Abstract
In this study, aqueous acrylic polyol dispersions with high stability for a two-component waterborne polyurethane were prepared. To improve the stability of acrylic dispersions, the influence of the acrylic acid (AA) addition method, neutralization, water addition rate during the dilution process, and dispersion equipment on the stability of the aqueous acrylic polyol dispersion was studied using dynamic light scattering and a thermal storage experiment. The acrylic resins’ structure was examined using Fourier transform infrared spectra, and the water resistance of the resultant films was investigated by electrochemical measurements and a water-swelling experiment. The dispersions prepared by two-step AA addition exhibited better particle size distribution, viscosity, and thermal storage compared with those prepared by one-step AA addition. Furthermore, the acrylic resin prepared by two-step AA addition was observed to possess a higher acid value. The corrosion currents of films based on dispersions prepared by two-step AA addition decreased to a smaller extent after 24 h of immersion in water. The dispersions afforded smaller particles when larger amounts of neutralizer and slower water addition rates were used. The dispersions prepared using a sawtooth disk dispersion machine displayed better performance than that prepared using a homogenizer dispersion machine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Two-component waterborne polyurethanes (2K-WPU) are environmentally friendly coatings with excellent properties rivaling those of traditional solvent polyurethanes. These polyurethanes have been rapidly developed and have a wide range of applications in the automotive industry, building and civil engineering, electrical engineering, and plastics1–4 owing to the present emphasis on environmental protection and energy savings. However, their further development is limited by several undesirable properties, including poor water resistance and side reactions between the crosslinker and water. Therefore, many methods have been proposed to improve the properties of 2K-WPU, such as molecular design,5 silicon modification,6,7 new polymerization techniques4 and organic–inorganic hybrids.8
Acrylic resins are functional polymers whose properties can be altered in a simple manner by adjusting the monomer compositions. Therefore, waterborne hydroxy-functional acrylic resins are widely used as a polyol component in 2K-WPU.3,9,10 Acrylic resins are prepared by solution polymerization or emulsion polymerization. However, dispersions obtained by emulsion polymerization have poor compatibility with isocyanate crosslinkers, and the films based on these dispersions have an unappealing appearance. Meanwhile, in the presence of an emulsifier, the films have poor water resistance. Consequently, solution polymerization is used to prepare the dispersions examined in this study.
At present, waterborne hydroxy-functional acrylic resins prepared by solution polymerization are synthesized by a combination of (meth)acrylic esters, styrene, a hydroxy-functional (meth)acrylate, and (meth)acrylic acid, as shown in Fig. 1. The acrylic dispersion is obtained by neutralizing the hydroxy-functional acrylic resin with an amine, leading to a water-reducible resin, which is then diluted with deionized water. In the dispersion, hydroxy groups provided by the hydroxy-functional monomers further react with polyisocyanate during ambient curing. The (meth)acrylic acid monomers allow carboxylic groups to react with amine, which plays a significant role in the stability of aqueous acrylic dispersions, one of their most important properties. The stability of acrylic dispersions can be improved in many ways such as by increasing the amount of (meth)acrylic acid or introducing other ionic groups.11 However, an excessive amount of (meth)acrylic acid leads to an acrylic resin with an overly high hydrophilicity, endowing the 2K-WPU with poor water and chemical resistance. Thus, there is an unavoidable tradeoff between the dispersion stability and resistance properties of the film.
To improve the corrosion resistance and water resistance of waterborne coatings, new resin synthesis techniques and the development of advanced dispersion technologies have become increasingly important. Therefore, the objective of this study is to investigate the effects of the preparation conditions on the synthesis of aqueous acrylic polyol dispersions and to further increase the stability of acrylic dispersions, thereby improving the water resistance of the films based on these dispersions.
The focus of this investigation is the hydrophilicity of the resin and the dilution process. The hydrophilicity of the resin increases with an increase in the amine salt content. Employing two-step acrylic acid (AA) addition can decrease the amount of carboxylic groups buried by molecular chains and maximize the neutralization of the carboxylic groups. Moreover, increasing the neutralization of the carboxylic group content increases the amine salt content, which is conductive to the microparticulation of acrylic resins. Thus, the stability of aqueous acrylic polyol dispersions can be improved by employing two-step AA addition rather than increasing the amount of (meth)acrylic acid used. The resin dilution process plays an important role in the properties of the dispersion. The effects of the water addition rate and dispersion equipment on the dispersion stability were studied. The morphology of the resin during the dilution process was investigated by the particle size analysis, and a dilution mechanism was proposed.
Experimental
Materials
All materials were purchased from commercial sources and used as received without further purification.
Methyl methacrylate (MMA), butyl acrylate (BA), 2-hydroxyethyl methacrylate (HEMA), and styrene (ST) (all of AR grade) were purchased from Kermel Chemicals. Cardura E10P and tert-amyl peroxypivalate were purchased from Huanda Paint Enterprise Group Co. Ltd. Acrylic acid (AA), propanediol butyl ether (PnB), and N,N-dimethyl ethanolamine (DMEA) were purchased from Kermel Chemicals.
Preparation of acrylic resin
In a 250-mL four-necked round-bottom flask equipped with a silicone oil bath, mechanical stirrer, dropping funnel, thermometer, reflux condenser, and nitrogen inlet, a predetermined quantity of PnB and E10P was charged as per the formulations shown in Table 1. The temperature was increased to 130°C, and a slow stream of N2 was passed to avoid oxidation by atmospheric oxygen. Thereafter, reactant 1 was added dropwise under constant stirring for 4 h. Subsequently, reactant 2 was added within 0.5 h. Finally, the rest of the initiator was added, and the reaction was allowed to proceed for an additional 1.5 h longer.
Preparation of the aqueous acrylic dispersion
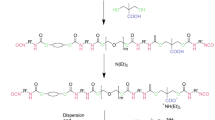
The reactants were cooled to 90°C. This temperature was maintained during the addition of the neutralizing agent (DMEA) and the subsequent stirring for 0.5 h. Next, the reactant was removed to a dispersion tank, and the temperature was maintained at 70°C. The aqueous acrylic dispersion was obtained by adding a predetermined quantity of deionized water at ambient temperature under stirring at 3000 r/min. In addition, the quantity of water used for the dispersion was determined on the basis of the theoretical dispersion solid content, and the solid content was typically approximately 35–40% (by mass) in this work. A schematic of the procedure for the preparation of the aqueous acrylic polyol dispersions is shown in Fig. 2.
Preparation of coating films
The acrylic dispersions were crosslinked by Bayhydur® XP2487/1 with an NCO:OH ratio of 1.2:1. Deionized water at ambient temperature was added under manual mixing to bring the solids to an acceptable viscosity. Clear films were prepared on carbon steel panels using a 30-μm (wet film thickness) wire bar coater. The films were allowed to dry and cure at room temperature, and the film properties were characterized after 7 days.
Characterization
Fourier transform infrared (FTIR) spectroscopy
The acrylic polymer was dissolved in carbon tetrachloride and placed on KBr pellets to create the samples for analysis. FTIR spectra were recorded using a Shimadzu FTIR 8300 spectrometer in the range from 400 to 4000 cm−1. The frequency and intensity of each band were obtained automatically using the Find Peak command in the instrument software.
Acid value
The acid values of the samples were determined by titration. First, 125 mL of a solvent mixture of isopropyl alcohol and toluene (1:1, v/v) containing 2 mL of phenolphthalein solution was added to accurately weighed oil samples. Titrations were performed using accurately standardized 5.6 g/L potassium hydroxide alcohol solution to the end point of definite pink color persisting for at least 30 s. The acid value was expressed as mg KOH/g sample.
Viscosity
The viscosities of the acrylic dispersions were measured with a Brookfield rotational viscometer equipped with a thermostat at 25°C using an LV.2 spindle.
Dispersion size
The number average particle diameters of the dispersions were measured by a light scattering method (Zetasizer Nano ZS, Malvern). The hydroxy-functional waterborne acrylic dispersions were diluted with deionized water to 0.12 wt% before the measurement.
Dispersion solid content
A dry clean dish was heated in an oven at 105 ± 2°C for 30 min, after which it was removed and placed in a dryer to cool to room temperature before being weighed. Subsequently, a 4- to 5-g sample was weighed with a grinding mouth bottle by the subtraction method and placed in the weighted dish such that the sample was spread uniformly over the bottom of the dish. The dish with the sample was then placed in a forced-air oven at 160 ± 2°C and baked for 2 h, after which it was placed in a dryer, cooled to room temperature, and weighed. Furthermore, the dish with the sample was placed in the oven for 30 min and weighed until the weight difference between consecutive weightings was not more than 0.01 g (with all weights rounded to the nearest 0.01 g). It was calculated as follows:
where X is the dispersion solid content, W 0 is the weight of the dish, W 1 is the weight of the dish and the sample, and G is the weight of the sample.
Zeta potential measurement
The zeta potential of the dispersions was measured using a dynamic light scattering method (Zetasizer Nano ZS, Malvern). The zeta potentials were measured at an angle of 90° at room temperature after the original dispersions were diluted by approximately 10,000-fold with deionized water.
Electrochemical measurements
Electrochemical measurements were performed using a CHI604D electrochemical workstation supplied by Shanghai Chenhua Instruments Inc. A standard 50-mL three-electrode cell was used at ambient temperature with 3.5% NaCl solution at pH 7.0 as the electrolyte and samples coated with 2K-WPU films as the working electrodes. The counter electrode was a semicylindrical platinum slice with a surface area of 10 cm2. Moreover, the reference electrode was a saturated calomel electrode (SCE), and all of the measured potentials were referred to this electrode.
Water-swelling measurement
Dried films were immersed in deionized water for 24 h at 25°C. The residual water was wiped from the films using filter paper, and the weight was measured immediately. The swelling was then calculated as follows:
where W 0 is the weight of the dried film and W is the weight of the film after water absorption.
Results and discussion
Effect of neutralization on stability
The ionic groups from the neutralization reaction between the carboxylic group and amine play an important role in the stability of acrylic dispersions. The presence of ionic groups at the particle–water interfaces reduces the particle size by increasing the hydrophilicity and improves the dispersion stability by generating electrical double layers.12 The stability of an acrylic dispersion is primarily determined by the electrostatic repulsion. According to Derjaguin–Landau–Verwey–Overbeek (DLVO) theory, when the electrostatic repulsion force is stronger than the aggregation-inducing van der Waals force, the dispersion is stable. The electrostatic repulsion energy (V R) between two particles can be described by the following formula:13
where φ 1 and φ 2 are the potentials on the particles surfaces, H is the distance between the particles, and ε is the dielectric constant. From the formula, it can be determined that increasing the surface potential improves the dispersion stability. The surface potential can be estimated by the zeta potential (ζ). Dispersions with zeta potentials valued between −30 and +30 mV are usually unstable, tending to form agglomerates and sediments.14 Both the hydrophilicity and zeta potential of acrylic resin can be improved by increasing the neutralization.
The particle sizes and zeta potentials of dispersions with different neutralizations are shown in Fig. 3. The zeta potentials of the aqueous dispersions increase with the degree of neutralization, whereas the particle size decreases. Moreover, the particle size decreases rapidly when the neutralization increases from 60% to 80%, which may be because the carboxylic groups on the surface are all neutralized in this stage, and the carboxylic groups inside the resin begin to be neutralized. Furthermore, water molecules can enter the particles during the dilution process and break the particles. As the neutralization increases further, the particle size remains almost constant because the carboxylic groups buried in the core by the hydrophobic chains cannot be neutralized. Overall, the zeta potential increases steadily with the particle size.
Effect of means of AA addition on stability
In general, the real neutralization of acrylic resin is always far below the designed value. The pH of an acrylic dispersion is commonly between 7 and 8, whereas the designed neutralization is only 80%. This may be because some of the carboxylic groups are buried by the chain of the acrylic resin and thus participate very less in the reaction. Fortunately, employing two-step AA addition can decrease the amount of buried carboxylic groups.
Acid values of acrylic resins
The variations in the acid of PA1, PA2, and PA3 during polymerization were studied by titration, and the results are shown in Fig. 4. In the first 4 h, the acid values of PA1 and PA2 increased uniformly when reactant 1 was added at a constant dropping rate. Meanwhile, the acid value of PA3 was zero because the AA was added in the second step. Over the next 30 min, the acid values of PA2 and PA3 increased rapidly due to the increase in the AA concentration, while that of the PA1 remained constant. Finally, the acid values of PA1, PA2, and PA3 plateau. The final acid value of PA3 was highest, being approximately 30.3 mg KOH/g and close to the theoretical value of 32.7 mg KOH/g.
Figure 5 shows the speculated distribution of carboxylic groups in PA1, PA2, and PA3. PA1 has the highest amount of buried carboxylic groups and PA3 has the lowest, though the distribution of carboxylic groups in the latter is not uniform. The distribution of carboxylic groups can be adjusted by two-step AA addition: the relative amount of buried carboxylic groups can be decreased, allowing more carboxylic groups to be neutralized.
FTIR analysis of acrylic resin
Figure 6 shows the representative FTIR spectra of PA1, PA2, and PA3 after neutralization. The FTIR spectra show the following bands: 3434 cm−1, corresponding to the stretching vibration of –OH; 2958 and 2873 cm−1, corresponding to the asymmetric stretching vibration of –CH2; and 1731 and 1164 cm−1, assigned to the C=O and C–O stretching vibrations of ester groups, respectively. The absence of bands of unsaturation at 1644 cm−1 confirms the complete polymerization of the monomers. The bands of COO− were investigated in greater detail to verify the increased neutralization. The asymmetric and symmetric stretching vibrations of COO− are expected to appear at 1610–1550 cm−1 and 1420–1335 cm−1, respectively.15 Fortunately, although the symmetric stretching band was masked by other bands, the asymmetric stretching band at 1575 cm−1 was not compromised.16 As expected, the bands corresponding to the asymmetric stretching vibration of COO− increase in intensity in the following order: PA1 < PA2 < PA3. This finding indicates that the concentration of the amine salt of the carboxylic group can be increased by employing two-step AA addition.
Characteristics of acrylic resin dispersions
Figure 7 shows the appearance of the dispersions. The dispersion of PA1 is pale yellow, whereas those of PA2 and PA3 are a beautiful milky white with light blue. The characteristics of the acrylic dispersions are shown in Table 2. The dispersion of PA1 has the smallest average particle size but poor performance in the thermal storage experiment, which may be explained by the existence of macroparticles. The polydispersity index (PdI) represents the uniformity of the particle size distribution of a dispersion. The smaller the PdI value, the more uniform is the particle size distribution. The PdI of the PA1 dispersion is three times greater than that of PA2. The PA3 dispersion has a larger particle size and higher PdI than PA2, but PA3 possesses a higher acid value. This result may be caused by the unbalanced distribution of carboxylic groups in the resin.
Figure 8 shows the particle size distributions of the dispersions. The difference in the PdI values of the dispersions is clearly observed. The curve for PA1 reveals the existence of macroparticles in the corresponding dispersion.
Water resistance of films
The water resistance of the films was studied by swelling test and electrochemical measurements. Figure 9 shows the Tafel plots of films based on dispersions of PA1, PA2, and PA3. The plots of films which were immersed in water for 24 h are shown in Fig. 9, too. The results of the swelling test and corrosion current are shown in Table 3. The PA2-based films show the smallest swelling rate and the smallest increase of the corrosion current, indicating that they have the best water resistance as compared to PA1 and PA3. This high water resistance may be a result of the PA2 dispersion, which has a more uniform particle size distribution, the best compatibility with the crosslinker, and the highest crosslinking density.
Effect of water addition rate on stability
Phase inversion is known to occur during the dilution process. In the vicinity of the phase inversion point, the viscosity is at a maximum, and the interfacial tension between the oil and water phase is at a minimum. In this stage, the resin tends to be highly deformable. Therefore, the effect of dispersion in this stage has a decisive effect on the properties of the final dispersion. Slowing down the speed of water addition rate in this stage tends to provide smaller particles. The particle size and zeta potential of the dispersions obtained by adding water at various rates are shown in Table 4. The particle size decreases with decrease in the water addition rate, whereas the zeta potential exhibits no obvious trend.
Effect of dispersion equipment on stability
The dispersion equipment plays an important role in the dilution process. In this part of the study, dispersions based on two-step AA addition with different solid contents were prepared by a homogenizer or a sawtooth disk dispersion machine, respectively. The properties of the resulting dispersions are presented in Table 5.
Dispersions with 33% solid content and 40% solid contents prepared by the sawtooth disk dispersion machine show the best heat storage stability, with negligible difference between them. However, the dispersions with different solid contents prepared by the homogenizer show a significant difference in heat storage stability. In this case, the dispersions with 40% solid content show excellent heat storage stability because of the higher acrylic monomer content, whereas the dispersions with 33% solid content have poor stability.
Effect of dilution process
The water dilution process was studied by determining the effects of water content on the particle size of the dispersion. The relationship between the water content and particle size is shown in Fig. 10. Initially, the particle size decreases with the water content. With further addition, the particle size begins to increase unexpectedly. The particle size then decreases, ultimately reaching a constant value. The variation of the particle size is similar to the variation of the viscosity, which exhibits a nadir during the dilution process.
A mechanism for the water dilution process is proposed. At the beginning of water addition, there is a lowering of the polymer concentration and the formation of micelles. Next, as more water is added, the hydrophobic groups on the micelles are attracted to each other, leading to aggregates of micelles and a higher viscosity. With further addition of water, the system becomes a dilute low-viscosity oil-in-water dispersion.
Conclusions
Acrylic polyol dispersions for 2K-WPU were obtained. The influence of factors, such as the means of AA addition, neutralization, water addition rate, and dispersion equipment on the dispersion stability was studied. The dispersion stability can be improved by employing two-step AA addition, increasing neutralization, and slowing the water addition rate. The distribution of carboxylic groups could be adjusted by choosing two-step AA addition instead of one-step AA addition. This two-step process leads to a lower relative content of buried carboxylic groups, allowing more carboxylic groups to participate in the neutralization and thereby improving the resin hydrophilicity. However, the addition of excessive AA in the second step can produce an excessively uniform distribution of carboxylic groups, potentially increasing the average particle size and broadening the particle size distribution. Moreover, the films based on the dispersions prepared by two-step AA addition show better water resistance because of their better compatibility with the crosslinkers and higher degree of crosslinking compared with films based on the dispersions prepared by one-step AA addition. The dilution process was studied, and a speculation of the mechanism was proposed.
References
Melchiors, M, Sonntag, M, Kobusch, C, Jurgens, Eberhard, “Recent Developments in Aqueous Two-Component Polyurethane (2K-PUR) Coatings.” Prog. Org. Coat., 40 (1–4) 99–109 (2000)
Wicks, ZW, Jr, Wicks, DA, Rosthauser, JW, “Two Package Waterborne Urethane Systems.” Prog. Org. Coat., 44 (2) 161–183 (2002)
Ley, DA, Fiori, DE, Quinn, RJ, “Optimization of Acrylic Polyols for Low VOC Two-Component Water Reducible Polyurethane Coatings Using Tertiary Isocyanate Crosslinkers.” Prog. Org. Coat., 35 (1–4) 109–116 (1999)
Huybrechts, J, Bruylants, P, Vaes, A, De Marre, A, “Surfactant Free Emulsions for Waterborne Two-Component Polyurethane Coatings.” Prog. Org. Coat., 38 (2) 67–77 (2000)
Park, SH, Chung, D, Hartwig, A, Kima, BK, “Hydrolytic Stability and Physical Properties of Waterborne Polyurethane Based on Hydrolytically Stable Polyol.” Colloids Surf. A, 305 (1–3) 126–131 (2007)
Ge, Z, Luo, Y, “Synthesis and Characterization of Siloxane-Modified Two-Component Waterborne Polyurethane.” Prog. Org. Coat., 76 (11) 1522–1526 (2013)
Zhang, FA, Yu, CL, “Application of a Silicone-Modified Acrylic Emulsion in Two-Component Waterborne Polyurethane Coatings.” J. Coat. Technol. Res., 4 (3) 289–294 (2007)
Akbarian, M, Olya, ME, Ataeefard, M, Mahdavianc, M, “The Influence of Nanosilver on Thermal and Antibacterial Properties of a 2K Waterborne Polyurethane Coating.” Prog. Org. Coat., 75 (4) 344–348 (2012)
Billiani, J, Wilfinger, W, “New Low-VOC Acrylic Polyol Dispersions for Two-Component Polyurethane Coatings.” Surf. Coat. Int. Part B Coat. Trans., 85 (1) 191–195 (2002)
Xinhua, Z, Weiping, T, Jianqing, H, “Preparation and Characterization of Two-component Waterborne Polyurethane Comprised of Water-soluble Acrylic Resin and HDI Biuret.” Chin. J. Chern. Eng, 14 (1) 99–104 (2006)
Lee, H-T, Wu, S-Y, Jeng, R-J, “Effects of Sulfonated Polyol on the Properties of the Resultant Aqueous Polyurethane Dispersions.” Colloids Surf. A Physicochem. Eng. Aspects, 276 (1–3) 176–185 (2006)
Kim, DH, Lee, YH, “Synthesis and Surface Properties of Self-crosslinking Core-Shell Acrylic Copolymer Emulsions Containing Fluorine/Silicone in the Shell.” Colloid Polym. Sci., 9 (1) 1435–1536 (2013)
Socrates, G, Infrared Characteristic Group Frequencies. Wiley, Great Britain, 1980
Lee, SK, Kim, BK, “High Solid and High Stability Waterborne Polyurethanes Via Ionic Groups in Soft Segments and Chain Termini.” J. Colloid Interface Sci., 336 (1) 208–214 (2009)
Hunter, RJ, Zeta Potential in Colloid Science: Principles and Applications. Academic Press, London, 1981
Stamm, M, Polymers Surface and Interfaces, 1st ed. Springer, Berlin, 2008
Acknowledgments
The authors would like to acknowledge the support of the Key Scientific and Technological Project of Zhejiang Province, China (Grant No. 2013C01095).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Xu, F., Li, H. et al. Preparation and stability of aqueous acrylic polyol dispersions for two-component waterborne polyurethane. J Coat Technol Res 14, 215–223 (2017). https://doi.org/10.1007/s11998-016-9845-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-016-9845-x