Abstract
The approach of converting waste to wealth has become a crucial topic and intense research has been ongoing globally for their proper management and utilization. With an aim to follow up this concept, this study is aimed to valorize Citrus limetta peels by extracting pectin using green organic acid. A Box-Behnken experimental design was used to optimize the extraction process and study the effect of process parameters, i.e., temperature (70–100 °C), extraction time (30–120 min), pH (1–2.5), and liquid:solid ratio (10–40 v/w) on the yield of pectin. The maximum pectin yield of 22.03 ± 0.13% was obtained at optimum extraction conditions of 90 °C temperature, 95-min time, 1.8 pH, and 30 v/w LSR which was observed to be in close agreement to the predicted value of 22.7%. Furthermore, the pectin extracted under optimal conditions was low methoxy pectin with a degree of esterification of 45.62 ± 0.64% and methoxyl content of 5.45 ± 0.04%. The structural properties of pectin were characterized using analytical techniques including X-ray diffraction and Fourier transform infrared spectroscopy. The morphological analysis of peel powder before and after pectin extraction examined using scanning electron microscope indicated that the peel structure after extraction treatment was damaged and disrupted. Besides, the emulsifying activity, emulsifying stability, water holding, and oil holding capacities of extracted pectin were evaluated to determine its potential to be considered as a natural food additive. The results suggested that C. limetta peels have a great potential to be used as a novel low-cost source for the pectin extraction having good properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Citrus limetta, also known as sweet lime, is one of the well-known indigenous citrus fruits which is mainly cultivated in Southern and Southern East Asia. The world’s leading producers of C. limetta include India, China, Pakistan, southern Japan, Egypt, Vietnam, Malaysia, Thailand, Indonesia, tropical America, and Mediterranean countries [1]. In India, annual production of approximately 3.26 million tons of C. limetta was observed in 2018 (http://nhb.gov.in/statistics/Publication/Horticulture%20Statistics%20at%20a%20Glance-2018.pdf). Furthermore, considering numerous promising health benefits due to the presence of a wide array of nutrients and bioactive compounds along with their exceptional sensorial properties, consumption, and processing of this fruit have increased exponentially. Processing of C. limetta generates an enormous quantity of peels accounting for 8–10% of fresh fruit which is arbitrarily discarded into the landfills without any prior treatment, causing environmental pollution [2]. However, ironically C. limetta peels (CLP) are being recognized as an alluring repository of high-value compounds, especially pectin, which makes it preferable to be used in functional foods and nutraceuticals. Therefore, the extraction of pectin from this by-product can not only help in reducing the volume of citrus waste followed by its subsequent ecological effects but can also increase the financial profits obtained from conversion plants.
Pectin is a mixture of complex heteropolysaccharides distributed extensively in the cell wall and middle lamella of plants. Chemically, pectin is composed of α-(1-4)-D-galacturonic acid units which are partially esterified with acetic acid or methanol at some carboxylic acid groups [3]. It is a popular natural food additive (INS 440) used in food industries as a fat replacer as well as thickening, emulsifying, stabilizing, and gelling agents [4]. Furthermore, it is also used in pharmaceutical industries for drug encapsulation and tablet formation [5]. In addition, previous studies have reported numerous pharmacological benefits of pectin, such as lowering of cholesterol levels [6], antioxidant, immunity modulation [7], anti-cancer [8], and reduction in the postprandial concentration of glucose and insulin [9]. Therefore, considering the increasing demand for natural pectin owing to the wide array of health and technological applications, the global market size of pectin is expected to reach USD 1.5 billion by 2025, at a CAGR of 6.5% from the year 2019 (https://www.marketsandmarkets.com/Market-Reports/pectin-market-139129149.html#:~:text=%5B181%20Pages%20Report%5D%20The%20pectin,USD%201.5%20billion%20by%202025).
Commercially, pectin is commonly extracted from citrus peel and apple pomace. Despite the fact that pectin obtained from by-products is chemically equivalent, citrus fruits are generally preferred for pectin extraction as it contains almost twice pectin content than apple, i.e., 20–30% and 10–15%, respectively. Moreover, due to the presence of more phenolic compounds, apple pectin is darker in color than citrus pectin and is therefore not preferred for application in clear food products [10]. Previous studies have reported pectin extraction from most common citrus species, such as C. sinensis, C. aurantifolia, C. reticulata, C. paradisi, and C. limon using different techniques [11]. However, to meet the increasing demand of consumers for pectin, researchers are currently exploring other citrus sources for pectin extraction.
The traditional method of pectin extraction involves the use of strong mineral acids, i.e., hydrochloric, sulfuric, or nitric acid [12]. However, due to increasing concerns of mineral acids due to their corrosive nature and production of toxic compounds, green organic acids, such as citric acid have recently gained attention for pectin extraction as they are environment friendly [13, 14]. Besides, because of their low dissociation constant, organic acids have a low hydrolyzing capacity which will reduce the risk of pectin depolymerization which could lead to pectin with high yield, molecular weight, and viscosity as compared to mineral acids [15].
During the acid extraction method, several factors such as temperature, pH, time, and liquid:solid ratio (LSR) affects the extraction yield and quality of pectin [16]. So, in order to achieve the maximum extraction efficiency, optimization of these conditions is needed. For this purpose, response surface methodology (RSM) is applied extensively to determine the relationship among independent and response factors using a group of statistical and mathematical procedures [17].
Studies on the optimization of pectin extraction using organic acids from CLP are meager. Therefore, this study is aimed to optimize the different process variables for efficient extraction of pectin from CLP using citric acid using Box-Behnken RSM and further study the physicochemical and structural properties of extracted citrus pectin.
2 Materials and methods
2.1 Plant material and chemicals
C. limetta peels (CLP) were procured from the local juice vendors in Longowal, Punjab, India. The peels were first washed and dried in a tray drier (SICO House, Patiala, India) at 45 °C until a constant weight was achieved. The dried peels were then grounded using an electric grinder (Philips, India), and it was passed through a mesh sieve (BSS 44) to obtain fine powder. Finally, the samples were packed into air-tight containers and were stored in a dry environment (24–24 °C) for further use.
Citric acid, hydrochloric acid (37%), sodium hydroxide, phenolphthalein reagent, and ethanol (99%) were purchased from Merck (Germany) and Sigma chemicals (USA). Ultrapure water for analysis was collected from the Milli-Q purification system (Millipore, France). All the other chemicals used for further analysis were of analytical grade.
2.2 Design of experiment for optimization
RSM was utilized to evaluate the optimal conditions for maximum extraction of pectin from CLP powder. A Box-Behnken design (BBD) with three levels was used to evaluate the effect of four extraction variables, i.e., temperature (°C, X1), time (min, X2), pH (X3), and LSR (v/w, X4) on the extracted pectin yield, selected as a response. The range of four independent variables, i.e., temperature, time, pH, and LSR was selected as 70–100 °C, 30–120 min, 1–2.5, and 10–40 v/w, respectively. The complete RSM design consisted of 27 experiments including 3 replicates at the center point to determine any possible pure error. All the experiments were performed randomly in triplicates, and the data obtained was fitted to the second-order polynomial equation (expressed as Eq. 1) to demonstrate the relationship among independent variables and response.
where Y is the dependent variable (pectin yield), β0 is model constant, β1, β2, β3, and β4 are the linear coefficients, β11, β22, β33, and β44 are quadratic coefficients, and β12, β13, β14, β23, β24, and β34 are interaction coefficients of the independent variables (X1, X2, X3, and X4). The outcomes obtained were analyzed statistically using analysis of variance (ANOVA), and any difference was considered to be significant at a level of p < 0.05 [18]. Furthermore, the competency of the model was determined valuating the coefficient of determination (R2), adjusted coefficient of determination (adj. R2), lack of fit test, and F test value generated from ANOVA. In addition, the model was verified using the observed vs. predicted values, and regression coefficients were used to obtain the response surface. It must be noted that all the calculations and graphical analysis were done using the Design-expert software (Stat-Ease, Version 11, USA).
2.3 Preparation of alcohol-insoluble reside from CLP powder
The process for pectin extraction is supervened by preparing alcohol insoluble residues, with the purpose to remove the compounds with low molecular weight, such as traces of galacturonic acid, partly polar compounds, and proteins. The alcohol insoluble residue was prepared by following the method of Oliveira et al. [19]. CLP (100 g) powder was washed twice, firstly using boiling 70% v/v ethanol solution (600 mL) for 10 min and then by absolute ethanol (600 mL) for 10 min at room temperature. Finally, the residue was washed using acetone (200 mL). Amidst all the washings, the residue was filtered via 10-μm nylon mesh and was allowed to dry at room temperature for 24 h.
2.4 Citric acid-assisted pectin extraction
Pectin was extracted from alcohol insoluble residue using citric acid solution according to different extraction conditions using a water bath under stirring (150 rpm). The mixture was allowed to cool at room temperature, and it was then centrifuged (3000 rpm) for 20 min at 10 °C. Thereafter, the supernatant was filtered through cheesecloth, followed by precipitation by the addition of ethanol in a ratio of 1:2 v/v, and was left overnight at 4 °C. The precipitated pectin was then collected, washed twice with ethanol, and oven-dried at 50 °C until a constant weight was achieved. After complete drying of pectin, it was made into powder using a pestle mortar and was stored in a container for further analysis. The complete process of pectin extraction from CLP has been depicted in Fig. 1 [20].
The pectin yield was determined using the formula (Eq. 2) below:
2.5 Physicochemical characterization
2.5.1 Degree of esterification
Degree of esterification (DE) of extracted pectin was demonstrated using the titration method, as reported by Wai et al. [21] with minor modifications, and the result was calculated using the following formula (Eq. 3):
2.5.2 Determination of equivalent weight, methoxyl content, and total anhydrouronic acid content
The equivalent weight (Eq. W.), methoxyl content (MeO), and total anhydrouronic acid content (AUA) were determined using the method as reported by Khan et al. [22]. The Eq. W. and MeO were then calculated using the following formulas (Eqs. 4 and 5, respectively):
Finally, total AUA was calculated using the following formula (Eq. 6):
where: x = titer volume (ml) of NaOH obtained from equivalent weight determination, and y= titer volume (ml) of NaOH obtained from MeO content determination.
2.5.3 Color
For the color measurement of pectin, a hunter calorimeter (Model D25 optical sensor, Hunter Associates laboratory Inc., USA) was used on the basis of L*, a*, and b* values. The pectin powder was placed in a glass cell kept above the source of light covered with a white plate and the color values were noted.
2.6 Technological properties
2.6.1 Emulsification properties
Emulsion activity (EA) and emulsion stability (ES) of pectin powder were assessed by the method of Jafari et al. [14] with slight modifications. For the preparation of emulsions, aqueous pectin solution (2%, 5 mL) was homogenized with olive oil (5 mL). The pre-emulsions were then centrifuged (3500 rpm) for 5 min. Finally, the EA was calculated using the formula as mentioned in Eq. 7:
Furthermore, ES of pectin was determined by storing the prepared emulsions at 24 °C for 1 and 30 days. ES was then calculated using the formula (Eq. 8):
2.6.2 Water holding capacity and oil holding capacity
Water holding capacity (WHC) and oil holding capacity (OHC) were determined by the procedure of Shchekoldina et al. [23] with minor modifications. Dried pectin (1 g) was added in distilled water/olive oil (10 mL), followed by vigorous stirring for 5 min, and the mixture was allowed to stand at room temperature for 30 min. The solution was then centrifuged (4500 rpm) for 30 min, and the supernatant was discarded, and the tube was drained on a filter paper to remove extra water/oil. WHC and OHC were calculated using the formula (Eq. 9):
where
- x:
-
weight of tube with sample and water/oil (g)
- y:
-
weight of tube and sample (g)
- z:
-
weight of sample (g)
2.7 Structural properties
2.7.1 Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FT-IR) of extracted CLP pectin powder was analyzed using an FT-IR spectrometer (RX-U, FTIR, USA) using the KBr disc method in the wavelength range of 500–3500 cm−1.
2.7.2 X-ray diffraction analysis
The X-ray diffraction (XRD) pattern of CLP pectin powder was analyzed using an X-ray diffractometer (PAN-analytic-Xpert PRO MRD, Almedo, Netherlands) where the sample was scanned at a diffraction angle (2θ) within the range of 10 to 80° with a step size of 0.05°.
2.8 Scanning electron microscopy
The surface morphology of CLP powder before and after the pectin extraction process was observed by scanning electron microscope (SEM, JSM-7610 F plus, JEOL, Japan). The dried peel powder was dispersed uniformly on a conductive sample table and was coated using a thin gold layer under argon atmospheric conditions by an iron sputter coater. The images of both samples were obtained at a magnification of 3000 ×.
2.9 Statistical analysis
All the experiments were carried out in triplicates, and the results were demonstrated as the mean values with standard deviation.
3 Results and discussion
3.1 RSM model fitting and statistical analysis
The effect of four extraction conditions, i.e., temperature, time, pH, and LSR in the range of 70–100 °C, 30–120 min, 1–2.5, and 10–40 mL/g, respectively were studied using a 3-level BBD design in order to achieve a maximum pectin yield from CLP. A total of 27 different experiments were obtained having a different set of input for process parameters to evaluate their combined effect on the pectin yield. The experimental and predicted values of pectin yield are shown in Table 1. A multiple regression analysis was utilized on the achieved experimental data to develop a quadratic second-order polynomial equation that includes linear, interactive, and quadratic terms (Eq. 10), to establish the relationship between process parameters and response, i.e., pectin yield.
The reliability and adequacy of the developed experimental model were analyzed using ANOVA and R2, which were acceptable statistically at a confidence level of 95% (p < 0.05). The results of ANOVA are represented in Table 2. Statistical results showed that the p value of the experimental model was less than 0.0001 and lack-of-fit (0.4002) was insignificant, which indicated that the suggested model was significantly well-fitted. Furthermore, the high value of R2 (0.9915), adjusted R2 (0.9815), and predicted R2 (0.9537) along with the low value of the coefficient of variation (C.V. = 4.93%) confirmed the higher accuracy and reliability of the model in determining the relationship among pectin yield and process parameters [24]. In addition, the variables in each coefficient including linear (X1, X2, X3, and X4), quadratic (X12, X22, X32, and X42), and interactive (X1X2, X1X3, X1X4, X2X3, X2X4, and X3X4) were also found to be lower than 0.05, suggesting their significant effect on the extraction pectin yield.
3.1.1 Effect of process variables on CLP pectin yield
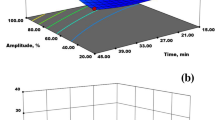
The yield of pectin extracted from CLP ranged from 3.97 to 22%. The linear and interactive effects of all the process variables on pectin yield were visualized using three-dimensional (3D) plots (Fig. 2). It was observed that the extraction temperature had a significant effect on pectin yield (Fig. 2a, b, and f). Results showed that the pectin yield increased with an increase in temperature, which might be due to increased solvent penetration into the plant matrix, thereby increasing the solubility as well as diffusivity of pectin from the solid matrix into the solution through the disruption of hydrogen bonds and ester linkages [3, 14]. Moreover, extraction at lower temperatures might not be sufficient for complete hydrolysis of the insoluble fraction of pectin (protopectin) by solvent, therefore achieving low pectin yields [13]. However, further increase in temperature beyond the optimum limit can result in thermal depolymerization of pectin which cannot be precipitated by alcohol, thereby reducing the pectin yields [25]. These findings were similar to the results obtained by extraction of pectin from peels of orange and passion fruit, as reported by previous researchers [26, 27].
Another important parameter affecting pectin extraction yield is time, and the results showed that with an increase in the extraction time, yield of pectin increased (Fig. 2a, c, and e). This increase in pectin yield at the initial stage of the extraction process is probably due to the prolonged reaction time between plant matrix and solvent, thereby providing high mass transfer from solid material into the solution [14]. This result was in agreement with the work of Chaharbaghi et al. [28] and Colodel et al. [29] who studied the influence of extraction parameters on pectin yield from pistachio green hull and grape pomace, respectively. However, after a certain duration, the pectin yield decreased with an increase in extraction time which could be due to the hydrolysis of glycosidic bonds and methyl ester of pectin due to the effect of citric acid, thereby leading to an overall reduction in yield [30].
The surface plots in Fig. 2b, c, and d depict a considerable increase in the yield of pectin with a decreasing value of pH. The acidic solvent used for the extraction process hydrolyzes the insoluble portions of pectin into the soluble form; therefore, the pectin recovery is increased at a low pH of 2. Moreover, lower pH values could reduce the molecular weight of pectin and, therefore, improve its release from plant matrix with minimum degradation [31]. Nonetheless, at high pH levels, the pectin yield was reduced considerably, which was probably due to the accumulation of pectin, thus reducing its release [32]. These results were in close accordance with the results obtained from the extraction of pectin from pomegranate peel [33], carrot pomace [14], and citron peel [17].
The effect of LSR on the pectin yield was demonstrated, and it was clearly evident from the results that it is an important parameter influencing the process yield (Fig. 2d, e, and f). As it can be seen that with an increase in LSR up to 30 mL/g, the pectin yield increased. A study on the effect of process parameters on the pectin yield extracted from ponkan peel reported that this increase in yield with increased LSR is due to the enhanced contact area between plant matrix and solvent, which further increased the driving force for the mass transfer of polysaccharides into the solution [10]. In addition to this, increased LSR might induce swelling in the cell wall of the plant matrix, leading to the disruption of cells and, therefore, promotes pectin solubilization [34]. On the other hand, an increase in LSR after an optimum value led to a reduction in the pectin yield. These results were in agreement with the work of previous researchers who extracted pectin from sugar beet pulp [35] and sour orange [31], respectively. The possible reason for this decreased pectin yield was that with an increase in LSR beyond a certain limit, the solution starts to get saturated with a solute which negatively affected the mass transfer of polysaccharides into the solution.
3.2 Validation of optimized experimental conditions
The adequacy of developed model equation in order to predict the maximum target CLP pectin yield was carried out using the selected experimental range of process parameters. The optimal conditions were computed by solving Eq. 10, and the highest yield (22.7%) was obtained at a temperature of 90 °C, pH of 1.8, LSR of 30 v/w after 95-min extraction time. Furthermore, under these optimal conditions, three verification experiments were then carried out to validate the developed experimental model, and the pectin yield was found to be 22.03 ± 0.13%, which was found to be in close proximity to the predicted values and validated the overall suitability of optimized experimental conditions.
3.3 Physicochemical characterization
Different physicochemical parameters of CLP pectin extracted under optimum conditions were determined (Table 3) and are discussed in detail below:
3.3.1 Degree of esterification
DE is a vital parameter influencing the classification of pectin having their respective applications in the food sector. It describes the limit to which the carboxyl groups are esterified in a pectin molecule [36]. On the basis of DE, pectin is being classified into two categories, namely low methoxyl pectin (DE less than 50%) and high methoxyl pectin (DE higher than 50%). The DE of pectin extracted using citric acid was found to be 45.62 ± 0.64%, indicating that the CLP pectin can be categorized as low methoxyl pectin (LMP). The reason for the low DE of extracted pectin is probably due to extraction at harsh conditions (high temperature, longer time, and low pH) which could lead to de-esterification of polygalacturonic acid chains in pectin structure [14]. This type of pectin can form gels even in the absence of sugar and therefore can be used for the development of low-calorie food products. Similar results were observed for the pectin extracted from other plant sources such as mulberry bark [37], Japanese plum [38], sour orange peel [31], and wild plum [22].
3.3.2 Equivalent weight, methoxyl content, and total anhydrouronic acid content
Eq. W. is one of the significant characteristics of pectin affecting its functional abilities, such as gelling properties. Pectin with high Eq. W. is known to have higher gel-forming capability, whereas, lower Eq. W. is probably due to the partial degradation of pectin which is unfavorable during gel formation. The Eq. W. of CLP pectin was observed to be 477.89 ± 9.43, and this low equivalent weight was probably due to extraction at high temperature, longer time, and low pH levels [39].
MeO is another key characteristic of pectin indicating the gel formation and its dispersibility in water. It has been reported that the methoxyl content of pectin obtained from different sources under different extraction conditions may vary from 0.2 to 12% [40]. In addition, pectin having methoxyl content less than 7% is considered as low methoxyl content, whereas, pectin with methoxyl content in the range of 8–12% lies in the category of high methoxyl pectin. According to the result obtained in this study, the methoxyl content of CLP pectin was 5.45 ± 0.04% which can be categorized as low methoxy pectin; therefore, it does not require sugar to form gels and can be used for the development of low-sugar food products. A similar result was reported by Fakayode et al. [26], where pectin extracted from orange peel was observed as low methoxyl with MeO of 6.23%.
AUA content is a vital parameter that determines the purity of the pectin and affects its gelling properties. According to the standards of the Food and Agriculture Organization (FAO) and Food Chemical Codex (FCC), pectin must contain an AUA of at least 65% to be used in food and pharmaceutical industries [41]. AUA of CLP pectin extracted at the optimized conditions was found to be 67.81 ± 0.64% which was higher than the recommended limit and therefore confirmed the purity of extracted pectin.
3.3.3 Color
Color is a vital characteristic influencing the final appearance of food products as well as its acceptance to be used as a food additive. The color of pectin is calculated using hunter color values (L*, a*, and b*), where L* value represents the lightness in the range of 0 (dark) to +100 (light); a* represents redness or greenness ranging from −100 (green) to +100 (red), and b* represents yellowness or blueness ranging from −100 (blue) to +100 (yellow). The color of pectin extracted from CLP using citric acid was observed to be light brown. As correlated by visual appearance, the L*, a*, and b* of CLP pectin extracted under optimum conditions were found to be 54.64 ± 0.03, 7.54 ± 0.02, and 17.45 ± 0.05, respectively. This light brown color of extracted pectin is probably due to the high temperature and long extraction times during conventional heating. A similar result was reported by Wang et al. [42], who observed the gray-brown color of pectin extracted from grapefruit using a conventional extraction process.
3.4 Technological properties
3.4.1 Emulsification properties
In order to evaluate the technological properties of extracted CLP pectin, the EA and ES of the sample were studied. EA is the measure of the capacity to form an emulsion using a macromolecule, whereas, ES is an indicator of their capability to form a stable emulsion in a defined time period [43]. As listed in Table 3, the EA of extracted CLP pectin was 41.31 ± 1.42%, which was observed to be higher than the reported result for sour orange peel pectin (40.7%) [31] and was close to the result for pectin extracted from C. medica peel (46.5%) [44]. Furthermore, these emulsions were found to be 70.22 ± 1.74% and 62.8 ± 1.39% stable at 24 °C after 1 day and 30 days of storage, respectively. It has been reported that due to the presence of reduced methyl ester groups in low-methoxy pectin, the surface activity of pectin is decreased which leads to the increase in stability of interfacial tension and particle size of emulsion, thereby positively affecting the emulsifying properties [45]. Therefore, it could be inferred from these observations that under the optimum extraction conditions, CLP pectin could be potentially used as an emulsifier and stabilizer in food industries.
3.4.2 Water holding capacity and oil holding capacity
WHC is a key technological property of many food products, and it represents the ability of 1-g pectin to retain water. Evaluation of WHC is an important parameter in food industries since pectin with high WHC is capable of holding a large quantity of water, thereby improving the textural and sensorial attributes of the product [46]. Therefore, WHC of pectin extracted from CLP under optimal conditions was evaluated, and it was observed that 1g of extracted pectin was able to hold 3.14 ± 0.11 g water (Table 3). The value was found to be higher than polysaccharides obtained from almonds (1.95 g/g) and pistachio (1.46 g/g) as well as pectin obtained from sour orange peel pectin (3.10 g/g) reported by Sila et al. [47] and Hosseini et al. [48], respectively. It has also been reported that numerous factors including the number of free hydroxyl groups present in pectin structure, pH, the porosity of dried pectin powder, and temperature could affect the WHC of the sample [49].
On the other hand, OHC is represented as the amount of oil retained by 1 g of pectin sample. Similar to WHC, OHC is also a paramount functional parameter since pectin exhibiting high OHC value can be potentially used as a stabilizer in high-fat food products [46]. The OHC of CLP pectin was observed to be 2.05 ± 0.17 g oil/g pectin (Table 3). The reported value was observed to be higher than the OHC value of commercial citrus pectin (0.93 g oil/g pectin) and Opuntia ficus indica pectin (1.23 g oil/g pectin) as reported by [49]. It has been reported that the high OHC value is probably due to the presence of some hydrophobic constituents in pectin structure [50].
3.5 Structural properties
The structural properties of extracted CLP pectin extracted under optimal conditions were observed using FT-IR and XRD spectroscopy which have been discussed below.
3.5.1 Fourier transform infrared spectroscopy
FT-IR is an important analytical technique used to study the primary structure of pectin by determining important functional groups in them [51]. FT-IR spectra of CLP pectin extracted under optimum conditions are depicted in Fig. 3. As observed from the spectra, broad and strong peaks were observed in the wavelength range of 3000–3500 cm−1 which was attributed to the stretching vibrations of inter-and intra-molecular O–H groups The appeared peak at 2982.78 cm−1 corresponds to the bending and stretching vibrations of C–H groups (CH, CH2, and CH3) of galacturonic acid. Furthermore, the peaks at 1623.08 cm−1 and 1782.38 cm−1 are mainly attributed to free and esterified carboxyl groups, respectively [46]. In addition, the DE of extracted pectin was related to the peak area intensities of 1623 cm−1 and 1782 cm−1. Therefore, as visualized by the spectra, the intensity of the peak at 1782.38 cm−1 was weaker than the peak of 1623.08 cm−1, which confirmed that DE of CLP pectin was low. The peaks observed within the range of 1000–1250 cm−1 were due to glycosidic linkages (C–O–C) of galacturonic acid units [52]. It should also be noted that several peaks were observed in the range of 500–1300 cm−1 which is unique for each type of pectin and is therefore considered as a finger-print region [53].
3.5.2 X-ray diffraction analysis
In order to further evaluate the structure (amorphous or crystalline) of extracted CLP pectin, XRD analysis was conducted and the diffractogram is presented in Fig. 4. In XRD patterns, the amorphous structure is usually identified by a broad background peak, whereas, the crystalline structure is characterized by numerous sharp signals. As depicted by the XRD pattern, the CLP pectin exhibited an amorphous nature, while some characteristic peaks were observed at 16.28, 18.05, 20.26, 26.09, 30.35, and 34.69° (2θ) which indicated the crystalline nature in the pectin structure. Therefore, the resulted diffractogram implied that CLP pectin exhibited both crystalline and amorphous nature. Similar results have been reported for pectin extracted from pistachio by-product [54], walnut processing waste [55], sour cherry pomace [56], and black mulberry pomace [57].
3.6 Scanning electron microscopy
SEM analysis was done to examine the detailed surface morphology and the effect of extraction treatment on the raw material at a specific magnification. The SEM results of CLP powder before and after pectin extraction treatment have been depicted in Fig. 5A and B, respectively. The microstructure of CLP powder prior to extraction was observed to be uniform, dense, and compact with no disruptions and cracks in the cell wall. However, significant morphological changes in the CLP powder were evident after the conventional heating extraction method. The surface of treated peel powder was found to be disrupted with a rough, wrinkled, and irregular texture. These fractural changes in the surface of CLP powder after conventional heating extraction at harsh conditions led to the disruption in middle lamella, thereby causing complete collapse and damage of the primary cell wall of CLP which resulted in enhanced pectin yield [58].
4 Conclusions
C. limetta peels have been successfully upgraded by the production of pectin using environment friendly organic acid. The results revealed that the CLP pectin yield was in the range of 3.97 to 22% and the highest pectin yield (22.03 ± 0.13%) obtained under 90 °C temperature, 95-min time, 1.8 pH, and 30 v/w LSR, which was in close proximity to the predicted value of 22.7%, thereby validating the reliability of the developed model. The extracted pectin was classified as low-methoxyl pectin with DE of 45.62 ± 0.64% and MeO of 5.45 ± 0.04%, whereas, the equivalent weight and total AUA content of pectin were observed to be 477.89 ± 9.43 and 67.81 ± 0.64%. SEM analysis showed a significant effect of the extraction process on the raw material, and the XRD pattern demonstrated the amorphous as well as crystalline nature of extracted pectin. Besides, the FTIR characterization evidenced the presence of characteristic bands of the extracted pectin. In addition, CLP pectin exhibited promising technological and functional properties such as emulsifying properties (ES and EA), WHC, and OHC. Therefore, the pectin extracted from CLP using organic acid can be utilized as a natural additive providing a wide array of technological applications in the food sector. The proposed method for the valorization of citrus peels is in congruence with the concept of circular economy as it can help in reducing environmental pollution as well as increasing the economic benefits for industries.
References
Anwar F, Naseer R, Bhanger MI, Ashraf S, Talpur FN, Aladedunye FA (2008) Physico-chemical characteristics of citrus seeds and seed oils from Pakistan. J Am Oil Chem Soc 85:321–330. https://doi.org/10.1007/s11746-008-1204-3
Maurya AK, Mohanty S, Pal A, Chanotiya CS, Bawankule DU (2018) The essential oil from Citrus limetta Risso peels alleviates skin inflammation: in-vitro and in-vivo study. J Ethnopharmacol 212:86–94. https://doi.org/10.1016/j.jep.2017.10.018
Maran JP, Priya B (2015) Ultrasound-assisted extraction of pectin from sisal waste. Carbohydr Polym 115:732–738. https://doi.org/10.1016/j.carbpol.2014.07.058
Kumar M, Tomar M, Saurabh V, Mahajan T, Punia S, del Mar CM, Rudra SG, Kaur C, Kennedy JF (2020) Emerging trends in pectin extraction and its anti-microbial functionalization using natural bioactives for application in food packaging. Trends Food Sci Technol 105:223–237. https://doi.org/10.1016/j.tifs.2020.09.009
Munarin FABIOLA, Tanzi MC, Petrini PAOLA (2012) Advances in biomedical applications of pectin gels. Int J Biol Macromol 51:681–689. https://doi.org/10.1016/j.ijbiomac.2012.07.002
Zhu RG, Sun YD, Li TP, Chen G, Peng X, Duan WB, Zheng ZZ, Shi SL, Xu JG, Liu YH, Jin XY (2015) Comparative effects of hawthorn (Crataegus pinnatifida Bunge) pectin and pectin hydrolyzates on the cholesterol homeostasis of hamsters fed high-cholesterol diets. Chem Biol Interact 238:42–47. https://doi.org/10.1016/j.cbi.2015.06.006
DKD d NS, da Silva Barros BR, da Cruz Filho IJ, NDSB J, da Silva PR, do Bomfim Nascimento PH, MDCA d L, Napoleao TH, CML d M (2021) Pectin-like polysaccharide extracted from the leaves of Conocarpus erectus Linnaeus promotes antioxidant, immunomodulatory and prebiotic effects. Bioact Carbohydr Diet Fibre 26:100263. https://doi.org/10.1016/j.bcdf.2021.100263
Bian Y, Zeng H, Tao H, Huang L, Du Z, Wang J, Ding K (2020) A pectin-like polysaccharide from Polygala tenuifolia inhibits pancreatic cancer cell growth in vitro and in vivo by inducing apoptosis and suppressing autophagy. Int J Biol Macromol 162:107–115. https://doi.org/10.1016/j.ijbiomac.2020.06.054
Inngjerdingen KT, Patel TR, Chen X, Kenne L, Allen S, Morris GA, Harding SE, Matsumoto T, Diallo D, Yamada H, Michaelsen TE (2007) Immunological and structural properties of a pectic polymer from Glinus oppositifolius. Glycobiology 17:1299–1310. https://doi.org/10.1093/glycob/cwm088
Colodel C, Vriesmann LC, Teófilo RF, de Oliveira Petkowicz CL (2018) Extraction of pectin from ponkan (Citrus reticulata Blanco cv. Ponkan) peel: optimization and structural characterization. Int J Biol Macromol 117:385–391. https://doi.org/10.1016/j.ijbiomac.2018.05.048
Yapo BM (2009) Lemon juice improves the extractability and quality characteristics of pectin from yellow passion fruit by-product as compared with commercial citric acid extractant. Bioresour Technol 100:3147–3151. https://doi.org/10.1016/j.biortech.2009.01.039
Koubala BB, Mbome LI, Kansci G, Mbiapo FT, Crepeau MJ, Thibault JF, Ralet MC (2008) Physicochemical properties of pectins from ambarella peels (Spondias cytherea) obtained using different extraction conditions. Food Chem 106:1202–1207. https://doi.org/10.1016/j.foodchem.2007.07.065
Chan SY, Choo WS (2013) Effect of extraction conditions on the yield and chemical properties of pectin from cocoa husks. Food Chem 141:3752–3758. https://doi.org/10.1016/j.foodchem.2013.06.097
Jafari F, Khodaiyan F, Kiani H, Hosseini SS (2017) Pectin from carrot pomace: optimization of extraction and physicochemical properties. Carbohydr Polym 157:1315–1322. https://doi.org/10.1016/j.carbpol.2016.11.013
Cho EH, Jung HT, Lee BH, Kim HS, Rhee JK, Yoo SH (2019) Green process development for apple-peel pectin production by organic acid extraction. Carbohydr Polym 204:97–103. https://doi.org/10.1016/j.carbpol.2018.09.086
Raji Z, Khodaiyan F, Rezaei K, Kiani H, Hosseini SS (2017) Extraction optimization and physicochemical properties of pectin from melon peel. Int J Biol Macromol 98:709–716. https://doi.org/10.1016/j.ijbiomac.2017.01.146
Pasandide B, Khodaiyan F, Mousavi Z, Hosseini SS (2018) Pectin extraction from citron peel: optimization by Box–Behnken response surface design. Food Sci Biotechnol 27:997–1005. https://doi.org/10.1007/s10068-018-0365-6
Guandalini BBV, Rodrigues NP, Marczak LDF (2019) Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res Int 119:455–461. https://doi.org/10.1016/j.foodres.2018.12.011
Oliveira TÍS, Rosa MF, Cavalcante FL, Pereira PHF, Moates GK, Wellner N, Mazzetto SE, Waldron KW, Azeredo HM (2016) Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem 198:113–118. https://doi.org/10.1016/j.foodchem.2015.08.080
Maran JP, Sivakumar V, Thirugnanasambandham K, Sridhar R (2014) Microwave assisted extraction of pectin from waste Citrullus lanatus fruit rinds. Carbohydr Polym 101:786–791. https://doi.org/10.1016/j.carbpol.2013.09.062
Wai WW, Alkarkhi AF, Easa AM (2010) Effect of extraction conditions on yield and degree of esterification of durian rind pectin: an experimental design. Food Bioprod Process 88:209–214. https://doi.org/10.1016/j.fbp.2010.01.010
Khan M, Nandkishor (2019) Optimization of extraction condition and characterization of low methoxy pectin from wild plum. J Package Technol Res 3:215–221. https://doi.org/10.1007/s41783-019-00070-z
Shchekoldina T, Aider M (2014) Production of low chlorogenic and caffeic acid containing sunflower meal protein isolate and its use in functional wheat bread making. J Food Sci Technol 51:2331–2343. https://doi.org/10.1007/s13197-012-0780-2
Shivamathi CS, Moorthy IG, Kumar RV, Soosai MR, Maran JP, Kumar RS, Varalakshmi P (2019) Optimization of ultrasound assisted extraction of pectin from custard apple peel: potential and new source. Carbohydr Polym 225:115240. https://doi.org/10.1016/j.carbpol.2019.115240
Masmoudi M, Besbes S, Chaabouni M, Robert C, Paquot M, Blecker C, Attia H (2008) Optimization of pectin extraction from lemon by-product with acidified date juice using response surface methodology. Carbohydr Polym 74:185–192. https://doi.org/10.1016/j.carbpol.2008.02.003
Fakayode OA, Abobi KE (2018) Optimization of oil and pectin extraction from orange (Citrus sinensis) peels: a response surface approach. J Anal Sci Technol 9:1–16. https://doi.org/10.1186/s40543-018-0151-3
Dao TAT, Webb HK, Malherbe F (2021) Optimization of pectin extraction from fruit peels by response surface method: conventional versus microwave-assisted heating. Food Hydrocoll 113:106475. https://doi.org/10.1016/j.foodhyd.2020.106475
Chaharbaghi E, Khodaiyan F, Hosseini SS (2017) Optimization of pectin extraction from pistachio green hull as a new source. Carbohydr Polym 173:107–113. https://doi.org/10.1016/j.carbpol.2017.05.047
Colodel C, Vriesmann LC, Teófilo RF, de Oliveira Petkowicz CL (2020) Optimization of acid-extraction of pectic fraction from grape (Vitis vinifera cv. Chardonnay) pomace, a winery waste. Int J Biol Macromol 161:204–213. https://doi.org/10.1016/j.ijbiomac.2020.05.272
Liew SQ, Chin NL, Yusof YA (2014) Extraction and characterization of pectin from passion fruit peels. Agric Agric Sci Procedia 2:231–236. https://doi.org/10.1016/j.aaspro.2014.11.033
Hosseini SS, Khodaiyan F, Yarmand MS (2016) Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr Polym 140:59–65. https://doi.org/10.1016/j.carbpol.2015.12.051
Maran JP, Sivakumar V, Thirugnanasambandham K, Sridhar R (2013) Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr Polym 97:703–709. https://doi.org/10.1016/j.carbpol.2013.05.052
Moorthy IG, Maran JP, Muneeswari S, Naganyashree S, Shivamathi CS (2015) Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. Int J Biol Macromol 72:1323–1328. https://doi.org/10.1016/j.ijbiomac.2014.10.037
Santos JDG, Espeleta AF, Branco A, de Assis SA (2013) Aqueous extraction of pectin from sisal waste. Carbohydr Polym 92:1997–2001. https://doi.org/10.1016/j.carbpol.2012.11.089
Li DQ, Jia X, Wei Z, Liu ZY (2012) Box–Behnken experimental design for investigation of microwave-assisted extracted sugar beet pulp pectin. Carbohydr Polym 88:342–346. https://doi.org/10.1016/j.carbpol.2011.12.017
Gnanasambandam R, Proctor A (2000) Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem 68:327–332. https://doi.org/10.1016/S0308-8146(99)00191-0
Liu L, Cao J, Huang J, Cai Y, Yao J (2010) Extraction of pectins with different degrees of esterification from mulberry branch bark. Bioresour Technol 101:3268–3273. https://doi.org/10.1016/j.biortech.2009.12.062
Basanta MF, Ponce NM, Rojas AM, Stortz CA (2012) Effect of extraction time and temperature on the characteristics of loosely bound pectins from Japanese plum. Carbohydr Polym 89:230–235. https://doi.org/10.1016/j.carbpol.2012.03.001
Yadav SD, Bankar NS, Waghmare NN, Shete DC (2017) Extraction and characterization of pectin from sweet lime. 4th Intern Confer on Multidis Resear & Prac 58-63
Kanmani P, Dhivya E, Aravind J, Kumaresan K (2014) Extraction and analysis of pectin from citrus peels: augmenting the yield from Citrus limon using statistical experimental design. Iran. J Ener Environ 5:303–312. https://doi.org/10.5829/idosi.ijee.2014.05.03.10
Khamsucharit P, Laohaphatanalert K, Gavinlertvatana P, Sriroth K, Sangseethong K (2018) Characterization of pectin extracted from banana peels of different varieties. Food Sci Biotechnol 27:623–629. https://doi.org/10.1007/s10068-017-0302-0
Wang W, Ma X, Xu Y, Cao Y, Jiang Z, Ding T, Ye X, Liu D (2015) Ultrasound-assisted heating extraction of pectin from grapefruit peel: optimization and comparison with the conventional method. Food Chem 178:106–114. https://doi.org/10.1016/j.foodchem.2015.01.080
Gharibzahedi SMT, Hernández-Ortega C, Welti-Chanes J, Putnik P, Barba FJ, Mallikarjunan K, Escobedo-Avellaneda Z, Roohinejad S (2019) High pressure processing of food-grade emulsion systems: antimicrobial activity, and effect on the physicochemical properties. Food Hydrocoll 87:307–320. https://doi.org/10.1016/j.foodhyd.2018.08.012
Pasandide B, Khodaiyan F, Mousavi ZE, Hosseini SS (2017) Optimization of aqueous pectin extraction from Citrus medica peel. Carbohydr Polym 178:27–33. https://doi.org/10.1016/j.carbpol.2017.08.098
Chen HM, Fu X, Luo ZG (2016) Effect of molecular structure on emulsifying properties of sugar beet pulp pectin. Food Hydrocoll 54:99–106. https://doi.org/10.1016/j.foodhyd.2015.09.021
Ezzati S, Ayaseh A, Ghanbarzadeh B, Heshmati MK (2020) Pectin from sunflower by-product: optimization of ultrasound-assisted extraction, characterization, and functional analysis. Int J Biol Macromol 165:776–786. https://doi.org/10.1016/j.ijbiomac.2020.09.205
Sila A, Bayar N, Ghazala I, Bougatef A, Ellouz-Ghorbel R, Ellouz-Chaabouni S (2014) Water-soluble polysaccharides from agro-industrial by-products: functional and biological properties. Int J Biol Macromol 69:236–243. https://doi.org/10.1016/j.ijbiomac.2014.05.052
Hosseini SS, Khodaiyan F, Kazemi M, Najari Z (2019) Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int J Biol Macromol 125:621–629. https://doi.org/10.1016/j.ijbiomac.2018.12.096
Bayar N, Friji M, Kammoun R (2018) Optimization of enzymatic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal. Food Chem 241:127–134. https://doi.org/10.1016/j.foodchem.2017.08.051
Elleuch M, Bedigian D, Roiseux O, Besbes S, Blecker C, Attia H (2011) Dietary fibre and fibre-rich by-products of food processing: characterisation, technological functionality and commercial applications: a review. Food Chem 124(2):411–421. https://doi.org/10.1016/j.foodchem.2010.06.077
Yang JS, Mu TH, Ma MM (2019) Optimization of ultrasound-microwave assisted acid extraction of pectin from potato pulp by response surface methodology and its characterization. Food Chem 289:351–359. https://doi.org/10.1016/j.foodchem.2019.03.027
Kazemi M, Khodaiyan F, Hosseini SS (2019) Eggplant peel as a high potential source of high methylated pectin: ultrasonic extraction optimization and characterization. LWT Food Sci Technol 105:182–189. https://doi.org/10.1016/j.lwt.2019.01.060
Yang JS, Mu TH, Ma MM (2018) Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem 244:197–205. https://doi.org/10.1016/j.foodchem.2017.10.059
Kazemi M, Khodaiyan F, Labbafi M, Hosseini SS, Hojjati M (2019) Pistachio green hull pectin: optimization of microwave-assisted extraction and evaluation of its physicochemical, structural and functional properties. Food Chem 271:663–672. https://doi.org/10.1016/j.foodchem.2018.07.212
Asgari K, Labbafi M, Khodaiyan F, Kazemi M, Hosseini SS (2020) High-methylated pectin from walnut processing wastes as a potential resource: ultrasound assisted extraction and physicochemical, structural and functional analysis. Int J Biol Macromol 152:1274–1282. https://doi.org/10.1016/j.ijbiomac.2019.10.224
Hosseini S, Parastouei K, Khodaiyan F (2020) Simultaneous extraction optimization and characterization of pectin and phenolics from sour cherry pomace. Int J Biol Macromol 158:911–921. https://doi.org/10.1016/j.ijbiomac.2020.04.241
Khodaiyan F, Parastouei K (2020) Co-optimization of pectin and polyphenols extraction from black mulberry pomace using an eco-friendly technique: simultaneous recovery and characterization of products. Int J Biol Macromol 164:1025–1036. https://doi.org/10.1016/j.ijbiomac.2020.07.107
Xu Y, Zhang L, Bailina Y, Ge Z, Ding T, Ye X, Liu D (2014) Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J Food Eng 126:72–81. https://doi.org/10.1016/j.jfoodeng.2013.11.004
Acknowledgements
Divyani Panwar acknowledge the fellowship support (ICMR Ref. Letter No. 3/1/2/181/2020-(Nut)) from Indian Council of Medical Research (ICMR), New Delhi, India and infrastructural support from Sant Longowal Institute of Engineering and Technology, Longowal, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Pectin was extracted from C. limetta peels using the organic acid-mediated heating extraction method.
• A maximum pectin yield of 22.03 ± 0.13% was achieved at optimal conditions of 90 °C temperature, 95-min time, 1.8 pH, and 30 v/w LSR.
• The extracted pectin exhibited a low degree of esterification with good emulsifying and water/oil binding properties.
• C. limetta peel can be potentially used as a new source of pectin production which can reduce and valorize citrus waste.
Rights and permissions
About this article
Cite this article
Panwar, D., Panesar, P.S. & Chopra, H.K. Green extraction of pectin from Citrus limetta peels using organic acid and its characterization. Biomass Conv. Bioref. 14, 159–171 (2024). https://doi.org/10.1007/s13399-021-02127-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02127-z