Abstract
Fruit waste has considerable potential for valorization as a pectin source. This research work concentrated on assessing the ability of kinnow (Citrus reticulata) peel as a raw material for pectin isolation. Pectin was isolated from kinnow peel through conventional methods (aqueous extraction and acid-assisted extraction) and novel green methods (microwave and ultrasound-assisted extraction). The pectin obtained with these techniques was analyzed and compared in terms of yield and physicochemical properties. The pectin yield ranged from 18.57% (aqueous extraction) to 30.59% (ultrasound-assisted extraction). The isolated pectin contained methoxyl (5.8 to 7.2%), anhydrouronic acid (57.6 to 73.4%), degree of esterification (55.7 to 80%), and galacturonic acid (64.5 to 68.2%). Structural analysis by Fourier transform infrared spectroscopy and X-ray diffraction showed that the pectin extracted was pure, crystalline, and of high methoxyl in nature. The scanning electron microscopy result showed that the ultrasonic treatment affected the pectin matrix, thus causing the disruption of bonds between pectin molecules. According to the result of characterization, it can be concluded that novel green technologies provide better pectin in terms of yield and quality in comparison with conventional techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Waste valorization is currently a prevalent topic across the world. It has the potential to give a country significant economic, social, and environmental benefits. Citrus fruit is the most widely eaten globally, accounting for roughly 18% of global fruit yield (Kamal et al., 2011). Citrus peels are rich in many phytochemicals and bioactive substances such as essential oils, pigments, carotenoids, and polyphenols (phenolic acids and flavonoids) that have positive effect on health. The monoterpene (i.e., limonene) is mainly responsible for the aroma of citrus fruits. The bioflavonoids found in citrus fruit are flavonones (mainly hesperidin) which shows antioxidant, anti-inflammatory, antiaging, antiallergen, and anti-tumor effect. They also contain significant amounts of potassium, folic acid, pectin, and vitamin C (Suri et al., 2022). Bioactive substances present in fruit peels, such as phenols, flavonoids, carotenoids, and vitamins, are often heat-labile substances whose degradation rates vary depending on the processing methods used (Shinwari & Rao, 2018). For juice extraction, a large number of citrus fruits are used, and as a result of processing, abundant solid debris is created, mostly in the form of peel and pomace. Separation of phytochemical substances, pectin, fibers, organic acids, enzymes, and other valuable goods from agro-industrial wastes is an effective waste valorization solution (Barcelos et al., 2020; Mehmood et al., 2021).
The kinnow (Citrus reticulata) fruit crop is a South East Asian hybrid of King and Willow leaf (Citrus nobilis Lour x Citrus deliciosa Tenora). Kinnow is high in ascorbic acid, vitamin B, and β-carotene and has a variety of medical and health benefits. The kinnow peel is excellent for the formation of cellulose (Oberoi et al., 2011).
Citrus juice production results in the creation of waste, which accounts for over 50% of the weight of fresh fruit. Peels (50–55 percent of the entire fruit mass), seeds (20–40 percent of the total fruit mass), pomace, and wastewater are all included in this waste (Suri et al., 2022). Kinnow peels are a rich source of cellulose, pectin, hemicelluloses, lignin, essential oils, and phenolic compounds; thus, dumping them can lead to a variety of disposal and environmental issues as well as a loss of nutrients (Singla et al., 2019). Additionally, the residual residue from the extraction of bioactive substances can be used to produce more energy, moving closer to the concept of zero waste (Sagar et al., 2018).
The term “pectin” refers to pectic compounds that are water-soluble and have the potential of producing gels under certain situations. Braconnot derived the name pectin from the Greek term pektikos, which means to coagulate (Kinnaert et al., 2017). Pectin is made up of linear chain of α-(1,4) galacturonic acid with partial esterification of carboxylic group using methanol or ethanol (Yang et al., 2019). Protopectin, pectic acid, pectinic acid, and pectins are the four classes of pectic compounds, all of which have high molecular weights. The global demand for pectin is increasing by 4 to 5% per year (Rezzadori et al., 2012). It is frequently used as a gel-forming agent, emulsifier, and stabilizer in the processing sector. It has also versatile uses in the pharmaceutical industry such as anti-tumor, antioxidant, anti-diabetic, and anti-cancer agent (Hosseini et al., 2019). Because of its biodegradability, biocompatibility, and non-toxicity, pectin is considered a useful biomaterial film (Rodsamran & Sothornvit, 2019a, b).
Citrus debris and apple pomace are the primary raw materials utilized in the commercial manufacturing of pectin, which has very rarely been done (Picot-Allain et al., 2022). It may be because of easy accessibility of raw materials and good gel-forming ability of the extracted pectin.
Nowadays, with the rise of “green chemistry,” many technologies have been found successful including microwave isolation, ultrasonic isolation, and enzymatic isolation in the extraction of pectin (Adetunji et al., 2017). The plant source and the methods used to isolate and purify the pectin determine the constitution of the pectin. Therefore, extraction is a crucial step in recovering pectins, and the conditions selected for extraction depend on the source material and the intended result (BeMiller & Whistler, 2012).
Pectins can be extracted traditionally or conventionally using solvents such as water; dilute acids such as H2SO4, HCl, and HNO3; and alkalis such as NaOH (Wandee et al., 2019). It adheres to the principle of “like dissolves like” and has a low viscosity so that it may circulate freely. The acids and water are used in extraction as they are cheaper and more efficient, but on the other hand, they are also time-consuming with poor pectin yield (Chan et al., 2017).
Microwave-assisted extraction (MAE) is an intriguing alternative with various benefits, including a faster extraction rate, reduced solvent use, and a shorter time in comparison to traditional extraction techniques (Amran et al., 2021). Since microwave energy does not cause molecular changes in a substance or break chemical bonds, it is regarded as an appropriate source of extraction energy (Liew et al., 2019).
In ultrasound-assisted extraction (UAE), a solvent is used to extract components from plant matrices using sound energy as the primary driving mechanism. Because UAE takes place quickly and uses less solvent, it is viewed as being more eco-friendly (Kumar et al., 2017).
Recognizing the necessity to find new resources of pectin and the limitation of knowledge regarding the pectin extraction from kinnow peel, the goal of this research was to utilize kinnow waste along with exploring the potential of different extraction methods such as aqueous extraction (AQE), acid-assisted extraction (AAE), microwave-assisted extraction (MAE), and ultrasound-assisted extraction (UAE) in isolation of pectin through kinnow peel (Citrus reticulata). In addition, the characterization and comparative analysis of the yield and physicochemical properties of pectin were also investigated.
Materials and Methodology
Materials
Kinnow (Citrus reticulata) peels were procured from the fruit juice center at the Banaras Hindu University (Varanasi, Uttar Pradesh, India). Procured kinnow peels were sorted, washed, sliced, dried using a tray drier at 50 °C up to 9% moisture content (Khera Instruments Pvt. Ltd., India), ground to powder using the grinder, and stored in vacuum-packed plastic pouches. Based on procedure (AOAC, 2000), dried kinnow peel powder consisted of (on dry weight basis) protein 0.67%, crude fat 1.59%, ash 0.55%, and crude fiber 0.64%.
Chemicals
All chemicals such as ethanol, citric acid, and hydrochloric acid utilized during the investigation were of analytical grade and were purchased from Sigma Chemical Co. Ltd, Mumbai, India.
Extraction of Pectin from Kinnow Peel
Aqueous Extraction (AQE)
The aqueous extraction of pectin was carried out as per the procedure of Hosseini et al. (2016a, b) with minor modification. Kinnow peel powder (40 g) was blended properly with the distilled water in a solid-solvent ratio (1:20) in the beaker. The pH of the mixture was kept up to 2.5 through 0.1N citric acid solution, and then the solution was warmed up at 90 °C with continual agitation for 30 min in a shaker water bath, and the solution was then left to cool down to ambient temperature. The slurry (sample + solvent mix) was filtered using a 420-µm PTFE (polytetrafluoroethylene) micromesh screen to separate the solid residue and liquid extract. The liquid extract was further subjected to centrifugation for 15 min at 8000 × g at 25 °C in a centrifuge (Model 3-30 k, Sigma Laborzentrifugen GmbH, Germany), and the supernatant was then collected in a beaker for further pectin purification.
Supernatant recovered was kept in a beaker, and absolute ethanol (95%) was added in the ratio (1:2) to precipitate the extract, mixed for 5 min, and stored for 12 h at 4 °C to provide sufficient time for pectin extraction. The precipitated pectin was found floating in the beaker which was recovered through filtration using a muslin cloth. The pectin was further washed using 90% ethanol for 2–3 times on the muslin cloth to separate unwanted constituents such as mono- and disaccharides. The kinnow peel pectin was then dried in a hot air oven (Caltar-NSW 143, Oven Universal, India) at 50 °C until moisture content 7–8%, then milled to powdered form, sieved using a 125-µm screen, and stored for further analysis.
Acid-Assisted Extraction (AAE)
The acid-assisted extraction of pectin was carried out as per the procedure of Kulkarni and Vijayanand (2010) with minor modification. The kinnow peel powder (40 g) was mixed with 0.5N HCl in a solid-solvent ratio (1:15) in the beaker. The pH of the mixture was kept up to 2.5, and then it was heated in a water bath shaker at 110 °C for 90 min, and the solution was left to cool down to ambient temperature. The mixture was strained using a 420-µm screen and centrifuged at 5000 × g (25 °C) for 20 min, and the supernatant was collected for pectin purification. After centrifugation, the steps for pectin purification were kept the same as that of aqueous extraction.
Microwave-Assisted Extraction (MAE)
It was carried out as per the technique suggested by Wang et al. (2007). Kinnow peel powder (40 g) was suspended in 0.05 M HCl in the solid-solvent ratio (1:20) in a beaker. It was kept in the center of a microwave equipment (Model-CE1047DRB, Samsung Electronics Co., Ltd., India) on a rotating plate and exposed to microwave power of 900W for 180 s. The intermittent heating was done, using on and off approach (at 60-s interval) to prevent the solution from coming out of the beaker. The other steps were kept the same as that of aqueous extraction.
Ultrasound-Assisted Extraction (UAE)
The ultrasound-assisted extraction of pectin was carried out as per the procedure of ) with slight modification. UAE was performed using an ultrasonicator (SonicsVCX800, Newtown, USA) with a flat tip probe operated at 20 kHz having a maximum power of 750W. The probe (25 mm diameter) of the ultrasonicator was immersed at 15 mm depth in the mixture containing kinnow peel powder (40 g) dissolved in 0.1 M citric acid in a solid-solvent ratio (1:20). The mixture was kept in a Pyrex beaker containing 800 ml of mixture. Sonication was done at 600W for 30 min. The other steps were kept the same as that of aqueous extraction.
Characterization of Kinnow Peel Pectin (Analysis of Quantitative and Qualitative Parameters)
Pectin Yield
The amount obtained after extraction was quantified using Eq. (1) (Ranganna, 1986):
Color
Hunter Color Lab Colorimeter (Model: Color Quest XE, USA) was used in analyzing the color of pectin. The standards were analyzed as L (lightness: 0, black and 100, white), a (− a, greenness and + a, redness), and b (− b, blueness and + b, yellowness) values.
Equivalent Weight (Eq. W)
The following approach (Ranganna, 1986) was applied to measure Eq.W of pectin. 0.5 gm kinnow peel pectin was suspended in 5 ml ethanol. To sharpen the endpoint, 1 g of NaCl was put into the solution, and then finally the volume was made up to 100 ml with distilled water. Six drops of phenol red as an indicator were added, and titration using 0.1N NaOH (sodium hydroxide) was done. The end value was analyzed by the persistence of the pink color for 30 s. The neutral solution obtained after titration was further used in the analysis of MeO. Eq.W was analyzed using Eq. (2):
Methoxyl Content (MeO)
The MeO value was calculated through the (Ranganna, 1986) procedure, and the neutralized solution obtained after titration in the analysis of Eq.W was used. In the solution, 25 ml of 0.25N NaOH were mixed and stored for 30 min at room temperature. After that 25 ml of 0.25N HCl were mixed, and titration was done using 0.1N sodium hydroxide until the pink color persisted for 30 s. The MeO was measured using Eq. (3):
Total Anhydrouronic Acid Content (AUA)
The AUA value of pectin was estimated using equivalent weight and methoxyl content measurements. Ranganna (1986) reported the following formula for calculating anhydrouronic acid content (Eq. (4)):
where Molecular weight of AUA = 176; z is the ml (titer) of alkali from Eq.W analysis; y is the ml (titer) of alkali from MeO analysis; w is the weight of pectin.
Degree of Esterification (DE)
The DE of pectin was calculated using Eq. (5) based on MeO and AUA contents (Lal et al., 2021):
Galacturonic Acid Content (GA)
The GA value was analyzed as per Dische (1947) procedure. One milliliter of pectin solution containing 200 µg/ml of pectin was taken and dissolved into 6 ml of concentrated sulfuric acid. After this, the tubes were allowed to stand for about 20 min. Then 200 µl of 0.1% carbazole solution (dissolved in absolute ethanol) were mixed and stored for 2 h for the development of pink color. The absorbance was taken at 520 nm through a spectrophotometer (Shimadzu UV–Visible 1800, Tokyo, Japan). D-Galacturonic acid (0–250 µg/ml) was used to determine the standard curve.
Solubility and Solvent Holding Capacity (SHC)
The solubility and SHC were analyzed as per the procedure of Guzel and Akpinar (2019). To analyze the pectin solubility, 250 mg of pectin were mixed in 5 ml of each solvent such as 1 M sodium hydroxide, acetone, methanol, and dimethyl sulfoxide. After mixing pectin into the solvents, they were left for 2 min, and the solubility of pectin in each solvent was noted.
To determine the solvent holding capacity (SHC), a solution was made by mixing 1 g of pectin in 100 ml of solvents mentioned above. The solutions were then left for 2, centrifuged at 3500 × g for 30 min and weighed. The SHC was calculated using Eq. (6):
where,
X = weight of pectin after centrifugation.
Y = weight of pectin before mixing in solvent.
Emulsifying Properties (Emulsifying Activity (EA) and Emulsion Stability (ES))
The emulsifying properties were evaluated using the procedure of Dalev and Simeonova (1995). For analysis of emulsifying activity (EA), an emulsion was prepared by mixing 3 ml of sunflower oil and 3 ml of pectin solution (0.5% w/w), and 0.03% sodium azide as bactericide was added. The solution was then homogenized for 3 min at 12,000 rpm through a homogenizer (IKA-T25 Digital ultra turrax, India). Samples after that were centrifuged at 527 rpm for 5 min at 27 °C. After centrifugation Eq. (7) was used to calculate EA:
To determine emulsion stability (ES), the above centrifuged mixtures were kept both at 4 °C and room temperature for 1 and 30 days. The ES was analyzed using Eq. (8):
Fourier Transform Infrared Spectroscopy (FTIR)
To gather chemical information on pectin, an FTIR spectrometer (Spectrum 3 FT-IR, PerkinElmer, Inc., USA) was used. To eliminate moisture, the pectin samples were kept in an infrared oven. Dried samples were then mixed with potassium bromate and processed into powder before being converted into tablet form. The spectra were then recorded at the resolution 4 cm−1 within the wavelength range of 4000–400 cm−1. All spectra were subjected to noise reduction and baseline smoothing (Guzel & Akpinar, 2019), and the spectra were obtained using ORIGIN software (Version 8.0).
X-ray Diffraction (XRD) Analysis
X-ray diffraction analysis was done with the help of an X-ray diffractometer (X’ Pert3 Powder, PANalytical, Netherlands). Half gram of pectin samples in powder form was scanned at a diffraction angle (2θ) of 90–5° with CuKα radiation at a voltage of 40 kV and current of 40 mA. The spectra were obtained using ORIGIN software (Version 8.0).
Thermogravimetric (TGA) Analysis
A thermogravimetric 176 analyzer (Pyris 1 TGA, Perkin Elmer, USA) was used to measure pectin (6.0 mg) thermal properties placed in an aluminum pan in a nitrogen atmosphere at a heating rate of 10 °C/min over a temperature range of 50–600 °C.
Scanning Electron Microscopy (SEM)
The morphological structure of pectin was investigated through SEM. The pectin samples were dusted on double adhering tape and after that inserted on an aluminum stub of the microscope. The stub after that was covered through gold using sputter coater up to a thickness of 300 Å and then spotted under a scanning electron microscope (JSM-6100, JEOL, Tokyo, Japan). The magnification range was kept 1.0–500X at 10 kV voltage (Jain et al., 2015).
Energy Consumption and Cost Economics of Extraction
The power consumption (watts) used by the extraction methods was taken into account from the output power of the equipment used during pectin extraction. The energy consumed (kJ) was calculated as per the formula reported by Misra and Yadav (2020) as mentioned below:
where Et is the energy consumed in kilojoules (kJ), G is the power consumed in watts (W), and t is the extraction time in seconds (s).
Statistical Analysis
The extraction of pectin from all the above-mentioned methods was carried out in triplicate, and the values were expressed as mean with standard deviation. The statistical analysis of data was brought out using single-factor analysis of variance (ANOVA) with a level of confidence of 95% using Excel software (Microsoft, USA). Differences between the values were estimated at a significant level of 0.05. Differences were expressed as statistically significant at p < 0.05.
Results and Discussion
Pectin Yield
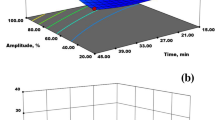
The yield of pectin from all the extraction methods, i.e., AQE, AAE, MAE, and UAE, is presented in Table 1. The ANOVA result showed that a significant difference was found (p < 0.05) amid the pectin yield of all isolation techniques. The chart shows yield% from all extraction methods (Fig. 1). The result obtained demonstrated that the pectin yield obtained from MAE (25.84 to 27.9%) and UAE (29.42 to 31.76%) was higher. Sharma et al. (2013) extracted pectin from kinnow peel using water acidified with HNO3 and obtained maximum pectin yield of 16.1%. The reason behind the highest pectin yield from UAE may be due to higher energy treatment by ultrasonic waves resulting in an accelerated breakdown of the side chain of dissolved pectin (Bhaskaracharya et al., 2009). The MAE also showed higher pectin yield which may be due to microwaves weakening the cell wall structure and causing parenchymal cells to cleave (Kratchanova et al., 2004). As a result, microwave radiation opens up skin tissues, increasing the contact between the solvent and the extracting substance (Kratchanova et al., 2004). The pectin yield from AAE (19.68 to 21.24%) also resulted in a slightly higher pectin yield because the acid caused hydrolysis of protopectin and converted it to pectin. However, the use of stronger acid (HCl) resulted in higher pectin yield than aqueous extraction, and it may be due to its higher affinity towards the cation such as Ca+ of the acid causing the stabilization of pectin molecule (Castillo-Israel et al., 2015). Pectin from passion fruit peel using conventional heating with high pressure as a pre-treatment was extracted, and the yield observed was around 8.97%, whereas the pectin extracted using only conventional heating was 7.4% (). The yield from kinnow peel using AQE (17.84 to 19.3%) was thus higher in comparison to the above-mentioned study. Numerous studies have shown that using a strong acid, like HCl, to extract pectin at a low pH (1.0–1.5), allows for the extraction of more pectin due to the improved capacity to remove the sugars (such as rhamnose, galactose, and arabinose) as well as D-galacturonic acid from the sample (Kliemann et al., 2009). Citrus peel pectin production varies from 11.1 to 21.3% when hydrochloric acid is used to extract it (Cui et al., 2020).
Color Determination
The color of the pectin is essential since it influences the appearance of the gel. Table 2 indicates the color parameters of pectin isolated from AQE, AAE, MAE, and UAE methods. All the color parameters of separation methods indicated significant differences (p < 0.05). The color variations have been shown in Fig. 2. The result conveyed that MAE had the highest lightness; UAE had the highest redness, and AAE had the highest yellowness. The brightness of extracted pectin showed the sequence: AQE < AAE < UAE < MAE whereas for redness, it was MAE < AAE < AQE < UAE and for yellowness AAE < AQE < UAE < MAE. This means that MAE-extracted pectin had a brighter appearance in comparison with AQE, AAE, and UAE. Masmoudi et al. (2012) extracted pectin from the date and lemon using acidified water and observed that the brightness of the pectin decreased due to an increase in extraction time and temperature. The darker color of pectin may be due to polyphenols or other water-soluble pigments which were confined in the inner side of pectin at the time of precipitation. Heating favors non-enzymatic browning, which changes the color of pectin and involves a variety of reactions such as the Maillard reaction, caramelization, and oxidation of phenolic components (Manzocco et al., 2000).
Equivalent Weight
The Eq.w is the amount of total free galacturonic acid (not esterified) in the pectin chain. Pectin having a higher equivalent weight will have a high gel-forming ability in comparison with pectin having a lower equivalent weight (Ranganna, 1986). Table 1 shows the equivalent weight of extracted pectin. Based on the ANOVA results, equivalent weight of UAE (726.07 mg) pectin was significantly higher (p < 0.05) than other extraction methods. The concentration of free fatty acid determines whether the equivalent weight of pectin increases or decreases (Nazaruddin, 2011). AQE-isolated pectin had the lowest equivalent weight (357 mg). The MAE-extracted pectin has higher equivalent weight (677.56 mg) than aqueous and acid-assisted extraction as the presence of stronger acid may lead to formation of smaller pectin particles due to an increase in the partial hydrolysis of the pectin at higher temperature (Rodsamran & Sothornvit, 2019a, b). The higher equivalent weight of pectin extracted from UAE could be attributed to higher ultrasonic power intensity, which allows for more pectin to be released.
Methoxyl Content
The amount of methyl groups in pectin impacts how long it will take to set and how well it can produce a gel. Table 1 indicates the methoxyl amount of extracted pectin. Based on the ANOVA result, methoxyl value of UAE (7.25%) was significantly higher (p < 0.05) than other extraction methods. The higher value of MeO in pectin isolated through UAE may be due to the presence of a freer esterified carboxyl group (Voragen et al., 2009). The least methoxyl content was observed in the pectin extracted from AQE (5.88%). The methoxyl content of MAE was 6.96% which is similar to the result of pectin extracted from lime peel (Rodsamran & Sothornvit, 2019a, b). Yadav et al. (2015) observed that the methoxyl content of pectin isolated from papaya, sweet lime, and orange was not much varied between the acid and conventional extraction. Research on the extraction of pectin from lemon and grapefruit showed that extracted pectin has a methoxyl concentration ranging from 0.22 to 12.0% (Aina et al., 2012). Commercial pectins typically have a methoxyl content between 8 and 11% and are capable of forming high sugar gels (over 65% sugar). On the other side, gels with lower sugar contents (less than 7.0%) can be produced by low methoxyl pectins (Rouse et al., 1964). Methoxyl content also affects how easily pectin dissolves in water, with higher methoxyl content being even more easily soluble in water than lower methoxyl content pectin (Constenla & Lozano, 2003).
Total Anhydrouronic Acid Content (AUA)
The quality or pureness of pectin is decided by the total anhydrouronic acid level, which should be at least 65% (Food Chemical Codex, 1996). Table 1 indicates the anhydrouronic acid content of extracted pectin. Based on the ANOVA result, AUA value of pectin isolated through UAE (73.40%) was significantly higher (p < 0.05) than other extraction methods. The higher anhydrouronic acid in pectin extracted from UAE was attributable to the presence of more galacturonic acid residues in the pectin structure. The least anhydrouronic acid content was found in the pectin isolated from AQE (57.61%) and AAE (60.54%). Similar values of 59.52 to 70.50% (Kumar & Chauhan, 2010) and 45.25 to 52.45% (Ismail et al., 2012), respectively, were reported in apple pomace pectin and dragon fruit pectin. The pectin extracted from tomato peel also reported the AUA content between 68 to 90% (Grassino et al., 2020). The AUA content increases with the decrease in pH (Perez et al., 2022). The pectic polysaccharide was extracted from cranberry pomace using microwave-assisted extraction, and the result yielded uronic acid (60.06 to 64.02%) (Davis et al., 2021). The result also showed that the extracted pectin is appropriately pure because of the absence of starch, sugar, and protein in precipitated pectin.
Degree of Esterification (%DE)
The DE determines whether extracted pectin contains a high methyl ester (HM pectin- DE > 50%) or a low methyl ester (LM pectin- DE < 50%). This is crucial in selecting the gel type. Based on the ANOVA result, degree of esterification of pectin isolated using UAE (80.09%) was significantly higher (p < 0.05) than other extraction methods. Higher DE in pectin isolated from UAE may be because of the higher intensity of ultrasound which leads to an increase in cavitation and thus increased the yield of pectin that has a higher amount of methyl ester groups (Grassino et al., 2016). The least degree of esterification was observed in the pectin extracted from AQE (55.74%) which may be due to the depolymerization of pectin chains, thus decreasing %DE (Wang et al., 2016). The higher %DE in AAE (66.03%) may be due to hydrolysis of carboxylic acid ester in the acidic solution (). The %DE of all the pectin samples was above 50% which means that extracted pectin was all high methoxyl pectin. The %DE of soluble pectin significantly affects by heating as higher temperatures > 100 °C give a higher degree of esterification (Eblaghi et al., 2021). As shown in Table 1, the DE% of MAE was lower than UAE, and this similar trend was also found by Bagherian et al. (2011). The result is similar to that produced from yellow passion fruit rind pectin (Yapo, 2009). The DE% of pectin extracted from passion fruit peel using MAE was in the range of 50–64% (Seixas et al., 2014). The DE% of kinnow peel extracted using MAE was thus much higher in comparison to the above-mentioned study. Furthermore, compared to conventional extraction pectin, microwave extraction of pectin from lime albedo, pulp, and flavedo produced greater DE values (Fishman et al., 2006).
Galacturonic Acid Content (%GA)
The purity of pectin is determined by its galacturonic acid content. The GA is regarded as the backbone of the pectin molecule (Wang et al., 2015). The Joint Food and Agricultural Organisation (FAO)/World Health Organisation (WHO) Expert Committee on Food Additives (JECFA) recommended that the pectin will only be considered pure when its galacturonic acid content will not be less than 65% (JECFA, 2007). Table 1 indicates the galacturonic acid amount of extracted pectin. Based on the ANOVA result, galacturonic acid value of UAE (68.21%) pectin was significantly higher (p < 0.05) than other extraction methods. The least GA content was observed in the pectin extracted from AQE (64.55%) and AAE (64.885) which indicates that the pectin had a lower affinity to gel (Kumar & Chauhan, 2010). Similar values were observed from the date and lemon pectin (41.5 to 74.5%) which were extracted using acidified water (Masmoudi et al., 2012). The GA content of pectin extracted from lime peel using microwave-assisted extraction was higher than the conventional extraction (Rodsamran & Sothornvit, 2019a, b). Similar results have been shown by the pectin extracted from sour orange peel using microwave extraction (Hosseini et al., 2016a, b). The GA content can also be estimated from the peak areas obtained from FTIR spectra (Fig. 1) at 1840 cm−1 and 1550 cm−1 showing the amount of carbonyl compound (C = O) found in the pectin molecule (Oliveira et al., 2016a, b, c). From the spectra, it can be determined that the pectin extracted using the UAE method had a higher amount of galacturonic acid content. This suggests that kinnow peels may be used to produce pure pectin.
Solubility and Solvent Holding Capacity (SHC)
Pectins are generally soluble in water and insoluble in organic solvents. Pectin can effectively lower the syneresis activity in food products due to its high water absorption capacity (Khedmat et al., 2020). Observing the solubility of extracted pectin, it was discovered that it was soluble in water and 0.1N NaOH but insoluble in methanol, dimethyl sulfoxide, and acetone (Table 1).
SHC was determined for every pectin extracted by AQE, AAE, MAE, and UAE techniques, and the results indicated that pectin had the highest SHC in dimethyl sulfoxide (DMSO), ranging from 231.1 to 290.2%, while the lowest SHC was recorded in acetone, ranging from 63.9 to 80.6%. The SHC of extracted pectin showed the sequence: acetone < dimethyl sulfoxide < methanol < NaOH < water (Table 1). Various characteristics, such as the number of O–H groups in the pectin network or the porosity of pectin granules, might influence the solvent holding capacity of pectin, as per Bayar et al. (2017).
Emulsifying Properties
Pectin is used as stabilizer or emulsifier due to its emulsifying activity in foods having large oil content such as meat products. Table 3 showed the EA and ES of pectin extracted from different extraction methods. After preparing the emulsion, three stages were observed: the pectin solution phase at the bottom, the emulsion phase in the center, and the oil phase on top. For emulsifying activity, it was observed that emulsion prepared from pectin extracted from AQE had the lowest EA, i.e., 65.8%, whereas MAE had the highest EA, i.e., 86.5%.
The particle arrangement does not vary much throughout the evaluation period, which is a sign of good ES. As shown in Table 3, the extracted pectin from MAE and UAE showed high emulsion stability (ES) at 1 day at 4 °C and 23 °C in comparison with the pectin from AQE and AAE, whereas after 30 days, the ES of the pectins was reduced, respectively. The reason for this could be that different isolation techniques result in the generation of pectins, which in turn result in various aqueous solution densities, altering emulsifying properties. The UAE method increases the galacturonic acid content which is an important factor in the emulsification (Bayar et al., 2017). The capacity for absorption in space between emulsion phases is due to the strong hydrophobicity of protein molecules attached to the arabinogalactan portion of pectin and prevents their flocculation; thus, enabling the pectin to form an emulsified system (Ngouémazong et al., 2015). The results also indicated that the emulsions were more stable at 4 °C than at 23 °C; therefore, it can be concluded that these emulsions are more stable at low temperature, whereas the emulsifying activity and emulsion stability decrease with time.
Fourier Transform Infrared Spectroscopy (FTIR) Analysis
In Fig. 3, FTIR graphs of pectin extracted from AQE, AAE, MAE, and UAE are presented. The purpose of the FTIR study was to confirm the properties of the isolated pectin and to demonstrate the impact of the separation procedure on the structural features of pectin. It was found that all the isolated pectin spectra had a similar transmittance pattern. The spectrum wavelength range of 950 to 1200 cm−1 is commonly known as the carbohydrate fingerprint zone because it detects the existence of key functional groups within polysaccharides (Fissore et al., 2012). From 1011 to 1220 cm−1, the absorption bands correspond to C–O–C glycoside ring bond expansion, O–H curving, and C-O bond expansion in COOH. This area is identical for all pectin and is unaffected by the method of extraction. UAE spectra indicated a stronger magnitude graph, which could be attributable to the inflated amount. Lower frequency vibrations that are deformed quivering of the pectin band skeleton are associated with the band from 625 to 640 cm−1 (Grassino et al., 2016). The protein amide in pectin molecules is shown by the region at 1540–1560 cm−1. The aromatic ring stretching is indicated by the areas between 1590 and 1600 cm−1. Moisture causes the hydroxyl (O–H) groups to stretch in the pectin causing the wider band from 2400 to 3600 cm−1. Similar findings reported by Sun et al. (2004) showed that ultrasonic exposure could dissolve the ether bonds that bound lignin and hemicelluloses, suggesting that ultrasound exposure could improve the quality of pectin that was extracted.
XRD Analysis
To understand more about the pectin structure, XRD was used. Figure 4 depicts the XRD graph of pectin obtained using various separation techniques. Due to the same source, samples from all extraction procedures produced similar results; however, the peak shifted to a higher 2θ. Crystalline polymer exhibits a set of distinct peaks in XRD diffractograms, whereas amorphous materials exhibit a wide backdrop sequence. There were very less notable attributed peaks shown in the graph, and by this, it could be inferred that the configuration of pectin developed as a result of all extraction methods is amorphous. It was observed that the distinctive peaks of pectins at 2θ were equal to 15.5°, 22.1°, 28.9°, and 81.3°, implying crystallinity in pectins (Sharma et al., 2015). As a result of the extraction treatments, several sharp peaks disappeared. A structured crystalline is strong and hard, whereas amorphous compounds are elastic and stretchable. Because there are fewer intermolecular interactions implicate, the amorphous compound can dissolve more quickly than the crystalline one. This outcome was in accordance with the XRD result of pectin derived from Musa sapientum L. (Suvakanta et al., 2014).
TGA Analysis
In Fig. 5, thermogravimetric analysis of pectin, i.e., the decrease in mass of pectin against temperature, was observed. All the thermogravimetric curves of AQE, AAE, MAE, and UAE extracted pectin were found to be identical, displaying three distinct areas (50–120 °C, 120–300 °C, and 300–600 °C). Evaporation and dehydration of adsorbed and surface water caused a slow weight deprivation in the first region, i.e., 50–120 °C (Combo et al., 2013). Wang et al. (2016) suggested that the degradation of the polysaccharide resulted in a rapid weight reduction in the second region (120–300 °C). The decomposition of the char resulted in a slow weight reduction in the third region, i.e., 300–600 °C (Guzel & Akpinar, 2019).
Scanning Electron Microscopic Imaging
The high scanning figures given by SEM (Fig. 6) showed dissimilarity among the effects of different isolation methods on the structure of pectin. Many morphological alterations in the pectin surface after separation were seen. All SEM images showed modification in the morphology of pectin’s surface. The pectin obtained from AQE had a smooth surface with some long fractures on their surface (Fig. 6a). The pectin acquired from the UAE method had uneven and rough surface which are flaky and compact in shape, and this may be due to the large content of neutral sugar sides in the pectin structure (Fig. 6d). Mahmoud et al. (2022) extracted pectin from apple waste using ultrasound and observed that the pectin surface was flaky, compact, and hard. MAE-treated pectin had a loosened structure and a flattened and rough surface, whereas ultrasound showed a smooth but wrinkled surface (Fig. 6c). Pectin extracted from AAE had a dense and compact structure (Fig. 6b). The different microstructures showed that ultrasound may have disrupted crosslinks between pectin molecules, causing the pectin matrix to rearrange. An earlier investigation of mango and apple pomace pectin also discovered uneven and abrasive surfaces (Jiang et al., 2012).
Cost Economics
It can be observed from Table 4 that in terms of yield, acid extraction was least energy efficient followed by ultrasound and aqueous extraction, whereas microwave extraction was found to be most energy efficient. The data showed that the higher yield was obtained from UAE, but the energy requirement is also significantly higher. So, considering yield and energy consumption, microwave extraction (MAE) was found to be the most energy efficient among all the extraction methods used and thus valuable to investigate industrial upscaling for pectin extraction from kinnow peel. These estimations may vary based on the equipment’s efficiency, but the expenditure pattern is anticipated to remain valid.
Conclusion
This study accentuates the isolation of pectin using kinnow waste through conventional and novel green technologies and its characterization. The best result for pectin yield ranging from 26.8 to 30.5% was obtained through ultrasound extraction (UAE) and microwave extraction (MAE) in comparison with the conventional techniques which provided the reduced yield (18.5 to 20.4%). Microwave extraction at 900W for 180 s gives higher pectin yield; thus, it can be concluded that MAE decreases the extraction time making it an energy-saving technique. Based on the result of galacturonic acid and methoxyl content, the pectin was classified as high-methoxyl pectin. The SEM images showed that the method of extraction affects the pectin’s morphological structure. During the extraction process, a substantial volume of liquid (a mixture of an acidic solvent and ethanol) is considered waste; thus, research is needed so that ethanol can be recovered from this massive amount of liquid mix that ends up in the effluents.
Data Availability
Not applicable.
Abbreviations
- AQE:
-
Aqueous extraction
- AAE:
-
Acid-assisted extraction
- MAE:
-
Microwave-assisted extraction
- UAE:
-
Ultrasound-assisted extraction
- Eq.W:
-
Equivalent weight
- MeO:
-
Methoxyl content
- AUA:
-
Total anhydrouronic acid content
- DE:
-
Degree of esterification
- GalA:
-
Galacturonic acid
- SHC:
-
Solvent holding capacity
- EA:
-
Emulsifying activity
- ES:
-
Emulsion stability
- FTIR:
-
Fourier transform infrared spectroscopy
- XRD:
-
X-ray diffraction
- TGA:
-
Thermogravimetric analysis
- SEM:
-
Scanning electron microscopy
References
Adetunji, L. R., Adekunle, A., Orsat, V., & Raghavan, V. (2017). Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocolloids, 62, 239–250.
Aina, V. O., Barau, M. M., Mamman, O. A., Zakari, A., Haruna, H., Umar, M. H., & Abba, Y. B. (2012). Extraction and characterization of pectin from peels of lemon (Citrus limon), grape fruit (Citrus paradisi) and sweet orange (Citrus sinensis). British Journal of Pharmacology and Toxicology, 3(6), 259–262.
Amran, M. A., Palaniveloo, K., Fauzi, R., Satar, N. M., Mohidin, T. B. M., Mohan, G., ... & Sathiya Seelan, J. S. (2021). Value-added metabolites from agricultural waste and application of green extraction techniques. Sustainability, 13(20), 11432.
AOAC Int. (2000). Official methods of analysis.
Bagherian, H., Ashtiani, F. Z., Fouladitajar, A., & Mohtashamy, M. (2011). Comparisons between conventional, microwave-and ultrasound-assisted methods for extraction of pectin from grapefruit. Chemical Engineering and Processing: Process Intensification, 50(11–12), 1237–1243.
Barcelos, M. C., Ramos, C. L., Kuddus, M., Rodriguez-Couto, S., Srivastava, N., Ramteke, P. W., ... & Molina, G. (2020). Enzymatic potential for the valorization of agro-industrial by-products. Biotechnology Letters, 42, 1799–1827.
Bayar, N., Bouallegue, T., Achour, M., Kriaa, M., Bougatef, A., & Kammoun, R. (2017). Ultrasonic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal: Optimization of experimental conditions and evaluation of chemical and functional properties. Food Chemistry, 235, 275–282.
BeMiller, J. N., & Whistler, R. L. (Eds.). (2012). Industrial gums: Polysaccharides and their derivatives. Academic Press.
Bhaskaracharya, R. K., Kentish, S., & Ashokkumar, M. (2009). Selected applications of ultrasonics in food processing. Food Engineering Reviews, 1(1), 31–49.
Castillo-Israel, K. A. T., Baguio, S. F., Diasanta, M. D. B., Lizardo, R. C. M., Dizon, E. I., & Mejico, M. I. F. (2015). Extraction and characterization of pectin from Saba banana [Musa'saba'(Musa acuminata x Musa balbisiana)] peel wastes: A preliminary study. International Food Research Journal, 22(1).
Chan, S. Y., Choo, W. S., Young, D. J., & Loh, X. J. (2017). Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydrate Polymers, 161, 118–139.
Combo, A. M. M., Aguedo, M., Quiévy, N., Danthine, S., Goffin, D., Jacquet, N., ... & Paquot, M. (2013). Characterization of sugar beet pectic-derived oligosaccharides obtained by enzymatic hydrolysis. International journal of biological macromolecules, 52, 148–156.
Constenla, D., & Lozano, D. J. (2003). Kinetic model of pectin demethylation. Latin American Applied Research, 33(2), 91–95.
Cui, J., Ren, W., Zhao, C., Gao, W., Tian, G., Bao, Y., ... & Zheng, J. (2020). The structure–property relationships of acid-and alkali-extracted grapefruit peel pectins. Carbohydrate polymers, 229, 115524.
Dalev, P. G., & Simeonova, L. S. (1995). Emulsifying properties of protein–pectin complexes and their use in oil-containing foodstuffs. Journal of the Science of Food and Agriculture, 68(2), 203–206.
Davis, E. J., Spadoni Andreani, E., & Karboune, S. (2021). Production of extracts composed of pectic oligo/polysaccharides and polyphenolic compounds from cranberry pomace by microwave-assisted extraction process. Food and Bioprocess Technology, 14(4), 634–649.
de Oliveira, C. F., Gurak, P. D., Cladera-Olivera, F., Marczak, L. D. F., & Karwe, M. (2016a). Combined effect of high-pressure and conventional heating on pectin extraction from passion fruit peel. Food and Bioprocess Technology, 9(6), 1021–1030.
de Oliveira, C. F., Giordani, D., Lutckemier, R., Gurak, P. D., Cladera-Olivera, F., & Marczak, L. D. F. (2016b). Extraction of pectin from passion fruit peel assisted by ultrasound. LWT-Food Science and Technology, 71, 110–115.
Dische, Z. (1947). A new specific color reaction of hexuronic acids. Journal of Biological Chemistry, 167(1), 189–198.
Eblaghi, M., Bronlund, J. E., Yedro, F. M., & Archer, R. H. (2021). Kinetics of pectin reactions in apple pomace during hydrothermal treatment. Food and Bioprocess Technology, 14(4), 739–750.
Fishman, M. L., Chau, H. K., Hoagland, P. D., & Hotchkiss, A. T. (2006). Microwave-assisted extraction of lime pectin. Food Hydrocolloids, 20(8), 1170–1177.
Fissore, E. N., Rojas, A. M., & Gerschenson, L. N. (2012). Rheological performance of pectin-enriched products isolated from red beet (Beta vulgaris L. var. conditiva) through alkaline and enzymatic treatments. Food Hydrocolloids, 26(1), 249–260.
Food Chemical Codex. (1996). IV monographs (p. 283). National Academy Press.
Grassino, A. N., Brnčić, M., Vikić-Topić, D., Roca, S., Dent, M., & Brnčić, S. R. (2016). Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chemistry, 198, 93–100.
Grassino, A. N., Ostojić, J., Miletić, V., Djaković, S., Bosiljkov, T., Zorić, Z., ... & Brnčić, M. (2020). Application of high hydrostatic pressure and ultrasound-assisted extractions as a novel approach for pectin and polyphenols recovery from tomato peel waste. Innovative Food Science & Emerging Technologies, 64, 102424.
Güzel, M., & Akpınar, Ö. (2019). Valorisation of fruit by-products: Production characterization of pectins from fruit peels. Food and Bioproducts Processing, 115, 126–133.
Hosseini, S. S., Khodaiyan, F., & Yarmand, M. S. (2016a). Aqueous extraction of pectin from sour orange peel and its preliminary physicochemical properties. International Journal of Biological Macromolecules, 82, 920–926.
Hosseini, S. S., Khodaiyan, F., & Yarmand, M. S. (2016b). Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydrate Polymers, 140, 59–65.
Hosseini, S. S., Khodaiyan, F., Kazemi, M., & Najari, Z. (2019). Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. International Journal of Biological Macromolecules, 125, 621–629.
Ismail, N. S. M., Ramli, N., Hani, N. M., & Meon, Z. (2012). Extraction and characterization of pectin from dragon fruit (Hylocereus polyrhizus) using various extraction conditions. Sains Malaysiana, 41(1), 41–45.
Jain, A., Thakur, D., Ghoshal, G., Katare, O. P., & Shivhare, U. S. (2015). Microencapsulation by complex coacervation using whey protein isolates and gum acacia: An approach to preserve the functionality and controlled release of β-carotene. Food and Bioprocess Technology, 8(8), 1635–1644.
JECFA, J. F. (2007). Evaluation of certain food additives and contaminants: Sixty-eight report of the joint FAO/WHO expert committee on food additives. WHO Technical Report Series, 947.
Jiang, Y., Du, Y., Zhu, X., Xiong, H., Woo, M. W., & Hu, J. (2012). Physicochemical and comparative properties of pectins extracted from Akebia trifoliata var. australis peel. Carbohydrate polymers, 87(2), 1663–1669.
Kamal, G. M., Anwar, F., Hussain, A. I., Sarri, N., & Ashraf, M. Y. (2011). Yield and chemical composition of Citrus essential oils as affected by drying pretreatment of peels. International Food Research Journal, 18(4).
Khedmat, L., Izadi, A., Mofid, V., & Mojtahedi, S. Y. (2020). Recent advances in extracting pectin by single and combined ultrasound techniques: A review of techno-functional and bioactive health-promoting aspects. Carbohydrate Polymers, 229, 115474.
Kinnaert, C., Daugaard, M., Nami, F., & Clausen, M. H. (2017). Chemical synthesis of oligosaccharides related to the cell walls of plants and algae. Chemical Reviews, 117(17), 11337–11405.
Kliemann, E., De Simas, K. N., Amante, E. R., Prudêncio, E. S., Teófilo, R. F., Ferreira, M. M., & Amboni, R. D. (2009). Optimisation of pectin acid extraction from passion fruit peel (Passiflora edulis flavicarpa) using response surface methodology. International Journal of Food Science & Technology, 44(3), 476–483.
Kratchanova, M., Pavlova, E., & Panchev, I. (2004). The effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohydrate Polymers, 56(2), 181–185.
Kulkarni, S. G., & Vijayanand, P. (2010). Effect of extraction conditions on the quality characteristics of pectin from passion fruit peel (Passiflora edulis f. flavicarpa L.). LWT-Food Science and Technology, 43(7), 1026–1031.
Kumar, A., & Chauhan, G. S. (2010). Extraction and characterization of pectin from apple pomace and its evaluation as lipase (steapsin) inhibitor. Carbohydrate Polymers, 82(2), 454–459.
Kumar, K., Yadav, A. N., Kumar, V., Vyas, P., & Dhaliwal, H. S. (2017). Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresources and Bioprocessing, 4(1), 1–14.
Lal, A. N., Prince, M. V., Kothakota, A., Pandiselvam, R., Thirumdas, R., Mahanti, N. K., & Sreeja, R. (2021). Pulsed electric field combined with microwave-assisted extraction of pectin polysaccharide from jackfruit waste. Innovative Food Science & Emerging Technologies, 74, 102844.
Liew, S. Q., Teoh, W. H., Yusoff, R., & Ngoh, G. C. (2019). Comparisons of process intensifying methods in the extraction of pectin from pomelo peel. Chemical Engineering and Processing-Process Intensification, 143, 107586.
Mahmoud, M. H., Abu-Salem, F. M., & Azab, D. E. S. H. (2022). A comparative study of pectin green extraction methods from apple waste: Characterization and functional properties. International Journal of Food Science, 2022.
Manzocco, L., Calligaris, S., Mastrocola, D., Nicoli, M. C., & Lerici, C. R. (2000). Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends in Food Science & Technology, 11(9–10), 340–346.
Masmoudi, M., Besbes, S., Abbes, F., Robert, C., Paquot, M., Blecker, C., & Attia, H. (2012). Pectin extraction from lemon by-product with acidified date juice: Effect of extraction conditions on chemical composition of pectins. Food and Bioprocess Technology, 5(2), 687–695.
Mehmood, T., Nadeem, F., Qamar, S. A., Bilal, M., & Iqbal, H. M. (2021). Bioconversion of agro-industrial waste into value-added compounds. Sustainable bioconversion of waste to value added products, 349–368.
Misra, N. N., & Yadav, S. K. (2020). Extraction of pectin from black carrot pomace using intermittent microwave, ultrasound and conventional heating: Kinetics, characterization and process economics. Food Hydrocolloids, 102, 105592.
Nazaruddin, R. (2011). Effect of ammonium oxalate and acetic acid at several extraction time and pH on some physicochemical properties of pectin from cocoa husks (Theobroma cacao). African Journal of Food Science, 5(15), 790–798.
Ngouémazong, E. D., Christiaens, S., Shpigelman, A., Van Loey, A., & Hendrickx, M. (2015). The emulsifying and emulsion-stabilizing properties of pectin: A review. Comprehensive Reviews in Food Science and Food Safety, 14(6), 705–718.
Oberoi, H. S., Vadlani, P. V., Nanjundaswamy, A., Bansal, S., Singh, S., Kaur, S., & Babbar, N. (2011). Enhanced ethanol production from kinnow mandarin (Citrus reticulata) waste via a statistically optimized simultaneous saccharification and fermentation process. Bioresource Technology, 102(2), 1593–1601.
Oliveira, T. Í. S., Rosa, M. F., Cavalcante, F. L., Pereira, P. H. F., Moates, G. K., Wellner, N., ... & Azeredo, H. M. (2016c). Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chemistry, 198, 113–118.
Pérez, J., Gómez, K., & Vega, L. (2022). Optimization and preliminary physicochemical characterization of pectin extraction from watermelon rind (Citrullus lanatus) with citric acid. International journal of food science, 2022.
Picot-Allain, M. C. N., Ramasawmy, B., & Emmambux, M. N. (2022). Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: A review. Food Reviews International, 38(3), 282–312.
Ranganna, S. (1986). Handbook of analysis and quality control for fruit and vegetable products. Tata McGraw-Hill Education.
Rezzadori, K., Benedetti, S., & Amante, E. R. (2012). Proposals for the residues recovery: Orange waste as raw material for new products. Food and Bioproducts Processing, 90(4), 606–614.
Rodsamran, P., & Sothornvit, R. (2019a). Lime peel pectin integrated with coconut water and lime peel extract as a new bioactive film sachet to retard soybean oil oxidation. Food Hydrocolloids, 97, 105173.
Rodsamran, P., & Sothornvit, R. (2019b). Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chemistry, 278, 364–372.
Rouse, A. H., Atkins, C. D., & Moore, E. L. (1964). Evaluation of pectin in component parts of pineapple oranges during maturation. In Proceedings of the Florida State Horticultural Society (Vol. 77, pp. 271–273).
Sagar, N. A., Pareek, S., Sharma, S., Yahia, E. M., & Lobo, M. G. (2018). Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Comprehensive Reviews in Food Science and Food Safety, 17(3), 512–531.
Seixas, F. L., Fukuda, D. L., Turbiani, F. R., Garcia, P. S., Carmen, L. D. O., Jagadevan, S., & Gimenes, M. L. (2014). Extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) by microwave-induced heating. Food Hydrocolloids, 38, 186–192.
Sharma, H., Bhatia, S., & Alam, M. S. (2013). Studies on pectin extraction from kinnow peel and pomace. Journal of Research, Punjab Agricultural University, 50(3), 128–130.
Sharma, R., Kamboj, S., Khurana, R., Singh, G., & Rana, V. (2015). Physicochemical and functional performance of pectin extracted by QbD approach from Tamarindus indica L. pulp. Carbohydrate Polymers, 134, 364–374.
Shinwari, K. J., & Rao, P. S. (2018). Stability of bioactive compounds in fruit jam and jelly during processing and storage: A review. Trends in Food Science & Technology, 75, 181–193.
Singla, G., Krishania, M., Sandhu, P. P., Sangwan, R. S., & Panesar, P. S. (2019). Value additon of kinnow industry byproducts for the preparation of fiber enriched extruded products. Journal of Food Science and Technology, 56(3), 1575–1582.
Sun, J. X., Sun, R., Sun, X. F., & Su, Y. (2004). Fractional and physico-chemical characterization of hemicelluloses from ultrasonic irradiated sugarcane bagasse. Carbohydrate Research, 339(2), 291–300.
Suri, S., Singh, A., & Nema, P. K. (2022). Current applications of citrus fruit processing waste: A scientific outlook. Applied Food Research, 100050.
Suvakanta, D., Narsimha, M. P., Pulak, D., Joshabir, C., & Biswajit, D. (2014). Optimization and characterization of purified polysaccharide from Musa sapientum L. as a pharmaceutical excipient. Food Chemistry, 149, 76–83.
Voragen, A. G., Coenen, G. J., Verhoef, R. P., & Schols, H. A. (2009). Pectin, a versatile polysaccharide present in plant cell walls. Structural Chemistry, 20(2), 263–275.
Wandee, Y., Uttapap, D., & Mischnick, P. (2019). Yield and structural composition of pomelo peel pectins extracted under acidic and alkaline conditions. Food Hydrocolloids, 87, 237–244.
Wang, M., Huang, B., Fan, C., Zhao, K., Hu, H., Xu, X., ... & Liu, F. (2016). Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. International Journal of Biological Macromolecules, 91, 794–803.
Wang, S., Chen, F., Wu, J., Wang, Z., Liao, X., & Hu, X. (2007). Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. Journal of Food Engineering, 78(2), 693–700.
Wang, W., Ma, X., Xu, Y., Cao, Y., Jiang, Z., Ding, T., ... & Liu, D. (2015). Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food chemistry, 178, 106–114.
Yadav, S. R., Khan, Z. H., Kunjwani, S. S., & Mular, S. M. (2015). Extraction and characterization of pectin from different fruits. International Journal of Applied Research, 1(9), 91–94.
Yang, J. S., Mu, T. H., & Ma, M. M. (2019). Optimization of ultrasound-microwave assisted acid extraction of pectin from potato pulp by response surface methodology and its characterization. Food Chemistry, 289, 351–359.
Yapo, B. M. (2009). Pectin quantity, composition and physicochemical behaviour as influenced by the purification process. Food Research International, 42(8), 1197–1202.
Acknowledgements
The authors gratefully acknowledge the Institution of Eminence (IoE) scheme, Banaras Hindu University, Varanasi (U.P.) India, for financial support under the Incentive to Seed Grant under IoE Scheme (Dev. Scheme No 6031 & PFMS Scheme No 3254).
Author information
Authors and Affiliations
Contributions
Muskan Kumari (M.K.) and Anil Kumar Chauhan (A.K.C.) performed the experiment. Shubhendra Singh (S.S.) improved the theory and carried out the computations. Muskan Kumari (M.K.) and Anil Kumar Chauhan (A.K.C.) pioneered in writing the manuscript. All authors contributed critical feedback and prepared shape of the research, analysis, and manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, M., Singh, S. & Chauhan, A.K. A Comparative Study of the Extraction of Pectin from Kinnow (Citrus reticulata) Peel Using Different Techniques. Food Bioprocess Technol 16, 2272–2286 (2023). https://doi.org/10.1007/s11947-023-03059-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03059-4