organosilanes.” Chem. Commun., 49 11509–11511 (2013)
Wu, L, Zhang, J, Li, B, Wang, A, “Mimic Nature, Beyond Nature: Facile Synthesis of Durable Superhydrophobic Textiles Using Organosilanes.” J. Mater. Chem. B, 1 4756–4763 (2013)
Cao, C, Ge, M, Huang, J, Li, S, Deng, S, Zhang, S, Chen, Z, Zhang, K, Al-Deyab, SS, Lai, Y, “Robust Fluorine-free Superhydrophobic PDMS–ormosil@fabrics for Highly Effective Self-cleaning and Efficient Oil–Water Separation.” J. Mater. Chem. A, 4 12179–12187 (2016)
Pan, G, Xiao, X, Ye, Z, “Fabrication of Stable Superhydrophobic Coating on Fabric with Mechanical Durability, UV Resistance and High Oil-Water Separation Efficiency.” Surf. Coat. Technol., 360 318–328 (2019)
Stöber, W, Fink, A, Bohn, E, “Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range.” J. Colloid Interf. Sci., 26 62–69 (1968)
Arukalam, IO, Li, Y, “Anticorrosion and Barrier Properties Appraisal of Poly (Dimethylsiloxane)-ZnO Nanocoating Transition from Superhydrophobic to Hydrophobic State.” J. Coat. Technol. Res., 16 1077–1088 (2019)
Ghosh, UU, Nair, S, Das, A, Mukherjee, R, Gupta, SD, “Replicating and Resolving Wetting and Adhesion Characteristics of a Rose Petal.” Colloid Surf. A, 561 9–17 (2019)
Shen, L, Lai, Y, Fu, H, “Fabrication of Flower Clusters-like Superhydrophobic Surface via a UV Curable Coating of ODA and V-PDMS.” J. Appl. Polym. Sci., 136 48210 (2019)
Hou, K, Zeng, Y, Zhou, C, Chen, J, Wen, X, Xu, S, Cheng, J, Pi, P, “Facile Generation of Robust POSS-Based Superhydrophobic Fabrics via Thiol-ene Click Chemistry.” Chem. Eng. J., 332 150–159 (2018)
Chen, K, Zhou, J, Ge, F, Zhao, R, Wang, C, “Smart UV-Curable Fabric Coatings with Self-healing Ability for Durable Self-cleaning and Intelligent Oil/Water Separation.” Colloid Surf. A, 565 86–96 (2019)
Lee, TY, Roper, TM, Jonsson, ES, Kudyaov, I, Viswanathan, K, Nason, C, Guymon, CA, Hoyle, CE, “The Kinetics of Vinyl Acrylate Photopolymerization.” Polymer, 44 2859–2865 (2003)
Oster, G, Yang, NL, “Photopolymerization of Vinyl Monomers.” Chem. Rev., 68 125–151 (1968)
Goto, A, Fukuda, T, “Kinetics of Living Radical Polymerization.” Prog. Polym. Sci., 29 329–385 (2004)
Liu, C, Ma, C, Xie, Q, Zhang, G, “Self-repairing Silicone Coating for Marine Anti-biofouling.” J. Mater. Chem. A, 30 15855–15861 (2017)
Liu, C, Xie, Q, Ma, C, Zhang, G, “Fouling Release Property of Polydimethylsiloxane-Based Polyurea with Improved Adhesion to Substrate.” Ind. Eng. Chem. Res., 55 6671–6676 (2016)
Oktay, B, Kayaman-Apohan, N, “Polydimethylsiloxane (PDMS)-Based Antibacterial Organic-Inorganic Hybrid Coatings.” J. Coat. Technol. Res., 10 785–798 (2013)
Yan, YY, Gao, N, Barthlott, W, “Mimicking Natural Superhydrophobic Surfaces and Grasping the Wetting Process: A Review on Recent Progress in Preparing Superhydrophobic Surfaces.” Adv. Colloid Interface Sci., 169 80–105 (2011)
Wang, S, Liu, K, Yao, X, Jiang, L, “Bioinspired Surfaces with Superwettability: New Insight on Theory, Design, and Applications.” Chem. Rev., 115 8230–8293 (2015)
Machado, TO, Sayre, C, Araujo, PHH, “Thiolene Polymerisation: A Promising Technique to Obtain Novel Biomaterials.” Eur. Polym. J., 86 200–215 (2017)
Liu, C, Li, T, Zhang, J, Chen, S, Xu, Z, Zhang, A, Zhang, D, “Preparation and Properties of Phosphorous-Nitrogen Containing UV-Curable Polymeric Coatings Based on Thiol-ene Click Reaction.” Prog. Org. Coat., 90 21–27 (2016)
Martines, E, Seunarine, K, Morgan, H, Gadegaard, N, Wilkinson, CD, Riehle, MO, “Superhydrophobicity and Superhydrophilicity of Regular Nanopatterns.” Nano Lett., 5 2097–2103 (2005)
Tu, K, Wang, X, Kong, L, Guan, H, “Facile Preparation of Mechanically Durable, Self-healing and Multifunctional Superhydrophobic Surfaces on Solid Wood.” Mater. Design, 140 30–36 (2018)
Wang, H, Xue, Y, Ding, J, Feng, L, Wang, X, “Durable, Self-healing Superhydrophobic and Superoleophobic Surfaces from Fluorinated-Decyl Polyhedral Oligomeric Silsesquioxane and Hydrolyzed Fluorinated Alkyl Silane.” Angew. Chem. Int. Edit., 50 11433–11436 (2011)
Guo, F, Wen, Q, Peng, Y, Guo, Z, “Simple One-Pot Approach Toward Robust and Boiling-Water Resistant Superhydrophobic Cotton Fabric and the Application in Oil/Water Separation.” J. Mater. Chem. A, 5 21866–21874 (2017)
Li, S, Huang, J, Chen, Z, Chen, G, Lai, Y, “A Review on Special Wettability Textiles: Theoretical Models, Fabrication Technologies and Multifunctional Applications.” J. Mater. Chem. A, 5 31–55 (2017)
Acknowledgments
The work was financially supported by the Science and Technology Planning Project of Guangdong Province, China (2017B090915002, 2018A030313884) and the Science and Technology Planning Project of Guangzhou City, China (201804010381).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 2720 kb)
Supplementary material 2 (MP4 2772 kb)
Supplementary material 3 (MP4 1970 kb)
Supplementary material 4 (MP4 2745 kb)
Supplementary material 5 (MP4 4861 kb)
Rights and permissions
About this article
Cite this article
Gao, S., Li, H., Lai, X. et al. UV-curable superhydrophobic organosilicon/silica hybrid coating on cotton fabric for oil–water separation. J Coat Technol Res 17, 1413–1423 (2020). https://doi.org/10.1007/s11998-020-00362-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-020-00362-z