Abstract
The development of effective anticorrosion pretreatments for metallic substrates is an issue of great importance for durability of metal structures and components. In this work for improved corrosion protection properties of sol–gel derived hybrid coatings, encapsulated corrosion inhibitors [2-mercaptobenzothiazole (MBT) and 2-mercaptobenzimidazole (MBI)] in the presence of β-cyclodextrin (β-CD) have been incorporated into hybrid coatings. Inclusion complex formation of MBI or MBT with β-CD led to encapsulated corrosion inhibitors which became active in corrosive electrolytes, and could slowly diffuse out of the host material to ensure continuous delivery of the inhibitors to corrosion sites and long-term corrosion protection. Structural characterization of the hybrid coatings were performed using EIS, SEM coupled with EDX, and FTIR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminum is an important material for transportation and is used widely for automobile components. The development of effective anticorrosion coatings is of great importance for durability of metal structures and components. Incorporation of encapsulated corrosion inhibitors in the hybrid sol–gel systems has been proven to enhance the corrosion protection properties which lead to long-term corrosion protection via the slow release of inhibitors. The corrosion resistance of the coating strongly depends on the concentration and size of the nanoparticles.1,2 To design and create such hybrid coatings with desired environmental protection properties, sol–gel technology is the method of choice because of its simplicity.3,4 Sol–gel technology offers various ways to prepare functional hybrid coatings with unique chemically tailored properties. The addition of corrosion inhibitors into the hybrid sol can also improve the anticorrosion properties of hybrid sol–gel coating and design of functional nanostructured materials through the use of controlled hybrid organic–inorganic interfaces.5,6 However, slow and controllable release of inhibitors from coatings is important for anticorrosion properties which are strongly dependent upon the type of compound selected for encapsulation of corrosion inhibitor.7,8 Cyclodextrins are promising candidates to serve this purpose.9–11 Cyclodextrins (CDs) are cyclic oligosaccharides consisting of several glucopyranose units and are often described as truncated cone-shaped structures with a hydrophilic exterior surface and a hydrophobic interior cavity. Cyclodextrins are known as effective complexing agents, which have an ability to form inclusion complexes with various organic guest molecules that fit the size of the cyclodextrin cavity.12–14 Inclusion complexes of inhibitor and CD are effective delivery systems of organic inhibitors in active corrosion protection applications. In this work, we propose a novel approach for designing a self-repairing anticorrosion coating by using the encapsulated corrosion inhibitor in β-cyclodextrin (β-CD) as smart corrosion inhibitor nanocontainers in different conditions (at room temperature and under sonic energy). The encapsulated particles were used as reservoirs for repairing agent and chemical initiator, which impart anticorrosion ability to the protective system.
Experimental
Reagents and equipment
Tetramethoxysilane (TMOS), 3-glycidoxypropyltrimethoxysilane (GPTMS), 2-mercaptobenzimidazole (MBI), 2-mercaptothiazole (MBT), diethylenetriamine (DETA) and β-CD were purchased from Aldrich (98% pure). All chemicals were used as received. Ethanol was purchased from Merck (98% pure).
Sol preparation
The sol hybrid was prepared by mixing TMOS, GPTMS precursor, and the organic crosslinking agent, DETA, as follows:
GPTMS and TMOS were mixed (2:1 molar ratio) in a beaker with ethanol (12.33 cc) at ambient temperature. The resultant two-phase solution was vigorously stirred at a rate of 240 rpm for 1 h. Then acidic catalyst (water with pH 2) was used for hydrolysis of sol hybrid. Hydrolysis and condensation of the silane was conducted at stoichiometric and 50% substoichiometric water pH 2/ethanol ratio in the presence of an acid catalyst. After hydrolysis and condensation, sol was stirred at room temperature for 2 h.6,15,16
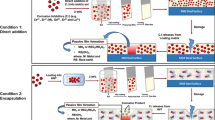
Encapsulated inhibitor was synthesized as reported previously.14 Encapsulated inhibitor solution (5% W/W) in ethanol was added to the sol and sonicated for 30 min and then a crosslinking agent (diethylentriamine, DETA, 0.5 mL) was added to the mixture. The final mixture was vigorously stirred and applied to the cleaned 2024 aluminum alloy panels. To prepare the samples for analytical tests, the coated panels were dried under ambient conditions for 24 h.
Electrochemical analysis of the smart corrosion inhibitor nanocontainers
Electrochemical measurements were performed under extreme environmental conditions, consisting of an aqueous, air exposed sodium chloride (5% NaCl) solution. Each sample was sealed with waterproof tape in order to prevent premature corrosion along the edges of the substrate. A 1 cm × 1 cm area within the center of each sample was exposed to the solution during testing. Corrosion analysis of bare and coated substrates was done using an AutolabPGSTAT30 potentiostat system connected to a corrosion analysis software program. Polarization measurements were carried out potentiostatically at room temperature using an Ag/AgCl/Cl− (0.222 V) reference electrode and a platinum counter electrode. The potentiodynamic measurements were taken within the range of −2000 to 2000 mV vs Ag/AgCl/Cl at a rate of 5 mV/s. Prior to the measurements, in order to reach steady potential, the electrodes were kept in the working solutions for at least 30 min.6,15,16
Characterization
Scanning electron microscopy (SEM) was performed on the coated substrates with smart corrosion inhibitor nanocontainers to characterize the surface morphology with a Jenavert optical microscope and Cambridge S.360 microscope using a backscattered or a secondary electron image detector at 10 kV and 2.85 A probe current. Infrared spectra were recorded using ATR objective of a Bruker microscope, between 400 and 4000 cm−1. UV–Vis spectrophotometer (Shimadzu UV-1650 PC) at the adsorption maximum of 242 nm was used.6
Results and discussion
Different parameters affected organic corrosion inhibitors performance such as inhibitor activity for corrosion protection of the high strength aluminum alloys, capability to form inclusion complexes with β-CD and compatibility of the corrosion inhibitor with the coating material. To the best of our knowledge, neither MBT nor MBI has been reported in a complexation reaction with β-CD, and no data on their stability constants are available in the literature. In this article, the capacity of MBT and MBI to form inclusion complexes with β-CD has been assumed based on certain structural similarities with other reported aromatic and heterocyclic organic compounds. Inclusion complex formation of MBT and MBI with α-cyclodextrin was described previously.14
FTIR of SNAP coatings showed that the distinct band at 1096 cm−1 was the characteristic of stretching of Si–O–Si. The band at 980 cm−1 was due to the stretching of Si–OH. The band at 980 cm−1 disappeared, indicating that Si–OH was transformed into Si–O–Si.
Morphology of the smart corrosion inhibitor nanocontainers
Corrosion inhibitor nanocontainers were synthesized via encapsulation of corrosion inhibitor in cyclodextrin core following an established method.14 The SEM images in Figs. 1, 2, 3, and 4 clearly show the spherical shape of the resultant inhibitor nanocontainers. Self-healing performance of inhibitor nanocontainers is greatly dependant to the diameter of embedded capsules.17 Results show that mixing, hydrolysis and condensation play an important role in the final product of sol–gel coating. Mixing at room temperature during inhibitor nanocontainers synthesis causes a reduction in the initial size and changed the hydrolyzed state of the inhibitor nanocontainers, which affected their subsequent evolution into stable sols or gels by agglomeration. It was found that the average diameter of MBT and MBI nanocontainers is lower when inclusion complexes are formed at room temperature than under sonic energy. It is therefore possible to conclude that mixing can be actively used and must be properly controlled in sol–gel processes in order to guarantee the final characteristics of the particulate product. Micro and nanocapsules were found to self-assemble into ordered film on the substrate surface.
When the ultrasound is used for making an inclusion complex of β-CD and inhibitors, the nucleation rate is further increased, so it is easy to get a larger nanoparticle due to high particle density, high impact probability, and the serious aggregation of particles. However, at room temperature, nucleation rate is much slower and results in nanocontainers with smaller size. The thickness of the coating was found to be ~100 nm. The degree of orderliness of these microspheres self-assembled on the surface of AA2024 alloy depends on both the dispersion of micro- and nanocapsules and the mixing condition.5
Self-healing mechanism of corrosion inhibitor nanocontainers
Structural changes of atoms or molecules may cause cracks and reduced strength, so the inverse reaction, i.e., recombination of the broken molecules, should be one of the repairing strategies.5 Reversible self-healing smart materials which are based on covalent and non-covalent bonding are more applicable and are able to undergo multiple repair cycles even upon damage at the same site. The large amount of energy which is needed to break and reform covalent bonds is a drawback for self-healing systems based on covalent bonding. In contrast, non-covalent bonds systems need low energy for reversible self-healing materials.5
In aqueous solutions, many organic and inorganic compounds are able to form inclusion complexes with cyclodextrins by taking up the organic compounds or some lipophilic moiety of the molecule, into the central cavity of cyclodextrin. Various driving forces are important in inclusion complex formation. No covalent bonds are formed or broken during complex formation, and the organic compounds in complex are in rapid equilibrium with free molecules in the solution. The driving forces for the complex formation include release of enthalpy-rich water molecules from the cavity, hydrogen bonding, Van der Waals interaction, charge transfer interaction, etc. The physicochemical properties of free cyclodextrin molecule differ from those which are involved in inclusion complex with cyclodextrin. Because of the special steric effects resulting from the occupation of β-CD’s cavity space by the MBT and MBI phenyl ring and with its thiazole ring protruding from the cavity (as opposed to the whole hydrophobic part of MBT and MBI), its hydrocarbon chain could interact with β-CD and had no choice but to enter the β-CDs cavity along its narrow rim to form a ternary inclusion complex of stoichiometry.12–14 Encapsulation of corrosion inhibitor via inclusion complex formation of MBI or MBT with β-CD is via reversible hydrogen bonds and when the nanocontainer is broken or cut, it can be simply repaired by bringing together the fractured ends for as little as few minutes at ambient temperature. It is crucial to bring the ends of the material together as quickly as possible to obtain sufficient self-healing as the hydrogen bonding units may react with the closest ones in their section.18–20
Electrochemical analysis
As complementary experiments, potentiodynamic polarization curves were plotted (Figs. 6 and 7) under extreme environmental conditions, consisting of an aqueous, air exposed sodium chloride (5% NaCl) solution (Fig. 5).
The potentiodynamic polarization curves in Fig. 6 show that the corrosive behavior of the alloys with coating containing encapsulated inhibitor coatings is very different than the curves observed for the alloy sample coated with the coating without any inhibitor (bare sample) and coatings containing MBT and MBI.
The protected alloys display very resistive behaviors with very low current density values (in the range from 10−14 to 5 × 10−11 A/cm2), which are typical of non-conductive materials, like the ceramic coatings. Note that the polarization curves of the alloys with coating containing encapsulated inhibitor were carried out in a very wide range of potentials, between −1.4 and 1.1 V vs SCE (2.5 V), which is higher than the commonly used range as observed for the bare alloy. For the corrosion protection of the alloy, corrosion current density must be decreased. The protective coating containing encapsulated inhibitor which was prepared at room temperature was very efficient in the passivation and protection of the alloy surfaces, since they displayed very small current density values in the polarization curves. In contrast, the alloy with protective coating containing encapsulated inhibitor which was prepared under sonic energy presented a lower protection.15,16
Such a result is often observed for a barrier-type coating placed under immersion when no corrosion inhibitors are present. Interestingly, coating samples with nanocontainers containing corrosion inhibitors displayed relatively low initial low-frequency impedance when compared to the bare sample and coating containing MBT and MBI. It is interesting to note that after an initial decrease in the low-frequency impedance, the samples then showed an increase in the low-frequency impedance, indicating improved corrosion protection. The impedance spectra of the SNAP coating with the MBI/β-CD and MBT/β-CD systems along with the spectra of the inhibitor-free sample (bare sample) and coatings containing MBT and MBI are shown in Fig. 7. In Table 1, corrosion rates of uncoated samples and samples coated with free inhibitor and nanocontainers are shown which indicate the SNAP coating containing MBI provides better protection than MBT. A comparison of these results indicates that both coating systems exhibit similar low-frequency impedance modulus values at the initial immersion in corrosive electrolyte. However, the impedance values for the inhibitor-free coating quickly decrease indicating that the coating is starting to delaminate, which is more likely to happen within the scribed area as a result of corrosion attack at the coating/substrate interface.5,6 Additionally, the coatings with the inhibitor/cyclodextrin complexes have shown visually much less pitting corrosion within the area of the scribe as compared to coating containing MBI and MBT and the inhibitor-free SNAP coatings (bare sample) (Fig. 6). Overall, the results of the EIS analysis support our findings on the corrosion protection properties of inhibitor-doped SNAP coatings. The results confirm our previous conclusions of promising corrosion protection of the cyclodextrin-based coating formulations.5,6
Controlled release of inhibitors from nanocontainers
Nanocontainer solubility and solution stability are important properties to be considered when selecting the dissolution medium. In this study, 0.05 M acetate buffer pH 5 is used as dissolution medium. UV-spectra of nanocontainers showed that the inhibitor absorbed appreciably at 237 nm and 239 for MBT and MBI, respectively, so these wavelengths were selected as the detection wavelength. The calibration curve was found to be linear which is shown in Fig. 7.
The amount of inhibitor released was measured by UV–Vis spectroscopy following the evolution of the absorption peaks of MBT (237 nm) and MBI (239 nm). UV-spectra showed the controlled released of inhibitor from nanocontainers with time which is based on reversible hydrogen bonds of inclusion complex formation of β-CD and inhibitors (Fig. 8).
Conclusions
Incorporation of various organic corrosion inhibitors into hybrid organo-silicate coatings has been investigated as smart corrosion inhibitor nanocontainers that are able to store an inhibitor and release it in the region of the damaged barrier coating, providing self-healing of the localized corrosion attack on the exposed metal. The incorporation of MBI and MBT corrosion inhibitors within β-CD and using these nanocontainers in coating has a pronounced effect on the reduction of a corrosion attack on aluminum substrate which has been observed by several electrochemical techniques. The inclusion complex formation of inhibitor corrosion with cyclodectrin is a result of reversible hydrogen bonds formation, and leads to improved anticorrosion properties of the coating, which can be simply repaired by bringing together the fractured ends for as little as few minutes at ambient temperature. Hydrogen bonding units may react with the closest ones in their section which is crucial to obtain a sufficient self-healing property. UV-spectra showed the controlled release of inhibitor from nanocontainers with time which is based on reversible hydrogen bonds of inclusion complex formation of β-CD and inhibitor.
References
Wu, Y, Meure, S, Solomon, D, “Self-healing Polymeric Materials: A Review of Recent Developments.” Prog. Polym. Sci., 33 (5) 479–522 (2008)
Wool, RP, “Self-healing Materials: A Review.” Soft Matter, 4 (3) 400–418 (2008)
Ghosh, S, Self-healing Materials: Fundamentals, Design Strategies, and Applications. Wiley-VCH, Weinheim, 2009
Syrett, J, Becer, C, Haddleton, D, “Self-healing and Self-mendable Polymers.” Polym. Chem., 1 (7) 978–987 (2010)
Pirhady Tavandashti, N, Sanjabi, S, “Corrosion Study of Hybrid Sol–Gel Coatings Containing Boehmite Nanoparticles Loaded with Cerium Nitrate Corrosion Inhibitor.” Prog. Org. Coat., 69 (4) 384–391 (2010)
Zandi-zand, R, Ershad-langroudi, A, Rahimi, A, “Silica Based Organic–Inorganic Hybrid Nanocomposite Coatings for Corrosion Protection.” Prog. Org. Coat., 53 (4) 286–291 (2005)
Khramov, AN, Voevodin, NN, Balbyshev, VN, Donley, MS, “Hybrid Organo-ceramic Corrosion Protection Coatings with Encapsulated Organic Corrosion Inhibitors.” Thin Solid Films, 447 –448 549–557 (2004)
Shchuki, DG, Helmuth, M, “Self-repairing Coatings Containing Active Nanoreservoirs.” Smart Mater., 3 (6) 926–943 (2007)
Khramova, AN, Voevodinb, NN, Balbysheva, VN, Mantzc, RA, “Sol–Gel-Derived Corrosion-Protective Coatings with Controllable Release of Incorporated Organic Corrosion Inhibitors.” Thin Solid Films, 483 (1–2) 191–196 (2005)
Mingxing, H, Zhang, H, Yang, J, “Synthesis of Organic Silane Microcapsules for Self-healing Corrosion Resistant Polymer Coatings.” Corros. Sci., 65 (16) 561–566 (2012)
Zheludkevich, ML, Shchukin, DG, Yasakau, KA, Mohwald, H, Ferreira, MGS, “Anticorrosion Coatings with Self-healing Effect Based on Nanocontainers Impregnated with Corrosion Inhibitor.” Chem. Mater., 19 (3) 402–411 (2007)
Semsarzadeh, MA, Amiri, S, “Preparation and Characterization of Inclusion Complexes of Poly(dimethylsiloxane)s with gamma-Cyclodextrin Without Sonic Energy.” Silicon, 4 (3) 151–156 (2012)
Semsarzadeh, MA, Amiri, S, “Preparation and Properties of Polyrotaxane from α-Cyclodextrin and Poly(ethylene glycol) with Poly(vinyl alcohol).” Bull. Mater. Sci., 36 (6) 989–996 (2013)
Amiri, S, Rahimi, A, “Preparation of Supramolecular Corrosion Inhibitor Nanocontainers for Self-protective Hybrid Nanocomposite Coatings.” J. Polym. Res., (2014). doi:10.1007/s10965-014-0566-5
Zandi-zand, R, Ershad-langroudi, A, Rahimi, A, “Synthesis and Characterization of Nanocomposite Hybrid Coatings Based on 3-Glycidoxypropyl-trimethoxysilane and Bisphenol A.” Iran. Polym. J., 14 (4) 371–377 (2005)
Zandi-zand, R, Ershad-langroudi, A, Rahimi, A, “Improvement of Corrosiom Resistance of Organic-Inorganic Hybride Coating Based on Epoxy-Silica via Aromatic Diol Curing Agent.” Iran. J. Polym. Sci. Technol., 17 (6) 359–367 (2005)
Rule, J, Sottos, N, White, S, “Effect of Microcapsule Size on the Performance of Self-healing Polymers.” Polymer, 48 (12) 3520–3529 (2007)
Rahimi, A, Gharazi, S, Ershad-Langroudi, A, Ghasemi, D, “Synthesis and Characterization of Hydrophilic Nanocomposite Coating on Glass Substrate.” J. Appl. Polym. Sci., 102 (6) 5322–5329 (2006)
Zheludkevich, ML, Shchukin, DG, Yasakau, KA, Mohwald, H, Ferreira, MGS, “Anticorrosion Coatings with Self-healing Effect Based on Nanocontainers Impregnated with Corrosion Inhibitor.” Chem. Mater., 19 (3) 402–411 (2007)
Kartsonakis, IA, Balaskas, AC, Koumoulos, EP, Charitidis, CA, Kordas, GC, “Incorporation of Ceramic Nanocontainers into Epoxy Coatings for the Corrosion Protection of Hot Dip Galvanized Steel.” Corros. Sci., 57 (26) 30–41 (2012)
Acknowledgments
Acknowledgments Financial support from IPPI nano-technology committee (Grant No. 24711149) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahimi, A., Amiri, S. Anticorrosion hybrid nanocomposite coatings with encapsulated organic corrosion inhibitors. J Coat Technol Res 12, 587–593 (2015). https://doi.org/10.1007/s11998-015-9657-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-015-9657-4