Abstract
The nanosilica is first treated with 3-mercaptopropyl trimethoxysilane (MPTMS) to introduce mercapto groups as growth points, and then hyperbranched polymer is grafted on the nanosilica surface via repeated step of thiol-ene click reaction between the acrylate double bond of trimethylolpropane triacrylate (TMPTA) and mercapto groups of trimethylolpropane tris 3-mercaptopropionate (Trithiol). FTIR results confirm that the grafting procedure is feasible, and TGA results indicate the grafting ratio is as high as 45.0 %. The curing kinetic, monitored by photo-DSC, shows that both terminal acrylate double bonds and mercapto groups can accelerate the curing speed of the UV-curing organic–inorganic hybrid coatings. The mechanical and physical properties of UV-curable hybrid coatings containing modified nanosilica at different generations are also investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanosilica plays an important role in UV-curable organic–inorganic hybrid coatings due to its special physical and chemical properties [1–3]. However, nanosilica is easy to aggregate and difficult to disperse in solvent and polymer matrix because of its high specific surface area and large amount of silanol, therefore, it is necessary to modify its surface prior to use in the UV-curable coatings. Grafting polymers onto nanosilica surface is one of the effective methods to improve the surface properties, and the grafted polymers claim on the surface interfere with the aggregation of these particles and increase the affinity of the surface for solvent and polymer matrix [4–6]. In recent years, preparation and application of nanosilica modified by polymers in UV-curable coatings has become an attractive field [7–9], especially the polymers with the terminal acrylate groups. Our previous works demonstrated that incorporation of nanosilica modified by acrylate polymers with tertiary structure is capable of increasing the curing speed and improving the mechanical properties of UV-cured films [10, 11]. Mori et al. have grafted hyperbranched (meth)acrylates onto nanosilica surface by one-step self-condensing atom transfer radical polymerization, the differences between linear polymer brush, the branched and the hyperbranched polymers are compared, and the feasibility of controlling the surface chemical functionalities is suggested [12].
Besides the polymerizable acrylate group grafted onto the nanosilica surface, the mercapto group is another potential functional group to graft onto the nanosilica surface for UV-curable coatings. The mercapto groups can not only reduce oxygen inhibition during UV-curable process, but also carry many other attributes for UV-curable materials; including inherently rapid reaction rate, low polymerization shrinkage, free-photoinitiator polymerization and formation of homogeneous polymer structure [13–16]. Nevertheless, low molecular thiol or linear polymer was grafted onto the nanosilica surface in the previous studies [17], and hyperbranched polymers with terminal mercapto groups grafted onto nanosilcia surface are yet to be explored. Recently, the thiol-ene click reaction was widely used in preparing polymers. It is anticipated that the hyperbranched polymer grafted from the nanosilica surface by thiol-ene click reaction might be feasible, and the synergistic effect of mercapto groups and nanosilica can be realized in UV-curable coatings. It can not only improve the dispersion of nanosilica, reduce the oxygen inhibition during UV-curing process, but also reduce the migration of small molecule thiol after UV curing.
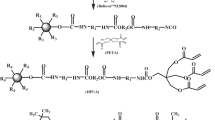
In this work, the nanosilica is treated with 3-mercaptopropyl trimethoxysilane (MPTMS) to introduce mercapto groups as the growth points on the surface, and then hyperbranched polymers is grafted onto nanosilica surface by a repeated thiol-ene click reaction between trimethylolpropane triacrylate (TMPTA) and trimethylolpropane tris 3-mercaptopropionate (Trithol). The preparation scheme is shown in Fig. 1. The structure, grafting efficiency, particle size distribution of nanosilica at different generations are investigated, respectively, and their influences on the properties of UV-curable coatings are also investigated.
Experimental
Materials
Nanosilica powder with an average particle size of 20–50 nm and silanol group content of 1.9 mmol/g was obtained from Zhejiang Hongsheng Materials Co., China. Prior to usage, the nanosilica was dried in an oven at 120 °C under vacuum for 24 h to get rid of the physically adsorbed water. 3-Mercaptopropyl trimethoxysilane (MPTMS) and trimethylolpropane tris 3-mercaptopropionate (Trithiol) were purchased from Sigma Chemical Co.
Epoxy acrylate (EA) with double functionality, tripropylene glycol diacrylate (TPGDA) and trimethylolpropane triacrylate (TMPTA) is provided by UCB chemicals. 2,4,6-Trimethyl benzoyl diphenylphosphine oxide (TPO) from Ciba-Geigy is used as a free-radical photoinitiator. All other chemicals are analytical pure reagents (AR) and are used as received without further treatment.
Grafting mercapto groups onto nanosilica surface
Ten grams of nanosilica (called as SiO2-0th) was dispersed in 150 mL anhydrous toluene under mechanical stirring and ultrasonic dispersing for 30 min. The mixture was stirred at 300 rpm for a further 1 h, and then added into another prepared mixture consisting of 10 mL water, 10 mL anhydrous ethanol, 2 mL formic acid (as pH adjuster) and 75 mL MPTMS under vigorously stirring. The detailed preparation is carried out according to our previous work [18]. After continuously stirring for 4 h at the reflux temperature, the obtained suspension was separated by centrifugation. After filtration and extraction with ethanol for 24 h to remove the excess silane adsorbed on the nanosilica, the MPTMS-treated nanosilica (called SiO2-1st) was dried in vacuum at 90 °C for 24 h. The thermal gravimetric analysis gave a grafting percentage of 16.8 % and the content of mercapto group (−SH) on the surface of SiO2-1st was determined with the method of Ellman [19], which provided a result of 0.9 mmol/g.
Grafting of hyperbranched polymer from nanosilica surface by thiol-ene click reaction
Hyperbranched polymer with terminal mercapto groups onto the nanosilica surface was achieved by a repeated thiol-ene click reaction between the acrylate groups of TMPTA and the mercapto groups of Trithiol in a toluene dry system.
A typical procedure was carried out as follows. 150 mL toluene and 10 g SiO2-1st were charged into a 500-mL three-necked flask. 50 g TMPTA (excess of surface mercapto group content) was sprayed, and the mixture was agitated at 300 rpm at room temperature and irradiated by UV light with the main wavelength of 365 nm. After 20 h, unreacted TMPTA was removed by extracting with toluene for 12 h. The obtained modified nanosilica with terminal acrylate groups was called as SiO2-2nd. Then, 150 mL toluene and 10 g SiO2-2nd were charged into a 500-mL three-necked flask. 50 g Trithiol (excess of surface acrylate group content) was added into the mixture. The mixture was agitated at 300 rpm under the same conditions as above. After 20 h, unreacted Trithiol was removed by extracting with toluene for 12 h. The obtained modified nanosilica with terminal mercapto groups was called as SiO2-3rd. The thiol-ene click reaction between TMPTA and Trithiol was repeated to allow the growth of hyperbranched polymers form the nanosilica surface. The modified nanosilica at different generations was called as SiO2-nth.
Characterizations
FTIR analysis was performed in potassium bromide on a Nicolet 360 FTIR spectrometer collecting 16 scans in the 400–4000 cm−1 range with 4 cm−1 resolution. The thermogravimetric analysis (TGA) was performed in a HCT-1 thermal gravimetric analyzer (Hnven Scientific Instument Co., China) under a nitrogen atmosphere from room temperature to 700 °C with a heating rate of 20 °C/min. The grafting ratio (R g) was calculated according to the following equation [20]:
where W 1 is the starting weight of the modified nanosilica particles, W 1′ is the residual weight of the modified nanosilica particles at 700 °C, W 0 is the starting weight of unmodified nanosilica particles, W 0′ is the residual weight of unmodified nanosilica particles at 700 °C.
Gel permeation chromatography (GPC) was used to determine the molecular weight after the grafted polymer was cleaved from nanosilica with hydrofluoric acid [21]. The GPC is Waters1515, which utilized a refractive index (RI, 2410) detector with THF as the eluent. Samples were firstly treated by centrifugal separation and then prepared by dissolving 10 mg of polymer in 1 mL of THF and passing them through a 0.22-μm organic filter to remove any particulate contaminants. Analysis was performed at 35 °C with a flow rate of 1 mL/min, and the poly(styrene) narrow standards were used.
The particle (or particle agglomeration) size and distribution of nanosilica at different generations were characterized by a laser particle analyzer Bettersize 2000 (Dandong Bettersize Instruments Corporation, Dandong, China). Toluene was used as the following liquid.
The morphology of nanosilica and modified nanosilica were visualized by a JEOL JSM-6700F field-emission scanning electron microscopy (FE-SEM) operating at an acceleration voltage of 10 kV and spatial resolution of 2 nm. The powder was dispersed in acetone to prepare 1 wt% solution, and the solution was dropped onto the surface of a support, dried and deposited on the sample with gold for SEM analysis.
Preparation and properties analysis of UV-curable coatings
The UV-curable coatings used in this study was a mixture of 30 wt % EA, 40 wt % TPGDA, 25 wt % TMPTA and 5 wt % TPO. 2 wt % of nanosilica at different generations was directly added into the above mixture, dispersed by ultrasonic irradiating for 1 h at room temperature, and the different hybrid coatings were obtained finally.
The photo-DSC experiments were carried out using a DSC (Q-1000 TA Instruments). The initiation light source was a 200-W medium pressure mercury lamp, which gave a UV light intensity at the sample of 80 mW/cm2 over a main wavelength of 365 nm. Samples weighing 20 ± 0.1 (mean ± SD) mg were placed in uncovered aluminum pans, and a reference aluminum pan was left empty. The conversion of acrylate double bonds in the UV-curable system was calculated by integrating the area of the exothermic peak.
The viscosities of coating were measured by an NDJ-79 rotation viscometer (Shanghai Scientific Instrument Co., China) at 25 °C. The pencil hardness was determined according to the testing method of ASTM D3363. The UV-curable system was processed by exposing to a medium pressure Hg lamp (1 kW, 80 W/cm2) for 2 min and putting them in a dark place for 5 h.
Results and discussion
Preparation and characterization of modified nanosilica
The functional groups of primary materials used in this study are characterized by FTIR to confirm the change of the functional groups on the nanosilica surface during the whole preparation processes, which is shown in Fig. 2. The characteristic peak at 2570 cm−1 corresponds to –SH of MPTMS and Trithiol in Fig. 2a, c, respectively. From the FTIR spectra of nanosilica as shown in Fig. 2b, the stretching vibration peaks of Si–OH were at 3430 cm−1, the asymmetric stretching vibration was at 810 cm−1, the symmetric stretching vibration and bending vibration of Si–O–Si were at 470 cm−1 [22].
The coupling agent treatment plays an essential role in chemically attaching polymer chains onto nanoparticles [23]. In this work, the mercapto groups are first introduced onto nanosilica surface as initiator site by the treatment of nanosilica with MPTMS, and then hyperbranched polymers is grafted from these mercapto groups. FTIR spectra of modified nanosilica at different generations are shown in Fig. 3. With the increase of generation and grafting polymer, the relate strength of peak at 3430 and 1650 cm−1 corresponding to −OH on the nanosilica surface decreases, the carboxyl groups at 1700 cm−1 increase and the methylene at 2938 and 2873 cm−1 is observed in the modified nanosilica, which proves that hyperbranched polymer chains have been chemically bonded to the nanosilica surface. However, the appearance of peak at 3430 cm−1 shows that –OH on the nanosilica surface has not reacted with MPTMS completely. The increasingly distinct peaks of organic chains on the nanosilica surface based on the FTIR spectra indicate that the repeated thiol-ene click reactions on the nanosilica surface have occurred.
The grafting ratio of organic materials on the nanosilica surface is determined by TGA, which is performed from room temperature to 700 °C with a heating rate of 20 °C/min. Figure 4 shows that the unmodified nanosilica has a weight loss (about 8 wt%) before 700 °C, which is related to the elimination of physically and chemical absorbed water on the surface. The physically adsorbed water was attributed to the weight loss up to 120 °C, while chemically bound water was attributed to the weight loss from 120 to 700 °C. As for modified nanosilica, there are two weight loss stages, the first weight loss occurred before 250 °C, which can be ascribed to the physically absorbed organic compounds. Compared with the different curves, it can be found that SiO2-2nd curve had lowest weight loss. The important reason is that TMPTA is not easy to absorb physically on the nanosilica surface, therefore, the ungrafted molecule is easy to extract and the weight loss is low in TGA experiment. While the MPTMS and trithiol is more easily physically absorbed on the nanosilica surface for their hydrogen bond effect. The sharp weight loss from 250 to 700 °C for modified nanosilica is probably attributed to large-scale thermal decomposition of grafted molecular chains. With the increase of hybranched polymer grafted onto nanosilica, the decomposition temperature is increasing and the TGA curve slope is growing from 400 to 700 °C. It can be ascribed that more hybranched polymer increased the cross-link density and the thermal properties were improved. Because the modified nanosilica was dried and extracted by the Soxhlet’s extraction apparatus using toluene for 12 h, the physical adsorbed organic compounds were removed and it could be thought that the decomposition ratio of organic compounds was equal to the grafting ratio. The grafting ratio increases from 16.8 % for MPTMS-modified nanosilica (SiO2-1st) to 45.0 % for the hyperbranched polymer-grafted nanosilica (SiO2-5th). And the corresponding number average molecular weight (Mn) of the grafted molecule changed from 500 to 2000 for modified stage from SiO2-1st to SiO2-5th. In addition, Fig. 2 indicates that the grafting ratio changes every time after grafting process indicated that new structure has grafted onto the nanosilica surface and the chemical reaction has occurred during grafting. During the post-grafting procedure, the grafting modes and chains propagation depends on the starting sites formed during the first-generation grafting rather than the propagation of grafted polymers [5]. Since there is strong steric hindrance effect, only a few grafted chains can grow from the particles and the grafting ratio change is limit in spite of excess monomer for modified nanosilica.
It is well established that the dispersion state of nanoparticles is a crucial factor in determining the final properties of nanocomposites. The dispersibility of modified nanosilica is better than that of bare nanosilica in organic solvent [24]. The particle size distributions of nanosilica at different generations are shown in Fig. 5. The mean size of modified nanosilica is about 2 μm in TPGDA, which is larger than the real size of nanosilica due to the particle agglomeration, but was smaller than the unmodified nanosilica dispersed by sonication in TPGDA (about 6 μm). There exists a shoulder peak at the lower size district, which is related to the single dispersion of nanosilica. The results indicate that the dispersion of nanosilica in organic solvent was improved progressively with the increase in generation of the modified nanosilica. Because the larger organic chains can form organic shell on the nanosilica surface, the self-agglomeration of nanosilica is reduced and the single dispersion of nanosilica is improved. The dispersion improvement of nanosilica in organic system also proves the feasibility of thiol-ene click reaction based on the nanosilica surface.
The SEM results are consistent with the distribution analysis; the morphology analysis of primary nanosilica and final hyperbranched grafted nanosilica is shown in Fig. 6. The unmodified nanosilica exists in irregular block and aggregated seriously because of the presence of silanol groups on their surface. After grafting with hyperbranched polymers, the silica exists in the state of irregular balls with a nanosized diameter about 200 nm. The results suggest that modified nanosilica form uniform cluster of particles and are homogeneously dispersed in the UV-curable polymer matrix without agglomeration.
Effect of modified nanosilica on the curing kinetics
The formulation of the UV-curable coatings used in this study was a mixture of 30 wt% EA, 40 wt% TPGDA, 25 wt% TMPTA and 5 wt% TPO. In this study, we fixed the concentration of nanoparticles at 2 wt% to the UV-curable coating, the effect of nanosilica at different modification stages and the surface functional group on the curing kinetics of UV-curable coatings were investigated. The content of modified nanosilica was calculated based on bare nanosilica.
The photopolymerization kinetics was performed using photo-DSC to clarify the photocuring process of the various systems. Photo-DSC experiments are capable of providing kinetics data in which the measured heat flow can be converted directly to the ultimate percentage conversion and polymerization rate. The photo-DSC method assumes that for a cure process the measured heat flow is proportional to the conversion rate [25–27].
where ΔH t is the reaction heat enthalpy released at time t and \(\Delta H_{ 0}^{\text{theor}}\) is the theoretical heat enthalpy for the complete conversion. For the mixture of EA, TPGDA and TMPTA, \(\Delta H_{ 0}^{\text{theor}}\) for EA, TPGDA and TMPTA was 339, 542 and 818 J/g, respectively [28]. So the theoretical heat enthalpy for the mixture \(\Delta H_{ 0}^{\text{theor}} = 550\, {\text{J/g}}\). The rate of polymerization (Rp) is related directly to the heat flow (dH/dt) using the following equation:
where da/dt is the conversion rate or the polymerization rate and dH/dt is the measured heat flow.
Generally, the photocuring process is considered as an autocatalyzed curing reaction for at least one of the reaction products that is also involved in the propagating reaction. The cure kinetics for an autocatalyzed reaction is described using the following equation [26–29].
where k is the Arrhenius-type reaction rate constant, m is the order of the initiation reaction, and n is the order of the propagation reaction. Values of k and n can be determined from a ln–ln plot of da/dt versus \(\alpha^{\text{m/n}} \left( {1 - \alpha } \right)\) derived from the equation
So as to enable comparison between more complex systems, the value of k was measured for the initial phase of polymerization. The value of n was fixed as 1.5 [27, 29] and then, m was calculated.
The photo-DSC exotherms for the polymerization of the UV-curable hybrid systems containing silica nanoparticles at different generations are illustrated in Fig. 7. The results from the photo-DSC analysis are summarized in Table 1.
Figure 7 and Table 1 indicate that the surface functional groups of nanosilica play an important role during the UV-curing process. The induction time of UV-curable coatings increased while the maximum heat flow decreased with the increase of generation of modified nanosilica. According to the experimental design, the terminal functional groups on the nanosilica surface are different for different modified step. The terminal group is mercapto group for SiO2-1st, SiO2-3rd, SiO2-5th, and acrylate double bonds for SiO2-2nd, SiO2-4th. Table 1 show that the UV-curable coatings containing modified nanosilica at different generations demonstrate more induction time and lower final percentage conversion than the pure UV-curable coatings, but the SiO2-1st is exception. Especially, the terminal mercapto group on the nanosilica surface had higher promoting effect than the acrylate groups. It can be ascribed to the reflection and scattering effect of nanosilica to UV light [11]. However, the final conversion percentage of coatings with modified nanosilica was higher than that with unmodified nanosilica, which can be illustrated by the effect of functional groups on the nanosilica surface.
Both the terminal acrylate double bonds and the mercapto groups can not only improve the dispersion of nanosilica in UV-curable coatings, but also promote the UV-curing process by either involving the UV-curing process or reducing oxygen inhibition. The acrylate double bonds on the nanosilica surface can provide polymerizable property for the nanosilica, therefore, the modified nanosilica can cross-link with the cured polymers as illustrated in Fig. 8a. While the mercapto groups on the nanosilica surface can transfer peroxide radicals into thiol radical, which provide more active radical for UV-curing process, therefore, the other acrylate structure can be initiated but not be terminated with other H atom. The illustrated scheme is shown in Fig. 8b, c [29].
In contrast, the SiO2-4th and SiO2-5th do not exhibit better curing properties than the former modified nanosilica beyond our expectation. The reason is that the surface of modified nanosilica at higher generation had higher branching degree. Despite the large organic molecule improved the dispersion, the grafted macromolecule limited the mobility of nanosilica, therefore, the surface functional groups do not play their effective role completely in the UV-curing process. The results are further proved by the more induction time and the more maximum peak time for SiO2-4th and SiO2-5th.
Effect of modified nanosilica on the properties of UV-curable coatings
The viscosity of a UV-curable system is considered as one of the most important parameters, affecting the processability and the photopolymerization rate of the cured film. So a suitable viscosity of the UV-curable system is very important to the properties of the final cured film [30]. Figure 9 shows the viscosities and pencil hardness of UV-curable coatings with 2 wt% silica nanoparticles at different generations. It was found that the nanosilica increased the viscosity of the UV-curable coatings at different degree. Compared with the coatings with unmodified nanosilica, the viscosity of UV-curable coating with modified nanosilica decreased progressively with the increase of modified generation. It can be concluded that unmodified nanosilica tended to self-agglomeration in UV-curable system and yielded highly viscous dispersions of UV-curable coatings ascribed to their poor compatibility to each other. After grafting with organic compounds, the organic shell which formed on the nanosilica surface, resulting in the transfer form hydrophilation to organophilation nanoparticles, thus decreased the viscosity of coatings. The more the polymers grafted onto the nanosilica surface, the better the dispersion of nanosilica in UV-curable coatings.
The results of the pencil hardness measurements indicated that the value increased with the increase of modified generation. This may be explained by the interaction of the nanosilica with the organic matrix. The difference of nanosilica at different generations can be ascribed to the better distribution of modified nanosilica at higher generation reducing the migration of inorganic nanoparticles during UV-curing process, the nanosilica without modification are not homogeneously distributed and dispersed in the hybrid matrix, this may lead to phase separation and result in poor pencil hardness [31]. The other reason can be attributed to uniform distribution of nanosilica in the UV-curable coatings and reinforcement by Si–O–Si linkage in the cured films [2].
Conclusions
The hyperbranched polymer has been successfully grafted from the nanosilica surface by a repeated step of thiol-ene click reaction between TMPTA and Trithiol. The final modified nanosilica is ended by mercapto groups with grafting ratio of 45.0 %. The self-agglomeration and the dispersion of nanosilica are improved progressively with the increase of grating polymer. The photo-DSC results show that both the terminal acrylate double bonds and mercapto groups grafted onto the nanosilica act as effective curing agent for UV-curable coatings. However, the nanosilica at higher generations are not found to further improvement in curing kinetics, which can be attributed to the influences of macromolecules grafted onto the nanosilica surface on the nanosilica mobility during the UV-curing process. Considering the viscosity and pencil hardness of UV-curable coatings with silica nanoparticles modification before and after, the viscosity decreased and the pencil hardness increased with the increase of modified generation.
References
Dastjerdi R, Montazer M (2010) A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Coll Surf B 79:5–18
Zhang SW, Yu AX, Song XQ, Liu XY (2013) Synthesis and characterization of waterborne UV-curable polyurethane nanocomposites based on the macromonomer surface modification of colloidal silica. Prog Org Coat 76:1032–1039
Ranjbar Z, Jannesari A, Rastegar S, Montazeri Sh (2009) Study of the influence of nano-silica particles on the curing reactions of acrylic-melamine clear-coats. Prog Org Coat 66:372–376
Laoutid F, Estrada E, Michell RM, Bonnaud L, Müller AJ, Dubois Ph (2013) The influence of nanosilica on the nucleation, crystallization and tensile properties of PP–PC and PP–PA blends. Polymer 54:3982–3993
Yu Y, Rong MZ, Zhang MQ (2010) Grafting of hyperbranched aromatic polyamide onto silica nanoparticles. Polymer 51:492–499
Seckin T, Gültek A (2003) Postgrafting of congo red dye onto hyperbranched mesoporous silica with terminal amino groups. J Appl Polym Sci 90:3905–3911
Wozniak M, Yde Hazan, Graule T, Kata D (2011) Rheology of UV curable colloidal silica dispersions for rapid prototyping applications. J Eur Ceram Soc 31:2221–2229
Taniguchi Y, Shirai K, Saitoh H, Yamauchi T, Tsubokawa N (2005) Postgrafting of vinyl polymers onto hyperbranched poly(amidoamine)-grafted nano-sized silica surface. Polymer 46:2541–2547
Mu B, Wang TM, Liu P (2007) Well-defined dendritic-graft copolymer grafted silica nanoparticle by consecutive surface-initiated atom transfer radical polymerizations. Ind Eng Chem Res 46:3069–3072
Ma GZ, Liu W, Liu XG, Wu JB, Yan T, Xu BS (2011) Preparation and properties of polymerizable silica hybrid nanoparticles with tertiary amine structure. Prog Org Coat 71:83–87
Ma GZ, Liu W, Yan T, Wei LQ, Xu BS (2011) Preparation of polymeric nanosilica hybrid materials and their properties. Acta Polym Sinica 2:203–209
Mori H, Böker A, Krausch G, Müller AHE (2001) Surface-grafted hyperbranched polymers via self-condensing atom transfer radical polymerization from silicon surfaces. Macromolecules 34:6871–6882
Sangermano M, Colucci G, Fragale M, Rizza G (2009) Hybrid organic–inorganic coatings based on thiol-ene systems. React Funct Polym 69:719–723
Clark TS, Hoyle CE, Nazarenko S (2008) Kinetics analysis and physical properties of photocured silicate-based thiol-ene nanocomposites: the effects of vinyl POSS ene on the polymerization kinetics and physical properties of thiol-triallyl ether networks. J Coat Tech Res 5:345–351
Colucci G, Mana S, Conzatti L, Sangermano M (2012) Hybrid organic–inorganic silicate/thiol-ene photocured coatings. Surf Coat Tech 206:2719–2724
Korthals B, Morant-Miñana MC, Schmid M, Mecking S (2010) Functionalization of polymer nanoparticles by thiol-ene addition. Macromolecules 43:8071–8878
Ren H, Qu YX, Zhao SH (2006) Reinforcement of styrene–butadiene rubber with silica modified by silane coupling agents, experimental and theoretical chemistry study. Chin J Chem Eng 14:93–98
Wu JB, Xie JB, Ling LX, Ma GZ, Wang BJ (2013) Surface modification of nanosilica with 3-mercaptopropyl trimethoxysilane and investigation of its effect on the properties of UV curable coatings. J Coat Tech Res 10(6):849–857
Shimada K, Cheftel JC (1989) Sulfhydryl group/disulfide bond interchange reactions during heat-induced gelation of whey protein isolate. J Agric Food Chem 37:161–168
Guo YK, Wang MY, Zhang HQ, Liu GD, Zhang LQ, Qu XW (2008) The surface modification of nanosilica, preparation of nanosilica/acrylic core-shell composite latex, and its application in toughening PVC matrix. J Appl Polym Sci 107:2671–2680
Cui WW, Tang DY, Gong ZL (2013) Electrospun poly (vinylidene uoride)/poly (methyl methacrylate) grafted TiO2 composite nanofibrous membrane as polymer electrolyte for lithium-ion batteries. J Power Sour 223:206–213
Chuayjuljit S, Boonmahitthisud A (2010) Natural rubber nanocomposites using polystyrene-encapsulated nanosilica prepared by differential microemulsion polymerization. Appl Surf Sci 256:7211–7216
Luo Y, Rong MZ, Zhang MQ, Friedrich K (2004) Surface grafting onto SiC nanoparticles with glycidyl methacrylate in emulsion. J Polym Sci Polym Chem 42:3842–3852
Liu P (2005) Hyperbranched aliphatic polyester grafted silica nanoparticles by a facile one-pot method. Surf Rev Lett 12:619–622
Cho JD, Hong JW (2005) Photo-curing kinetics for the UV-initiated cationic polymerization of a cycloaliphatic diepoxide system photosensitized by thioxanthone. Eur Polym J 41:367–374
Cho JD, Ju HT, Hong JW (2005) Photocuring kinetics of UV-initiated free-radical photopolymerizations with and without silica nanoparticles. J Appl Polym Sci 43:658–670
Esen DS, Karasu F, Arsu N (2011) The investigation of photoinitiated polymerization of multifunctional acrylates with TX-BT by photo-DSC and RT-FTIR. Prog Org Coat 70:102–107
Palanisamy A, Rao BS (2007) Photo-DSC and dynamic mechanical studies on UV curable compositions containing diacrylate of ricinoleic and amide derived from castor oil. Prog Org Coat 60:161–169
Cramer NB, Scott JP, Bowman CN (2002) Photopolymerizations of thiol-ene polymers without photoinitiators. Macromolecules 35:5361–5365
Qiu FX, Xu HP, Wang YY, Xu JC, Yang DY (2012) Preparation, characterization and properties of UV-curable waterborne polyurethane acrylate/SiO2 coating. J Coat Tech Res 9:503–514
Sangermano M, Messori M (2010) Scratch resistance enhancement of polymer coatings. Macromol Mater Eng 295:603–612
Acknowledgments
This study was supported by the Important Specialized Science and Technology Item of Shanxi Province of China (No. 20111101059) and the Postgraduate Innovation Item of Shanxi Province (20133030), which are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, J., Ma, G., Ling, L. et al. Grafting of hyperbranched polymer onto the nanosilica surface and their effect on the properties of UV-curable coatings. Polym. Bull. 73, 859–873 (2016). https://doi.org/10.1007/s00289-015-1523-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1523-0