Abstract
In order to improve the dispersion of nanosilica and the mechanical properties of UV-curable coating, nanosilica was modified with acrylsilane-containing tertiary amine structure, which was synthesized by the Michael addition reaction between 3-aminopropyl triethoxysilane and tripropylene glycol diacrylate. The prepared acrylsilane was characterized by 1H NMR, 13C NMR, and FTIR. The modified nanosilica was characterized by FTIR, TGA, and SEM. The TGA analysis showed that the grafting percentage of acrylsilane based on nanosilica was 72.4 wt%. The SEM results showed that the agglomeration of nanosilica was reduced and the dispersion was improved due to the acrylsilane modification. The viscosities of UV-curable coatings with modified nanosilica were determined and it was found that the viscosities of the coatings decreased in comparison with the viscosities of coatings with unmodified nanosilica. The photo-DSC results indicated that both nanosilica and modified nanosilica also decreased the UV-curing speed and final percentage conversion, while the conversion of the coatings containing modified nanosilica was faster than that with unmodified nanosilica owing to the tertiary amine structure and acrylate structure on the surface of the modified nanosilica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
UV curing technique is becoming increasingly important in the field of coatings due to its high-speed process, lower energy consumption, lower process costs, high chemical stability, and environmental friendliness by avoiding solvent exposure.1–5 However, the cured film of pure organic coating has poor mechanical properties, such as thermal stability, hardness, and scratch and abrasion resistance. Therefore, preparation of hybrid UV-curable coating by adding inorganic particles has drawn considerable attention in recent years.5
Nanosilica is available in industry and it has the potential for being used in UV coatings.6–8 However, the high specific surface area and hydrophilic property of nanosilica lead to its uneven dispersion in organic material and serious aggregation in coatings. Therefore, the mechanical properties cannot be improved greatly. Besides, the viscosity of coatings increases and the photopolymerization rate of coatings decreases.7 To resolve these problems, nanosilica must be modified before it is used in UV-curable coating.
Modified nanosilica can always be prepared by grafting nanosilica with suitable vinylsilanes or acrylsilane.8 The modification not only improves the miscibility of nanosilica and the organic coating, but also the presence of vinyl groups grafted onto nanosilica can crosslink with acrylate matrix leading to the silica phase interconnection with the polymer matrix after UV cure. As a result, the mechanical properties of films are improved, such as thermal stability, hardness, and scratch and abrasion resistance. Li et al.9 prepared modified nanosilica with 3-(trimethoxysilyl) propyl methacrylate with different sizes by sol–gel method and the results showed that the curing speed of UV-curable coatings containing modified nanosilica decreased. However, the influencing degree decreased with the increase of nanosilica size. Misra et al.10 prepared the modified nanosilica with acrylate functional silane, which was synthesized by the Michael addition reaction. After incorporation into UV curable materials, it was found that the abrasion resistance of coatings was greatly enhanced. To increase the photopolymerization rate and improve the mechanical properties of film, our research group had prepared polymeric nanosilica hybrid materials with nanosilica as node, IPDI and PPG as link, and acrylate as terminal group as well as the polymerizable silica hybrid nanoparticles with tertiary amine structure.11,12 Compared with pure organic coating or hybrid coating with unmodified nanosilica, the properties of hybrid coatings were improved greatly.
To our knowledge, previous literature reported only the grafting of small molecules onto the nanosilica surface for UV-curable coatings and the addition of small molecule tertiary amine, which can lead to residues after curing. In this work, we synthesized an acrylsilane-containing tertiary amine structure via Michael addition between trimethylolpropane triacrylate and 3-aminopropyl triethoxysilane and obtained the modified nanosilica with the above acrylsilane. The synthesized structure contained terminal acrylate, which can participate in the UV curing process. Effects of the modified nanosilica on their size distribution in organic solvent, viscosity, and curing speed of UV-curable coatings were discussed. The hardness and abrasion resistance of cured films were also evaluated.
Experimental
Materials
Epoxy acrylate (EA) with double functionality, tripropylene glycol diacrylate (TPGDA), 1,6-hexanediol diacrylate (HDDA), and trimethylolpropane triacrylate (TMPTA) were provided by UCB Chemicals. 3-Aminopropyl triethoxysilane (APTES) was purchased from Jiangsu Danyang Organosilicon Industrial Co., China. 1-Hydroxy-cyclohexyl-phenyl ketone (HCPK) from Ciba-Geigy was used as a free radical photoinitiator. Nanosilica with an average particle size of 20–50 nm and a specific surface area of 640 m2/g and silanol group content of 1.9 mmol/g was obtained from Zhejiang Hongsheng Materials Co., China. The other materials were obtained locally. Nanosilica was kept in a vacuum chamber for 24 h at 100°C and the other materials were used without further purification.
Preparation of acrylsilane-containing tertiary amine structure
Into a 500 mL four-neck flask equipped with a mechanical stirrer, thermometer, drop funnel, and nitrogen gas inlet, 150.1 g of TPGDA (0.5 mol) was introduced. Then, 55.4 g of APTES (0.25 mol) was dropped into the flask within 1 h and the solution was stirred at room temperature under nitrogen atmosphere. The reaction process was determined by the change of integrated area of the peak in the range of 1533–1673 cm−1 corresponding to acrylate double bonds in the FTIR spectra. After the conversion reached about 50%, a viscous transparent fluid of acrylsilane-containing tertiary amine structure was obtained and abbreviated as AS-TA.
Surface modification of nanosilica with AS-TA
In a 500 mL four-neck flask equipped with a mechanical stirrer, thermometer, drop funnel, and nitrogen gas inlet, 10 g nanosilica were dispersed in 150 mL toluene with vigorous stirring for 1 h. Into the flask, 20 g of AS-TA and 2 mL of 0.1 mol/L formic acid were added, and the reaction was continued for 10 h under refluxing temperature with the protection of nitrogen. After reaction, the modified nanosilica were filtered under suction and physically absorbed AS-TA compounds were removed by extracting with ethanol for 24 h and dried in an oven at 40°C for 72 h. A white powder was obtained, named modified nanosilica.
Preparation of UV-curable coatings
The organic coating in this study was a mixture of 30 wt% EA, 40 wt% HDDA, 25 wt% TMPTA, and 5 wt% HCPK. Into the above mixture 2, 4, and 6 wt%, respectively, of the unmodified nanosilica and the modified nanosilica were directly added and dispersed by ultrasonic irradiating for 1 h at room temperature. The different hybrid coatings were obtained.
Characterization and measurement
Samples were analyzed by a Nicolet 360 FTIR spectrometer (USA). Solid samples were ground with KBr and compressed into a pellet; liquid samples were coated on the KBr pallets. FTIR spectra were recorded in the 400–4000 cm−1 range with 16 scans. 1H NMR and 13C NMR spectra were performed with a Brucker AC300 nuclear magnetic resonance spectrometer using DCCl3 as solvent and TMS as reference.
The conversion of acrylate double bonds in Michael addition reaction was detected by FTIR. The conversions were identified by comparing the change of integrated area of acrylate double bonds in the range of 1533–1673 cm−1 with carbonyl group absorption peak in the range of 1688–1828 cm−1 as internal standard. At a subsequent time t, the area of the peak can be integrated and the conversion at the reaction time can be determined according our previous methods as follows13:
where A 0(C=C) and A t(C=C) are the integrated area of acrylate double bonds in the range of 1533–1673 cm−1 at the initial time and the time t, respectively. Meanwhile, A 0(C=O) and A t(C=O) are the integrated area of carbonyl group absorption peak in the range of 1688–1828 cm−1 at the initial time and the time t, respectively.
The thermal behavior of the nanosilica and the modified nanosilica was determined with a HCT-1 comprehensive thermal gravimetric analyzer (Hnven Scientific Instrument Co., China) with a heating rate of 20°C/min from 20 to 700°C and an air flow rate of 50 mL/min. The grafting percentage of organic compounds onto the nanosilica was calculated by the following equation14:
where W 1 is the starting weight of the modified nanosilica particles, \( W^{\prime}_{1} \) is the residual weight of the modified nanosilica particles at 700°C, W 0 the starting weight of unmodified nanosilica particles, and \( W^{\prime}_{0} \) the residual weight of unmodified nanosilica particles at 700°C.
Scanning electron microscopy (SEM) was performed on an S-530 scanning electron microscope (Hitachi Ltd., Japan). Powder was distributed evenly in acetone to prepare a 2% solution. The solution was dropped onto the surface of a support and dried for SEM analysis.
The polymerization processes of the UV-curable coatings were monitored by the photo-DSC (Q-1000 TA Instruments). The photo-DSC experiments were carried out by the above instrument under the UV light intensity of 50 mW/cm2 and an air atmosphere with the flow rate of 50 mL/min. The acrylate double bonds conversions of the UV-curable coatings were calculated by integrating the area of the exothermic peak using the following equation15:
where ΔH t is the reaction heat enthalpy released at time t and ΔH theor0 is the theoretical heat enthalpy for the complete conversion. For these calculations, ΔH theor0 (acrylate) = 86 kJ/mol. The rate of polymerization (R p) is related directly to the heat flow (dH/dt) using the following equation15:
The viscosities of coating were measured by an NDJ-79 rotation viscometer (Shanghai Scientific Instrument Co., China) at 25°C.
To measure the hardness and abrasion resistance of UV curable films, the coatings with a wet thickness of 100 ± 5 μm were applied on aluminum plates and then processed by exposure to a medium pressure Hg lamp with 10 cm distance (1 kW, 80 W/cm2) for 2 min and then being set in a dark place for 5 h. The Shore D hardness was determined according to the testing method of ASTM D2240. The abrasion resistance was measured by subjecting the cured film to a YL-3315 Taber Abrader in accordance with ASTM D4060 at room temperature. A 500 g load was placed on top of the rubber abrader wheel and allowed to spin with a speed of 60 rpm and the weight loss of the film was calculated from the weight change before and after 300 cycles of abrasion.
Results and discussion
Preparation and characterization of modified nanosilica
In order to prepare modified nanosilica with a high grafting percentage of organic compounds, we synthesized an organic acrylsilane-containing tertiary amine structure by Michael addition reaction. Michael reaction between primary amine and acrylate is a classical reaction and easily occurs. The influencing parameters, including catalyst, temperature, solvent, and substrate, have been studied systematically.16,17 The reaction will be accelerated and the side reaction will be induced by adding catalyst, raising temperature, and increasing concentration of primary materials. To obtain the desired acrylate, the adding rate of APTES and temperature were controlled at 1 h and room temperature, respectively. No solvent was used in the reaction process in order to reduce the post-treatment. The reaction scheme was selected and represented in Scheme 1.
The Michael addition of APTES and TPGDA was characterized by FTIR. In Fig. 1, the mixture of APTES and TPGDA showed the characteristic absorption peak of –CH=CH2 group at 1635 cm−1 and –NH2 group at 3447 cm−1. Increasing reaction time led to the decrease in the intensity of the two peaks progressively. The reaction was finished when the absorption peak of –NH2 at 3500 cm−1 almost disappeared and the acrylate double bonds conversion reached about 50%.
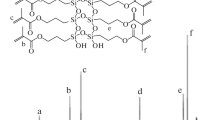
The 1H NMR and 13C NMR spectrum of AS-TA are given in Fig. 2.
1H NMR (CDCl3, ppm): 1.22–1.51 (m, 27H, –CH3); 2.36 (m, 4H, COO–CH2–); 2.73 (m, 6H, N–CH2–); 3.68 (m, 22H, O–CH2–CH–O, –CH2–); 4.43 (m, 6H, –O–CH(CH3)); 5.89 (d, 2H, CH2=CH–); 6.16 (m, 2H, CH2=CH–); 6.43 (d, 2H, CH2=CH–).
The peak assignment of 13C NMR of AS-TA was listed in the above spectrum. From the 1H NMR and 13C NMR, it indicated the desired product was synthesized according to the synthesis scheme.
The modification mechanism of nanosilica with acrylsilane was reported in previous literature.8 The influences of temperature,18 solvent,19 concentration,20 and pH21 on the nanosilica preparation modified by different silane coupling agents have been investigated and reported. Considering the literature reports, we chose toluene as solvent in order to improve the solubility of AS-TA. The temperature is controlled at refluxing temperature under nitrogen atmosphere to avoid yellowing of AS-TA, and the formic acid was used to ensure the complete hydrolysis of AS-TA therefore enhancing the grating ratio. Methoxy groups in AS-TA were available for the covalent linkage with abundant Si–OH groups from the surfaces of the nanosilica spheres. The scheme for modification of nanosilica with AS-TA is given in Scheme 2.
The FTIR spectra of the nanosilica before and after modification are given in Fig. 3. Compared with the FTIR spectrum of the unmodified nanosilica (Fig. 3a), the modified nanosilica (Fig. 3c) clearly showed the characteristic peak at 1720 cm−1 for –C=O group and the peak at 1664 cm−1 for –CH=CH2 group. The strong bands in the range of 2800–3000 cm−1 corresponded to the alkyl groups of AS-TA.22 The AS-TA physically absorbed on the nanosilica has been removed by extracting with ethanol solution, it proves that the AS-TA have been successfully grafted onto the nanosilica surface.23
The quantitative analysis of modified nanosilica and the grafting ratio were determined by TGA, which were performed from room temperature to 700°C by heating at 20°C/min. The result shows that the unmodified nanosilica had a weight loss (about 8 wt%) before 700°C, which is related to the elimination of physically and chemically absorbed water on the surface. The physically adsorbed water was attributed to the weight loss up to 120°C while chemically bound water was attributed to the weight loss from 120 to 700°C.24 As for modified nanosilica, there are two weight loss stages. The first weight loss occurred before 200°C, which can be ascribed to the physically absorbed organic compounds. The sharp weight loss from 200 to 700°C for modified nanosilica can probably be attributed large scale thermal decomposition of AS-TA chains.25–27 Drying the physically adsorbed organic compounds and then extracting by the Soxhlet’s extraction apparatus using ethanol for 24 h indicates that the decomposition ratio of organic compounds was equal to the grafting ratio. The grafting ratio for modified nanosilica was 72.4 wt% (Fig. 4).
In this study, the Si–OH group on the nanosilica was about 1.9 mmol/g. If methoxy groups in AS-TA structure reacted with only one hydroxyl group on the nanosilica surface, the theoretical amount of AS-TA for 1 g nanosilica would be 1.9 mmol = 1.4 g (the molecule weight of AS-TA is about 737 g/mol, supposing Si–OC2H5 fully hydrolyzed as Si–OH and without considering self-condensation of APTES).28 It means that the maximum theoretical grafting percentage of nanosilica was about 1.4 g/g = 140 wt%. The experimental grafting percentage for the modified nanosilica was about 72.4 wt%, which was lower than the theoretical grafting value of 140 wt% due to the steric hinder of the surface of nanosilica and using multiple grafting modes instead of only one AS-TA grafted onto one hydroxyl group of the nanosilica surface.
One of the main goals in this study was to improve the dispersion of the nanosilica in organic coatings. Therefore, the dispersion of the nanoparticles is a crucial factor. The SEM analysis of nanosilica before and after modification is shown in Fig. 5. The morphology analysis of nanosilica and modified nanosilica by SEM shows the effect of AS-TA on reducing nanosilica’s aggregation. Figure 5a shows that nanosilica existed in irregular blocks and aggregated extremely because of the large amounts of silanol groups on their surface. After being grafted with AS-TA, the silica existed in the state of irregular balls with a diameter of about 100 nm. After treatment with acrylsilane, the dispersion and self-aggregation of the modified nanosilica were improved.
Effect of modified nanosilica on the properties of UV-curable coating
The viscosity of a UV curable system is considered as one of the most important parameters because high viscosity affects the processability and the UV-curing speed of the coatings.29 Therefore, the viscosity of the UV coatings was evaluated and the results are shown in Fig. 6.
It was found that the viscosity of the UV-curable coatings increased with an increase in the amount of the modified nanosilica and the unmodified nanosilica. However, the viscosities of UV coatings with the modified nanosilica were smaller than those with the unmodified nanosilica at the same filling amount. The reason is that the unmodified nanosilica tended to self-aggregation in the UV-curable system and yielded high viscosities of UV-curable coatings due to the poor compatibility of unmodified nanosilica and organic matrix. The second reason is that the surface hydroxyl of unmodified nanosilica easily forms a hydrogen bond with the coatings. After grafting with organic compounds, the bonding between the silane and the silica surface removes the surface silanol groups and the organic shell forms on the nanosilica surface, leading to the transfer from hydrophilation to organophilation nanoparticles and the decrease in the viscosities of coatings.30,31
Incorporation of nanosilica into UV coatings affects the UV-curing speed and acrylate double bonds conversion rate because silica decreases the UV-ray intensity. The UV curable processes of castings were monitored by photo-DSC and the results are shown in Fig. 7.
The heat flow is proportional to the acrylate double bonds conversion rate at a constant temperature. It was found that both the unmodified nanosilica and the modified nanosilica also affected the UV curing process. With 2 wt% of silica nanoparticles, the UV curing systems exhibited lower exotherms while higher ultimate percentage conversion than that of pure organic UV coating. At the induction time, both the nanosilica and the modified nanosilica will absorb the UV light, which slightly increases the induction period of photoinitiator, and the photopolymerization rate would decrease.32 However, the nanosilica and the modified nanosilica behaved as an effective flow or diffusion aid agent for the photopolymerization process after induction time, which improved the mobility of propagating chains that gave an increase in ultimate percentage conversion.7 With the higher amount of silica nanoparticles (4 and 6 wt%), the results indicated that the nanosilica and the modified nanosilica apparently prolonged the UV curable time. It can be ascribed to the simultaneous action of some factors as follows: nanosilica in the coatings absorb the UV light during the polymerization process, with the higher concentration of nanosilica increasing the aggregation occurrence and hindering the radical mobility, which was confirmed by the viscosity variation of UV curable system containing the nanosilica as illustrated in Fig. 6. Compared with the effect of nanosilica before and after modification, the coatings with the modified nanosilica exhibited higher exotherms and ultimate percentage conversion, which can be ascribed to the tertiary amine and acrylate structure on the surface of the modified nanosilica. Tertiary amine structure in the modified nanosilica is a co-initiator for photopolymerization because α–H in the chain of amine group can absorb resolved oxygen in coating and it again initiates polymerization.33 The terminal acrylate double bonds on the nanosilica surface can increase the conversion of acrylate double bonds in the coating. The possible acting mechanism is shown in Fig. 8.
Effect of modified nanosilica on the mechanical properties of UV curable film
Incorporating the amount of the nanosilica and the modified nanosilica also affected the mechanical properties of the films of UV coatings. Figures 9 and 10 show the result of the effect of the modified nanosilica on the Shore D hardness and abrasion resistance of the cured film. The hardness of the cured films increased with the increasing amount of nanosilica, which can be ascribed to two reasons. One is that hard silica particles probably in part migrate toward the surface of the film during the UV-curable process.8 The difference of nanosilica before and after modification can be ascribed to the better distribution of modified nanosilica. The relatively small amount of hydrogen bond between modified nanosilica and the matrix coatings increased the migration of inorganic nanoparticles during the UV-curing process.34 The other reason may be uniform distribution of nanosilica in the UV-curable coatings and reinforcement by Si–O–Si linkage in the cured films.35 Increasing the amount of silica, the structure of the interpenetrating polymer network became much denser and the hardness of the material increased. The hardness increase is related to an increase in abrasion resistance, and similar trends were also seen in abrasion-resistance experiments. When various modified nanosilica were used in UV-curable coating, the abrasion loss of UV cured films decreased from 10.0 to 5.4 mg after 300 cycles, while the value for nanosilica decreased from 10.0 to 5.6 mg.
Conclusions
Surface modification and characterization of nanosilica with acrylsilane-containing tertiary amine structure synthesized from TPGDA and APTES were investigated. The modified nanosilica was used as an additive in the UV-curable coating, and the results showed that the surface modification of nanosilica with acrylsilane improved the dispersion of nanosilica in organic matrix, mechanical properties, and photocurable properties of UV curable hybrid silica coating.
The results revealed that the grafting percentage of acrylsilane onto the nanosilica surface was about 72.4 wt%, which was lower than the theoretical maximum grafting percentage due to the multiple grafting modes and being sterically hindered. Compared with the unmodified nanosilica, the modified nanosilica exhibited higher exotherms and ultimate percentage conversion in the UV curing process due to the tertiary amine decreasing the oxygen inhibition and acrylate double bonds increasing the concentration of polymerizable groups.
References
Karataş, S, Hoşgör, Z, Kayaman-Apohan, N, Güngör, A, “Preparation and Characterization of Phosphine Oxide Containing Organosilica Hybrid Coatings by Photopolymerization and Sol–Gel Process.” Prog. Org. Coat., 65 49–55 (2009)
Chen, YC, Zhou, SX, Yang, HH, Gu, GX, Wu, LM, “Preparation and Characterization of Nanocomposite Polyurethane.” J. Colloid Interface Sci., 279 370–378 (2004)
Zhou, SX, Wu, LM, Sun, J, Shen, WD, “The Change of the Properties of Acrylic-Based Polyurethane Via Addition of Nano-silica.” Prog. Org. Coat., 45 33–42 (2002)
Cui, XJ, Zhong, SL, Yan, J, Wang, CL, Zhang, HT, Wang, HY, “Synthesis and Characterization of Core–Shell SiO2-Fluorinated Polyacrylate Nanocomposite Latex Particles Containing Fluorine in the Shell.” Colloid Surf. A, 360 41–46 (2010)
Bauer, F, Flyunt, R, Czihal, K, Langguth, H, Mehnert, R, Schubert, R, Buchmeiser, MR, “UV Curing and Matting of Acrylate Coatings Reinforced by Nano-silica and Micro-corundum Particles.” Prog. Org. Coat., 60 121–126 (2007)
Chattopadhyay, DK, Zakula, AD, Webster, DC, “Organic–Inorganic Hybrid Coatings Prepared from Glycidyl Carbamate Resin, 3-Aminopropyl Trimethoxy Silane and Tetraethoxyorthosilicate.” Prog. Org. Coat., 64 128–137 (2009)
Cho, JD, Kim, YB, “The Effects of Silica Nanoparticles on the Photocuring Behaviors of UV-Curable Polyester Acrylate-Based Coating Systems.” Macromol. Res., 13 362–365 (2005)
Sangermanoa, M, Malucellia, G, Amerioa, E, Priola, A, Billi, E, Rizza, G, “Photopolymerization of Epoxy Coatings Containing Silica Nanoparticles.” Prog. Org. Coat., 54 134–138 (2002)
Li, FS, Zhou, SX, Wu, LM, “Preparation and Characterization of UV-Curable MPS-Modified Silica Nanocomposite Coats.” J. Appl. Polym. Sci., 98 2274–2281 (2005)
Misra, M, Guest, A, Tilley, M, “Hybrid Inorganic–Organic UV-Curable Abrasion-Resistant Coatings.” Surf. Coat. Int. B, 81 594–595 (1998)
Ma, GZ, Liu, W, Liu, XG, Wu, JB, Yan, T, Xu, BS, “Preparation and Properties of Polymerizable Silica Hybrid Nanoparticles with Tertiary Amine Structure.” Prog. Org. Coat., 71 83–87 (2011)
Ma, GZ, Liu, W, Yan, T, Wei, LQ, Xu, BS, “Preparation of Polymeric Nanosilica Hybrid Materials and Their Properties.” Acta Polym. Sin., 2 203–209 (2011)
Ma, GZ, Wu, JB, Xu, BS, “Study on the Conversion of Acrylic C=C Double Bonds During Dark Reaction After UV Curing Using Infrared Spectroscopy.” Spectrosc. Spect. Anal., 20 1780–1784 (2010)
Guo, YK, Wang, MY, Zhang, HQ, Liu, GD, Zhang, LQ, Qu, XW, “The Surface Modification of Nanosilica, Preparation of Nanosilica/Acrylic Core–Shell Composite Latex, and Its Application in Toughening PVC Matrix.” J. Appl. Polym. Sci., 107 2671–2680 (2008)
Palanisamy, A, Rao, BS, “Photo-DSC and Dynamic Mechanical Studies on UV Curable Compositions Containing Diacrylate of Ricinoleic and Amide Derived from Castor Oil.” Prog. Org. Coat., 60 161–169 (2007)
Mather, BD, Viswanathan, K, Miller, KM, Long, TE, “Michael Addition Reactions in Macromolecular Design for Emerging Technologies.” Prog. Polym. Sci., 31 487–531 (2006)
Wang, D, Liu, Y, Hu, ZC, Hong, CY, Pan, CY, “Michael Addition Polymerizations of Trifunctional Amines with Diacrylamides.” Polymer, 46 3507–3514 (2005)
Buyl, F De, Kretschmer, A, “Understanding Hydrolysis and Condensation Kinetics of γ-glycidoxypropyl Trimethoxysilane.” J. Adhes., 2 125–142 (2008)
Abel, ML, Joannic, R, Fayos, M, Lafontaine, E, Shaw, SJ, Watts, JF, “Effect of Solvent Nature on the Interaction of γ-Glycidoxy Propyl Trimethoxy Silane on Oxidised Aluminium Surface: A Study by Solution Chemistry and Surface Analysis.” Int. J. Adhes. Adhes., 26 16–27 (2006)
Bertelsen, CM, Boerio, FJ, “Effect of Processing Variables on the Solution Characteristics of γ-Glycidoxypropyltrimethoxysilane (γ-GPS).” J. Adhes., 3 259–279 (1999)
Yang, LX, Feng, J, Zhang, WG, Qu, JE, “Experimental and Computational Study on Hydrolysis and Condensation Kinetics of γ-Glycidoxypropyltrimethoxysilane (γ-GPS).” Appl. Surf. Sci., 257 990–996 (2010)
Lu, Q, Chen, XM, Nie, L, Luo, J, Jiang, HJ, Chen, LN, Hu, Q, Du, SH, Zhang, ZP, “Tuning of the Vinyl Groups’ Spacing at Surface of Modified Silica in Preparation of High Density Imprinted Layer-Coated Silica Nanoparticles: A Dispersive Solid-Phase Extraction Materials for Chlorpyrifos.” Talanta, 81 959–966 (2010)
Liu, HB, Chen, MC, Huang, ZT, Xu, K, Zhang, XJ, “The Influence of Silicon-Containing Acrylate as Active Diluent on the Properties of UV-Cured Epoxydiacrylate Films.” Eur. Polym. J., 40 609–613 (2004)
Mueller, R, Kammler, HK, Wegner, K, Pratsinis, SE, “OH Surface Density of SiO2 and TiO2 by Thermogravimetric Analysis.” Langmuir, 19 160–165 (2003)
Qiu, FX, Zhou, YM, Liu, JZ, Zhang, XP, “Synthesis, Structural and Morphological Characteristics of P(MA-MPTMS)/SiO2 Hybrid Nanocomposites.” Chem. J. Internet, 6 063020pe (2004)
Mikhailenko, S, Desplantier-Giscard, D, Danumah, C, Kaliaguine, S, “Solid Electrolyte Properties of Sulfonic Acid Functionalized Mesostructured Porous Silica.” Microporous Mesoporous Mater., 52 29–37 (2002)
Kim, SS, Park, JE, Lee, J, “Properties and Antimicrobial Efficacy of Cellulose Fiber Coated with Silver Nanoparticles and 3-Mercaptopropyltrimethoxysilane (3-MPTMS).” J. Appl. Polym. Sci., 119 2261–2267 (2011)
Sabzi, M, Mirabedini, SM, Zouhuriaan-Mehr, J, Atai, M, “Surface Modification of TiO2 Nano-particles with Silane Coupling Agent and Investigation of Its Effect on the Properties of Polyurethane Composite Coating.” Prog. Org. Coat., 65 222–228 (2009)
Yu, Y, Rong, MZ, Zhang, MQ, “Grafting of Hyperbranched Aromatic Polyamide onto Silica Nanoparticles.” Polymer, 51 492–499 (2010)
Sun, YY, Zhang, ZQ, Wong, CP, “Study on Mono-dispersed Nano-size Silica by Surface Modification for Underfill Application.” J. Colloid Interface Sci., 292 436–444 (2005)
Zhou, SX, Wu, LM, Sun, J, Shen, WD, “Effect of Nanosilica on the Properties of Polyster-Based Polyurethane.” J. Appl. Polym. Sci., 88 189–193 (2003)
Uhl, FM, Davuluri, SP, Wong, SC, Webster, DC, “Organically Modified Montmorillonites in UV Curable Urethane Acrylate Films.” Polymer, 45 6175–6187 (2004)
Decker, C, Jenkins, AD, “Kinetic Approach of Oxygen Inhibition in Ultraviolet- and Laser-Induced Polymerizations.” Macromolecules, 18 1241–1244 (1985)
Tahmassebi, N, Moradian, S, Ramezanzadeh, B, Khosravi, A, Behdad, S, “Effect of Addition of Hydrophobic Nano-silica on Viscoelastic Properties and Scratch Resistance of an Acrylic/Melamine Automotive Clearcoat.” Tribol. Int., 43 685–693 (2010)
Xu, JW, Pang, WM, Shi, WF, “Synthesis of UV-Curable Organic–Inorganic Hybrid Urethane Acrylates and Properties of Cured Films.” Thin Solid Films, 514 69–75 (2006)
Acknowledgments
This study was supported by the Important Specialized Science and Technology Item of Shanxi Province of China (No. 20111101059), the National Younger Natural Science Foundation of China (No. 21103120), and the Postgraduate Innovation Item of Shanxi Province (No. 20133030), which are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, J., Ma, G., Li, P. et al. Surface modification of nanosilica with acrylsilane-containing tertiary amine structure and their effect on the properties of UV-curable coating. J Coat Technol Res 11, 387–395 (2014). https://doi.org/10.1007/s11998-013-9552-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-013-9552-9