Abstract
C-phycocyanin is a high market value compound derived from cyanobacterium Spirulina platensis and has a wide range of applications in pharmaceutical and food industries as a useful biochemically active compound. In this study, essentially an aqueous two-phase system (ATPS) is presented for purification of C-phycocyanin. The phase behavior of ATPSs that include Pluronic copolymers and salts was evaluated systematically. After that, the effects of different parameters such as salt type, copolymer structure, pH of solution, tie line length (TLL), system’s temperature, and volume ratio for purification of C-phycocyanin were investigated. The results revealed that Pluronic 10R5/potassium phosphate buffer system is the most appropriate system for promoting the C-phycocyanin separation from other contaminants. Besides, the purity index of C-phycocyanin was enhanced by up to 3.92 at TLL of 46.31%, pH = 6, volume ratio of 0.34, and temperature of 35 °C without any loss of stability. At these conditions, the obtained C-phycocyanin recovery was 90%. After that, the purity factor reached to 5.9 by applying the ultrafiltration method. In addition, the results of circular dichroism (CD) spectroscopy proved that the C-phycocyanin structure remained intact during the purification step. Finally, 78% of 10R5 copolymer was recovered by increasing the temperature above 57 °C (micelle formation (which can be used in new ATPS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spirulina as a filamentous cyanobacterium is one of the earliest known life forms that date back billions of years. Spirulina belongs to a group of photosynthetic bacteria called cyanobacteria or green–blue algae (Huisman et al., 2018). These algae have always attracted the interest of research community due to their high nutritional value and their applications as antioxidant, antimicrobial, bioactive substances’ production, protein enrichment, and optimal color changing of various foods in the food industry (Bhat & Madyastha, 2001; Patel et al., 2018). One of the components attached to cyanobacterial membranes is phycobiliprotein, which is found in water-soluble form. Phycobiliproteins possess a consistent 40 to 60% of the total soluble protein in these cells (Khan et al., 2005; Kulshreshtha et al., 2008). They are composed of a valuable protein called C-phycocyanin which plays an important role in photo-systems (Begum et al., 2016). One of the functions of phycobiliproteins is their efficient energy transfer capability due to their unique macromolecular geometric structure. C-phycocyanin is a natural blue pigment that belongs to the phycobiliprotein family with Spirulina platensis being the main source of this protein. Due to the harmful effects of synthetic food colorants, natural pigments are significantly demanded by customers (Nowruzi et al., 2022). In addition to its wide application in the food and cosmetics industries as a natural nutrient and natural dye, C-phycocyanin is also implemented in the prevention of cancer and kidney diseases, and control of cardiovascular disease and diabetes, as well as strengthening the immune system and anti-tumor agents (Liao et al., 2016; Saini & Sanyal, 2015).
Many approaches have been proposed to purify C-phycocyanin, including membrane separation (de Amarante et al., 2020) and ion exchange chromatography (Chen et al., 2019). However, other techniques such as precipitation, microwave, and ultrasound-assisted isolation (Taragjini et al., 2022) have also been applied for this application. Some of these methods are costly and time-consuming due to their high required number of steps (Chandler, 2021). However, despite the relatively high purity obtained through most of these methods, the yield of C-phycocyanin is low. Lauceri et al. applied the ultrasound-assisted cell lysis process to obtain blue phycocyanin from biomass of Spirulina platensis (Lauceri et al., 2023). It represented an efficient process for production of this protein, but C-phycocyanin obtained had a moderate purity index (within 2.5 and 3.5) and low yield (28%). In another report, it has been shown that microwave and ultrasound technologies are often applied in the extraction process and do not have a noticeable ability in purification (Prandi et al., 2022). Therefore, it is necessary to apply an efficient and cost-effective method by scaling-up bioseparation while maintaining the high purification performance and high product yield. In recent years, aqueous two-phase systems (ATPSs) have emerged as an alternative method in isolation of biomolecules due to their simplicity, environmental compatibility, easier scale-up, and small processing time. It is noteworthy that ATPS reduces the subsequent downstream processing volume in complete phycocyanin separation and purification (Mittal et al., 2019; Phong et al., 2018). ATPS is a liquid–liquid extraction method that does not use organic solvents. A high percentage of this system is water and consists of two heterogeneous phases with partially immiscible components. As a result, it can be more useful for separation of proteins. The aqueous mixtures split into two phases above certain concentrations of components. The basis of extraction in these systems is the uneven distribution of biomolecules between two phases due to the difference in physicochemical properties of the phases (Darani et al., 2021). The effective parameters on phase behavior and partitioning of the biomolecules in the ATPS are the interaction between the phase components and biomolecule, phase forming properties, temperature, pH, and so on.
Common types of aqueous two-phase systems such as polymer–polymer, polymer-salt, and ionic liquid-salt systems have previously been applied for separation of a wide range of biomolecules (Ferreira et al., 2018; Nandini & Rastogi, 2011; Pei et al., 2012; Wang et al., 2022). Nascimento and coworkers (2020) have investigated the ATPS based on polymer (PEG)-salt to purify the C-phycocyanin with a low purity index of 1.88 and extraction efficiency of 80%. In this way, polymer-based systems showed poor performance in protein separation due to high viscosity and less hydrophobicity difference between the two formed phases. Another disadvantage was that the used polymers (PEG and PPG) were not recoverable, so the cost of separation increased. Moreover, in some articles, in order to achieve high purity index of the biomolecules, multiple steps of PEG/salt ATPS extraction were needed, which led to high polymer consumption. Thus, the large-scale purification process may not be economical for these systems. For example, the purification of C-phycocyanin (Patil & Raghavarao, 2007) and lipase (Nandini & Rastogi, 2011) was carried out using multiple steps of PEG/salt ATPS extraction to achieve high purity. However, a study on the purification of C-phycocyanin using imidazolium ionic liquid–based ATPS has been reported by Chang et al. (2018). The extraction efficiency and the partition coefficient of C-phycocyanin were 99% and 36.6, respectively, at pH = 7. Despite the high extraction efficiency, these imidazolium ionic liquids are toxic and expensive and have negative effects on the environment. Therefore, in this study, PEG-PPG block copolymers as environmentally friendly are utilized to overcome these impediments. These Pluronic copolymers are biocompatible, relatively cheap, and excellent co-solvents with low volatility and are used in various applications in medicine as pharmaceutical ingredients and drug delivery carriers (Pitto-Barry & Barry, 2014). One of the basic features of these copolymers is the ability to adjust their hydrophobicity, which is due to the presence of hydrophilic and hydrophobic regions and depends on weight ratio of \(\frac{\mathrm{PEG}}{\mathrm{PPG}}\), structure, and molecular weight of copolymer (Ahsaie et al., 2021; Bakshi et al., 2004). Haraguchi et al. (2004) studied the effect of copolymer hydrophobicity on phase equilibrium behavior of insulin in an ATPS based on block copolymer. They found that copolymer’s hydrophobicity would magnify the biphasic region and had higher efficacy on partitioning of insulin. Therefore, applying the block copolymers as phase forming agents of an ATPS causes excess partitioning and enhances extraction efficiency (de Oliveira et al., 2007; Shiran et al., 2020). Vicente et al. (2019) indicated that an ATPS based on block copolymer was the most effective downstream process to isolate different proteins. Moreover, Pluronic copolymers present self-association to micellar formation at the cloud point temperature of ATPS, i.e., the temperature at which the separation of the two phases (copolymer-rich phase and water phase) occurs (Hou & Cao, 2014). Thus, the temperature-induced phase separation has great potential for the recovery of Pluronic copolymers. Hence, it is clear that the reuse of the copolymers in new ATPS can significantly reduce the cost of purification.
The main purpose of this work is to establish the ATPS based on Pluronic copolymers for the extraction and purification of C-phycocyanin from Spirulina platensis which has been investigated for the first time. Therefore, the partition coefficient, recovery, and purity of this protein were determined. Two different Pluronic copolymers (normal and reverse) were selected to study the effect of copolymer’s structure on ATPS for C-phycocyanin purification. The phase behaviors of six different salts and a buffer with these copolymers were studied to develop more efficient partitioning of C-phycocyanin. The effects of different parameters such as, pH value, tie line length, phase volume ratio, temperature, and salt type on purification of C-phycocyanin were studied. Finally, isolation of phase components was investigated.
Materials and Methods
Materials
Pluronic L35 copolymer of Mw = 1900 g mol−1 and Pluronic 10R5 of Mw = 2000 g mol−1 were purchased from Sigma-Aldrich (99 wt% of purity). Number of ethylene glycol (EG) monomers and propylene glycol (PG) monomers, molecular weight (MW), percentage of EG monomer, cloud point temperature (Tcp), density, and viscosity of copolymers are presented in Table 1. Ammonium sulfate, sodium sulfate, magnesium sulfate, sodium citrate, potassium citrate, potassium phosphate, and potassium carbonate were analytical grade with 99% purity and supplied from Merck. Fresh biomass of Spirulina platensis was obtained from Ariyangostar (Iran). Samples were prepared in double distilled water with conductivity of 0.08 \(\frac{\mathrm{\mu s}}{\mathrm{cm}}\).
Methods
Solid–Liquid Extraction (SLE)
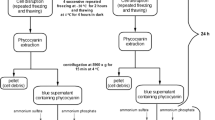
One of the factors influencing a successful extraction of C-phycocyanin from Spirulina platensis biomass is the acquisition of molecules placed behind cell walls (connected via disulfide bridge to polysaccharides) (Favas, 2021). An extraction agent was applied to break the cells. The solvent used in this extraction was sodium phosphate buffer aqueous solution at 20 mM. In accordance with the method applied by Sintra et al. (2021), fresh biomass and sodium phosphate buffer were mixed with a solid–liquid ratio of 0.1 (1 g of fresh biomass is added to 10 mL of buffer). Then, the mixture was placed in a thermo-mixer set at 25 °C and operated at 1500 rpm for 45 min. The mixture was not exposed to light during all stages of extraction. At the end, the samples were centrifuged at 12,000 rpm for 10 min to remove the cell debris and collect the crude extract containing mostly the C-phycocyanin. This solution was kept at 4 °C for subsequent experiments.
Phase Diagrams and Tie Lines
The cloud point titration procedure was applied to obtain the binodal curve for ATPS based on copolymer-salt at 25 °C (Baghbanbashi et al., 2022). In order to indicate the pH effect on the binodal curve, the phase diagrams of ATPS containing potassium buffer at different pH values (6.5, 7.2, 8.0) were developed. This method consisted of two basic parts to determine the binodal curve. First, a copolymer solution with a known mass fraction was provided gravimetrically (as a stock solution) and then, a salt solution of known mass fraction was added to the base solution dropwise until a cloudy mixture was detected. The composition of this solution was calculated. In the second part, distilled water was added to the base solution to return its opaque color to that of a clear monophasic system and this methodology was repeated. Note that each point of binodal curve was determined by weight quantification. The experimental binodal curves were fitted by the following three-parameter equation (Eq. (1)) proposed by Merchuk et al. (1998):
A, B, and C are adjustable parameters determined by least squares regression of experimental data. The compositions of top and bottom phases and tie lines were determined by the lever-arm rule and using gravimetric method (Eqs. (2)–(5)) (Merchuk et al., 1998):
The subtitles F, Top, and Bot indicate the feed, top, and bottom phases, respectively. Parameter \(\alpha\) indicates the mass ratio of top phase to feed. Tie line length (TLL) and its slope (STL) are defined by Eqs. (6) and (7) (Jamshidi & Pazuki, 2018):
Partitioning of C-Phycocyanin
The ATPSs consisting of copolymer, salt, and water were prepared gravimetrically (the characteristics of scale made in Japan are ± 10−4 g, AND, model HR200). One gram of crude extract containing C-phycocyanin was added to 9 g of each mixture point (10 wt%). In order to complete the phase separation and achieve thermodynamic equilibrium, the mixture was stirred vigorously, centrifuged (at 2700 rpm for 15 min) and incubated for 24 h at 25 °C. Note that an ATPS without crude extract was used as a blank solution to eliminate the component interference. After that, the copolymer-rich (top phase) and salt-rich (bottom phase) phases were carefully separated and the mass and the volume of each phase were measured. C-phycocyanin and total protein in each phase were determined by a UV–Vis spectrophotometer (double beam spectrometer, Shimadzu, model UV-200S Japan) at 615 nm, 652 nm, and 280 nm, respectively. The concentration of C-phycocyanin (CPC) was determined according to the following equation (Moraes et al., 2010):
where OD652 and OD615 represent the absorbance at 652 nm and 615 nm, respectively.
Then, partition coefficient (K), recovery of top and bottom phases (R), selectivity factor (SF), and purity index of C-phycocyanin were determined by applying Eqs. (9)–(16) (Rito-Palomares & Benavides, 2017):
Statistical Analysis
It should be noted that three replicates were carried out for each experiment. The presented results were the average of obtained partition coefficient, purity index, selectivity factor, and recovery with the respective standard deviations. Significant differences between the results were treated by applying Statistical Product and Service Solutions (SPSS) 19.0 software. The p values less than 0.05 were considered statistically significant. The resulting errors were indicated in the relevant figures using error bars that represented the standard deviation of the experiments.
Circular Dichroic Spectra
To study the structure of C-phycocyanin after purification, the spectroscopy of circular dichroism (CD) was used. First, the sample containing the appropriate concentration of C-phycocyanin was selected according to the optimal feed (bottom phase) criteria and then, this solution was passed through Amicon ultrafiltration. The solution was then centrifuged at 4000 rpm for 30 min. The CD spectrum was recorded on a Jasco J-810 spectropolarimeter. Amicon ultra device employs the Ultracel regenerated cellulose ultrafiltration membrane with a wide range of molecular weight cut-offs to concentrate or purify proteins. The membranes are designed to provide high recovery performance and reliable results for various biological solutions.
Results and Discussion
Determination of Phase Diagrams and Tie Lines
In this research, ATPSs based on thermo-separating triblock copolymers have been used in order to create an adjustable and efficient process. For this purpose, the effects of different organic/inorganic salts as well as copolymer’s structure impact on biphasic region were investigated. All binodal curves of ATPSs composed of Pluronic L35 and Pluronic 10R5 copolymers with six salts and a buffer at pH = 6.5, 7.2, and 8.0 are shown in Figs. 1, 2, 3, 4, and 5. These diagrams were obtained at a temperature of 25 °C and atmospheric pressure. The experimental binodal data and the constant Merchuk parameters (A, B, and C) are reported in Tables S1 through S19 (Supplementary Information). The tendency and ability of different salts on formation of two phases were investigated. Salting-out ability affects the extent of the biphasic region. Higher salting-out strength leads to larger two-phase region. As depicted in Figs. 1 and 2, the salts’ ability in promoting two-phase formation at 8 wt% of salt follows the order \({\mathrm{Na}}_{2}{\mathrm{SO}}_{4}\) > \({\mathrm{K}}_{2}{\mathrm{HPO}}_{4}/{\mathrm{KH}}_{2}{\mathrm{PO}}_{4}\) (pH = 7.2) > \(({\mathrm{NH}}_{4}{)}_{2}{\mathrm{SO}}_{4}\) > \({\mathrm{K}}_{2}{\mathrm{CO}}_{3}\) > \({\mathrm{Na}}_{3}{\mathrm{C}}_{6}{\mathrm{H}}_{5}{\mathrm{O}}_{7}\) > \({\mathrm{K}}_{3}{\mathrm{C}}_{6}{\mathrm{H}}_{5}{\mathrm{O}}_{7}\) > \({\mathrm{MgSO}_{4}}\) for both ATPSs based on Pluronic L35 and 10R5. A similar trend has been reported for these salts in several papers (Silvério et al., 2013; Wang et al., 2010).
Generally, the salting-out effect of ions follows the Hofmeister series and the free energy of ion hydration (\(\Delta {G}_{\mathrm{Hyd}})\). Increasing the valence of the ions (more negative \(\Delta {G}_{\mathrm{Hyd}}\)) leads to an increase in their salting-out effect.The reported \(\Delta {G}_{\mathrm{Hyd}}\) values of the magnesium, ammonium, and sodium cations in literatures are − 1850\(\frac{\mathrm{kJ}}{\mathrm{mol}}\), − 285\(\frac{\mathrm{kJ}}{\mathrm{mol}}\), and − 365\(\frac{\mathrm{kJ}}{\mathrm{mol}}\) respectively (Pimentel et al., 2017; Wang et al., 2016). It was expected that magnesium sulfate would show more salting-out effect than sodium sulfate and ammonium sulfate salts (including similar \({\mathrm{SO}}_{4}^{2-}\) anion), but the results illustrate an inverse trend (Ahsaie & Pazuki, 2021; Da Silva et al., 2006; Khayati & Alizadeh, 2013; Zhao et al., 2011). The multi-valence cations (\({\mathrm{Mg}}^{2+}\)) show more interactions with ethylene oxide monomer in Pluronic L35/10R5, which lead to a salting-in phenomenon (Shiran et al., 2020; Zhao et al., 2011). Figure 3 shows the effect of the structure of Pluronic 10R5 and Pluronic L35 copolymers on the binodal curves in the presence of potassium phosphate buffer and sodium sulfate salt. As can be seen, the larger biphasic area of Pluronic 10R5 indicates its capability to form two phases when compared to L35 copolymer. This could be due to structural differences between the two copolymers. The results also reveal that the reverse copolymer (Pluronic 10R5) has higher hydrophobicity than normal copolymer (Pluronic L35). Similar results were also reported by Ahsaie and Pazuki (2021). They explained that normal copolymer is more hydrophilic due to the two PEG units in its structure compared to the two PPG units of the reverse copolymer, resulting in a lower ability to form two phases. The variation between the compositions of both phases is described by TLL and it is commonly applied to fit trends in the partition of the biomolecule between two phases. The concentrations (wt%) of each component in the top and bottom phases and both TLLs and STLs are reported in Tables S20 and S21. In the following, the phase behaviors of ATPSs composed of potassium phosphate buffer in ATPS based on Pluronic L35 and Pluronic 10R5 at different pH values are presented in Figs. 4 and 5. Since the stability of C-phycocyanin depends on the pH value and it is more stable at pH values between 5 and 8, the salting-out effect was evaluated based on this pH range (Patil & Raghavarao, 2007). Note that pH can be altered by changing the ratios of K2HPO4 and KH2PO4 in potassium phosphate buffer (Peng et al., 1994). Generally, the results demonstrated that as the conditions change from acidic to alkaline, the binodal shifts to lower concentrations of copolymer and salt and the two-phase region becomes larger which is mainly explained by the strength of salting-out of salt and the ion capacity in that pH. In other words, ions with higher valence \(({\mathrm{HPO}}_{4}^{2-}>{\mathrm{H}}_{2}{\mathrm{PO}}_{4}^{-})\) have more salting-out capability to form the two-phase system and a wider two-phase region is obtained at higher pH.
Partitioning and Purification of C-Phycocyanin
Effect of Salt Type and Copolymer Structure
After binodal curves and tie lines were specified for each system, the effects of salt type and structure of copolymer on the bioseparation of C-phycocyanin from contaminants were studied. The mixture points of 8 wt% of salt and 20 wt% of copolymers were considered to represent the type of ATPS. After that, crude extract (including mainly C-phycocyanin, chlorophylls, and other proteins) was added to copolymer/salt-based ATPSs to purify C-phycocyanin as a target protein. Note that the initial purity index of C-phycocyanin in crude extract was 0.53. The obtained partition coefficient results for C-phycocyanin, chlorophyll and total proteins, purity index of C-phycocyanin at bottom phase, and selectivity factor are presented in Table 2 (p < 0.05). It can be seen that the partition coefficient of C-phycocyanin is less than 1, indicating high tendency of C-phycocyanin to the salt-rich phase in all studied systems. C-phycocyanin is a hydrophilic molecule, which justifies the highest affinity of this protein to the most hydrophilic (salt-rich phase) phase. Among the studied salts that include magnesium sulfate, sodium sulfate, ammonium sulfate, potassium phosphate, sodium citrate, potassium citrate, and potassium carbonate, the highest selectivity and purification is obtained by the ATPS composed of potassium phosphate buffer, in which C-phycocyanin is more compatible and stable (stability pH is between 5 and 8) (Patil & Raghavarao, 2007). The structure of C-phycocyanin was decomposed in alkaline conditions and as a result, its concentration and its blue color decreased, which is clearly apparent in biphasic systems that include sodium citrate and potassium carbonate salts. Fluorescence disappearance was also observed in PEG-sodium citrate ATPS (Patil & Raghavarao, 2007). It should be mentioned that C-phycocyanin precipitated at the interphase in the sodium, ammonium, and magnesium sulfate-ATPSs leading to reduced C-phycocyanin’s purity in the bottom phase. Patil et al. reported that C-phycocyanin precipitated at interphase in most of PEG-sulfate ATPSs. They have studied the effect of pH on precipitation of C-phycocyanin, and reported that precipitation occurred at pH = 5 which can be related to the changes in water properties (Patil & Raghavarao, 2007). The pH values of all studied systems are reported in Table 3. According to the reported results in Tables 2 and 3 considering the highest purity index of C-phycocyanin, potassium phosphate buffer was selected for the further studies. As can be seen in Table 2, the purity index was at the minimum value (0.19) at extreme basic values (pH = 11.6) for Pluronic 10R5-K2CO3 ATPS. Also, in acidic values (pH = 5 or lower), C-phycocyanin precipitated at the intermediate phase and the purity index decreased. Lauceri1 and coworkers reported that the use of CaCl2 solution in ultrasound-assisted extraction can hardly reach the purity index of C-phycocyanin to 2 (Lauceri et al., 2023), while this study shows that the purity index of this protein by potassium phosphate buffer in respect to other solutions was undoubtedly higher than 2.
In this study, Pluronic L35 and Pluronic 10R5 were applied as normal and reverse copolymers, respectively, to investigate the influence of copolymer structure on C-phycocyanin purification. The \(\frac{\mathrm{PEG}}{\mathrm{PPG}}\) ratio and molecular weights of these copolymers are almost the same. The only difference between these two copolymers is their hydrophilic-lipophilic balance (HLB) and relative solubility number (RSN) values, which depend on copolymer’s structure as well as the molecular weight and \(\frac{\mathrm{PEG}}{\mathrm{PPG}}\) ratio. The higher these values follow, the greater the hydrophilicity of the copolymer (Table S22). Wu et al. studied the effect of copolymer structure on hydrophilic-lipophilic balance (HLB) and relative solubility number (RSN). They showed that normal Pluronics have higher HLB and RSN than corresponding reverse copolymers (Wu et al., 2005). They also reported that for small copolymers with molecular weight less than 3000\(^{\mathrm g}/{_\mathrm {mol}}\), the difference between normal and reverse Pluronics is not significant. Therefore, there is a slight difference between Pluronic L35– and Pluronic 10R5–based ATPSs because of their structures. According to the reported results, the largest selectivity (\(\frac{{K}_{\mathrm{total Protein}}}{{K}_{\mathrm{C}-\mathrm{Phycocyanin}}}\) and \(\frac{{K}_{\mathrm{Chlorphyl }}}{{K}_{\mathrm{C}-\mathrm{Phycocyanin}}} )\) and purity were observed in the aqueous two-phase system that include Pluronic 10R5-potassium phosphate buffer. This ATPS can be used to create an extremely effective purification system in which C-phycocyanin is concentrated in one phase with the purity index of 2.68. Similar to our work, the effect of copolymer structure (normal and reverse) on partitioning of other hydrophilic and hydrophobic proteins (cytochrome c (cyt) and azocasein (azo)) was investigated by Vicente et al. (2019). Cytochrome c as a hydrophilic protein was partitioned into the salt-rich phase (95% recovery), while azocasein was completely recovered in the copolymer-rich phase in the ATPS based on Pluronic 10R5-potassium phosphate buffer. In this ATPS, the selectivity (Sazo/cyt) was 1428. The high hydrophobic property of Pluronic 10R5 pushed more cytochrome c to the salt-rich phase (more hydrophilic phase).
Effect of pH
In order to better understand the impact of system pH on partition coefficient of biomolecules, it is necessary to consider the nature of charged amino acid residues on the biomolecules surface. The net charge (positive, negative, or neutral) of the surface-exposed amino acid residues determines the protein surface properties and, more importantly, can be altered by changing the pH system.
In this study, the effect of pH as an effective parameter in protein purification and recovery was investigated and the results are shown in Fig. 6. Since C-phycocyanin is stable in pH range 5 to 8, the effect of pH was investigated in this range. System pH was adjusted by changing the potassium phosphate ratio (Table S23). The pH value can affect protein partitioning by changing the solute charge or the proportion of charged species. At pH values lower than iso-electric point (PI), the protein has a positive charge because the amine groups acquire an extra proton, while pH ˃ PI, a net negative charge protein is created because the proton of carboxyl is released. The iso-electric point for C-phycocyanin is 5.8 (Narayan & Raghavarao, 2007), so at pH > 5.8, C-phycocyanin is directed to positively charged copolymer-rich phase due to the electrostatic force.
According to the obtained partition coefficients in Table 4, it can be concluded that with an increase in pH (6 to 8), C-phycocyanin shows more tendency towards the top phase (increasing the k value) while the contaminants partition in favor of the bottom phase (decreasing the k value). The purity index and recovery of C-phycocyanin in the bottom phase decrease by increasing the pH value due to favorable charge-charge interaction between PEG block in copolymer and C-phycocyanin (Fig. 6). Several authors have also observed that negatively and positively charged biomolecules tend to concentrate in polymer- and salt-rich phases, respectively (Chew et al., 2019; Chow et al., 2015). As shown in Fig. 6B, since C-phycocyanin was precipitated at interphase at pH = 5, the bottom purity index was reduced to a minimum value)0.91(. It should be noted that the highest recovery and purity index of C-phycocyanin in the bottom phase were 81% and 3.14 at pH = 6, respectively. The values obtained were statistically significant (p < 0.05). Therefore, the credibility of the results was acceptable.
Effect of Tie Line Length (TLL)
In this study, the effect of TLL on the purity index of C-phycocyanin in Pluronic 10R5/potassium phosphate ATPS was investigated by considering the highest purity index at pH = 6. Partition coefficients of total proteins, chlorophyll, and C-phycocyanin, volume ratio, purity index, and recovery of C-phycocyanin in top and bottom phases are reported in Table 5 and Fig. 7. An increase in TLL from 21.53 to 57.19% w/w (further from critical point) causes an incremental difference of physio-chemical properties (hydrophobicity and free volume) between top and bottom phases leading to migration of C-phycocyanin and other proteins to the top phase (except from TLL 46.31 to 57.19) (Enriquez-Ochoa et al., 2020; Rito-Palomares & Hernandez, 1998). This observation can be justified by the reduction of water content, hydrophilicity, and free volume in the bottom phase (Rito-Palomares & Hernandez, 1998). It should be mentioned that the purity index in the bottom phase increases since migration of contaminants to the top phase is higher than that of C-phycocyanin. Chethana et al. (2015) observed a similar behavior for the purification of C-phycocyanin in PEG-potassium phosphate ATPS. In this system, with an increase in TLL, C-phycocyanin showed a high tendency to migrate to the top phase along with the contaminants. Therefore, the increasing amount of contaminants due to increasing of TLL is supposed to reduce the purity index of C-phycocyanin in the top phase. However, the maximum purity of obtained C-phycocyanin was 3.5.
Based on the results, the greatest purification of the C-phycocyanin was obtained at TLL 46.31% w/w, with the purity of 3.79 in the bottom phase. It was observed that increasing TLL to 46.31% in the ATPS based on Pluronic 10R5-potassium phosphate increased hydrophobicity of the top phase more efficaciously, so the migration of C-phycocyanin to the top phase was less than that of the contaminants. As a result, the purity of target product was increased in the salt-rich phase and also increasing TLL did not significantly change the recovery.
As shown in Fig. 7, further incremental increase of TLL from 46.31 to 57.19% causes a decline in the purity index of C-phycocyanin reaching to a value of 1.29. By increasing TLL, copolymer and salt concentrations increase in both the top and bottom phases. Hence, the volume exclusion and salting-out effects increase, which results in precipitation at the interface (Suarez Ruiz et al., 2018). It should be mentioned that interfacial tension has a strong relation with TLL (Atefi et al., 2015; Grilo et al., 2016). As reported in several literatures (Atefi et al., 2015; Chow et al., 2013; Kee et al., 2022), precipitation is more probable in ATPSs with higher interfacial tension. Visual observation also emphasizes the accuracy of this phenomenon. Considering the results of purity index obtained for C-phycocyanin at different TLLs, the TLL at 46.31% with pH = 6 was selected to study the effect of temperature. The results obtained were statistically significant (p < 0.05).
Effect of Temperature
The influence of temperature on recovery and purification of C-phycocyanin in Pluronic 10R5/potassium phosphate ATPS at pH = 6 and TLL at 46.31% was investigated at five selected temperatures 5, 15, 25, 35, and 45 °C. The results of recovery and purity index are depicted in Figs. 8 and 9, respectively. As the temperature increases, the hydrophobicity of Pluronic 10R5 increases due to hydrophilicity reduction of its monomers. This results in diffusion of water molecules from the top to the bottom phase, making top and bottom phases more hydrophobic and hydrophilic, respectively. This factor promoted the C-phycocyanin to partition into the bottom phase. As a result, the bottom recovery of the target protein was significantly enhanced up to 92% with the temperature reaching 45 °C (Fig. 8). As can be seen in Fig. 9, the purity index would be higher in the bottom phase reaching to 3.92 at 35 °C, while it started a downward trend again after this temperature. The results obtained were statistically significant (p < 0.05). The reason may be that at high temperatures, the degradation rate increased dramatically and conformation of the C-phycocyanin changed and consequently its concentration decreased. Therefore, decreased purification is a result of low stability of protein at high temperatures. Chaiklahan et al. (2012) also showed that a significant rate of degradation of C-phycocyanin occurs at temperatures higher than 40 °C.
Effect of Volume Ratio
One of the key important factors considered in any purification step is volume ratio (VR) of both phases and high purity index and recovery could be achieved by changing this parameter. Volume ratio will change by moving along the tie line while the compositions of top and bottom phases are constant. In this case, the pH and TLL values are kept constant at 6 and 46.31% w/w, respectively. According to the reported results in Table 6, it can be seen that an increase in the volume ratio from 0.21 to 1.60 has increased the purity index of C-phycocyanin while the recovery decreases at the bottom phase. It can be reasoned that decreased available volume in the extracting phase (salt-rich phase) causes more affinity of C-phycocyanin to the top phase, and thus, an incremental change of C-phycocyanin’s K value leads to a reduction of bottom phase recovery. In the case of purity, the trend was different, so that initially, by increasing VR to 0.73, the purity varied slightly, while with further increase of VR, the purification of C-phycocyanin increased significantly to 4.29. This behavior is due to the fact that large amounts of contaminant proteins migrate into the top phase compared to C-phycocyanin, which is more noticeable at higher VR values. This was confirmed by observing that in Table 6 with VR = 1.6, the partition coefficient of the total protein was about 4 times more than the corresponding value for C-phycocyanin. Note that the results were found to be statistically significant (p < 0.05).
CD Spectra Analysis
Circular dichroic (CD) spectroscopy was employed to investigate any alterations in the configuration of C-phycocyanin. These spectra provided proper information on the secondary structure of C-phycocyanin in the far UV region between 190 and 270 nm. The obtained results of CD spectra for the bottom phase of optimum ATPS and a standard sample of C-phycocyanin are shown in Fig. 10. The location of the peaks for samples is one of the important aspects of this spectrum. According to this figure, the spectrum indicated the maximum and minimum peaks at 198 nm and 221 nm, respectively, for both the optimum and standard samples. It can be concluded that the protein structure of the optimum sample was in good agreement with the standard sample and consequently, the secondary structure of C-phycocyanin had remained unchanged after the purification step. The obtained secondary structures of α-helix, β-sheet, β-turn, and random coil for C-phycocyanin in both samples are presented in Table 7. There are no visible significant changes in protein structure compared with the standard and optimum samples. Therefore, there has been no indication of C-phycocyanin’s stability loss. A similar pattern has been reported in other works (Patil et al., 2006). It was observed that the secondary structure of the standard sample was completely consistent with that of the purified C-phycocyanin using the ATPS method.
Isolation of Phase Components
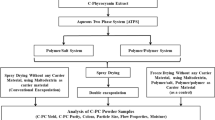
First, for phase component isolation, the top and bottom phases were carefully separated. Considering that the ATPS top phase is rich in 10R5 copolymer, the recovery and reuse of this copolymer can significantly reduce the cost of separation of C-phycocyanin. The copolymer-rich phase (top phase) was recovered by heating the solution above the cloud point temperature. The molecules of PEG-PPG copolymer are amphiphilic and undergo self-assembly to create micellar-like aggregates at a certain temperature. In other words, at above the lower critical solution temperature (LCST), i.e., 57 °C, the 10R5 copolymer makes spherical micelles and a biphasic micellar system including a copolymer phase and a water phase was formed. Therefore, the increase in temperature causes a more concentrated Pluronic 10R5–rich phase, and an enhancement in excluded volume effect. The contaminants partition to the water phase and thus lead to the recycling of copolymer after thermoseparation. The obtained 10R5 copolymer recovery was 78%, and this can be used to compose a new ATPS. As mentioned, the ATPS bottom phase mostly contained C-phycocyanin, potassium phosphate buffer, and other contaminants. Thus, an ultrafiltration step was applied to decrease the buffer concentration and isolate the C-phycocyanin from contaminants at the ATPS bottom phase. For this, an Amicon Ultra Centrifugal Filter device was used to upgrade the purification process of target protein with a 30 kDa cut-off membrane. After that, the final purity index of C-phycocyanin was achieved to 5.9. It should be noted that there is no need to completely remove the phosphate buffer at the bottom phase because this buffer is used to stabilize C-phycocyanin as a common media. The diagram for operating process is shown in Fig. 11.
Conclusion
In this research, the phase behaviors of ATPSs composed of two triblock copolymers and different salts/buffer were studied. Their binodal curves and tie lines were obtained experimentally. According to the results, the potassium phosphate buffer (pH = 6) showed higher potential for phase separation. However, Pluronic 10R5 was selected for C-phycocyanin purification due to its higher hydrophobicity compared to Pluronic L35, which caused the target protein to migrate unevenly to the salt-rich phase in the presence of 10R5 copolymer. The highest protein purification obtained for the ATPS based on Pluronic 10R5/potassium phosphate buffer was 4.29 in the salt-rich phase at pH = 6, VR = 1.6, TLL = 46.31%, and 35 °C. The isolation of components was investigated by an ultrafiltration step as well as a temperature increase step (above LCST) to enhance the purity index of C-phycocyanin up to 5.9 and 10R5 copolymer’s recovery up to 78%. Moreover, the result of CD spectroscopy conducted at the above conditions indicated no change in the structure of C-phycocyanin.
Data Availability
Data will be made available on request.
References
Ahsaie, F. G., & Pazuki, G. (2021). Separation of phenyl acetic acid and 6-aminopenicillanic acid applying aqueous two-phase systems based on copolymers and salts. Scientific Reports, 11(1), 1–10. https://doi.org/10.1038/s41598-021-82476-x
Ahsaie, F. G., Pazuki, G., Sintra, T. E., Carvalho, P., & Ventura, S. P. (2021). Study of the partition of sodium diclofenac and norfloxacin in aqueous two-phase systems based on copolymers and dextran. Fluid Phase Equilibria, 530, 112868. https://doi.org/10.1016/j.fluid.2020.112868
Atefi, E., Joshi, R., Mann, J. A., Jr., & Tavana, H. (2015). Interfacial tension effect on cell partition in aqueous two-phase systems. ACS Applied Materials & Interfaces, 7(38), 21305–21314. https://doi.org/10.1021/acsami.5b05757
Baghbanbashi, M., Pazuki, G., & Khoee, S. (2022). One pot silica nanoparticle modification and doxorubicin encapsulation as pH-responsive nanocarriers, applying PEG/lysine aqueous two phase system. Journal of Molecular Liquids, 349, 118472. https://doi.org/10.1016/j.molliq.2022.118472
Bakshi, M. S., Sachar, S., Yoshimura, T., & Esumi, K. (2004). Association behavior of poly (ethylene oxide)–poly (propylene oxide)–poly (ethylene oxide) block copolymers with cationic surfactants in aqueous solution. Journal of Colloid and Interface Science, 278(1), 224–233. https://doi.org/10.1016/j.jcis.2004.05.025
Begum, H., Yusoff, F. M., Banerjee, S., Khatoon, H., & Shariff, M. (2016). Availability and utilization of pigments from microalgae. Critical Reviews in Food Science and Nutrition, 56(13), 2209–2222. https://doi.org/10.1080/10408398.2013.764841
Bhat, V. B., & Madyastha, K. (2001). Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: Protection against oxidative damage to DNA. Biochemical and Biophysical Research Communications, 285(2), 262–266. https://doi.org/10.1006/bbrc.2001.5195
Chaiklahan, R., Chirasuwan, N., & Bunnag, B. (2012). Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochemistry, 47(4), 659–664. https://doi.org/10.1016/j.procbio.2012.01.010
Chandler, E. (2021). Multi-stage aqueous two-phase extraction. University of Sheffield.
Chang, Y.-K., Show, P.-L., Lan, J.C.-W., Tsai, J.-C., & Huang, C.-R. (2018). Isolation of C-phycocyanin from Spirulina platensis microalga using ionic liquid based aqueous two-phase system. Bioresource Technology, 270, 320–327.
Chen, K.-H., Wang, S.S.-S., Show, P.-L., Hsu, S.-L., & Chang, Y.-K. (2019). Rapid and efficient recovery of C-phycocyanin from highly turbid Spirulina platensis algae using stirred fluidized bed ion exchange chromatography. Separation and Purification Technology, 209, 636–645.
Chethana, S., Nayak, C. A., Madhusudhan, M., & Raghavarao, K. (2015). Single step aqueous two-phase extraction for downstream processing of C-phycocyanin from Spirulina platensis. Journal of Food Science and Technology, 52, 2415–2421.
Chew, K. W., Chia, S. R., Krishnamoorthy, R., Tao, Y., Chu, D.-T., & Show, P. L. (2019). Liquid biphasic flotation for the purification of C-phycocyanin from Spirulina platensis microalga. Bioresource Technology, 288, 121519.
Chow, Y. H., Yap, Y. J., Anuar, M. S., Tejo, B. A., Ariff, A., Show, P. L., Ng, E.-P., & Ling, T. C. (2013). Interfacial partitioning behaviour of bovine serum albumin in polymer-salt aqueous two-phase system. Journal of Chromatography B, 934, 71–78. https://doi.org/10.1016/j.jchromb.2013.06.034
Chow, Y. H., Yap, Y. J., Tan, C. P., Anuar, M. S., Tejo, B. A., Show, P. L., Ariff, A. B., Ng, E.-P., & Ling, T. C. (2015). Characterization of bovine serum albumin partitioning behaviors in polymer-salt aqueous two-phase systems. Journal of Bioscience and Bioengineering, 120(1), 85–90.
Da Silva, M., Da Silva, L. M., Amim, J., Guimarães, R. O., & Martins, J. P. (2006). Liquid-liquid equilibrium of aqueous mixture of triblock copolymers L35 and F68 with Na2SO4, Li2SO4, or MgSO4. Journal of Chemical and Engineering Data, 51(6), 2260–2264. https://doi.org/10.1021/je0603401
Darani, S. F., Ahsaie, F. G., Pazuki, G., & Abdolrahimi, S. (2021). Aqueous two-phase systems based on thermo-separating copolymer for partitioning of doxorubicin. Journal of Molecular Liquids, 322, 114542.
de Amarante, M. C. A., Braga, A. R. C., Sala, L., Moraes, C. C., & Kalil, S. J. (2020). Design strategies for C-phycocyanin purification: Process influence on purity grade. Separation and Purification Technology, 252, 117453.
de Oliveira, M. C., de Abreu Filho, M. A. N., & de Alcântara Pessôa Filho, P. (2007). Phase equilibrium and protein partitioning in aqueous two-phase systems containing ammonium carbamate and block copolymers PEO–PPO–PEO. Biochemical Engineering Journal, 37(3), 311–318. https://doi.org/10.1016/j.bej.2007.05.010
Enriquez-Ochoa, D., Sánchez-Trasviña, C., Hernández-Sedas, B., Mayolo-Deloisa, K., Zavala, J., Rito-Palomares, M., & Valdez-García, J. E. (2020). Aqueous two-phase extraction of phenolic compounds from Sedum dendroideum with antioxidant activity and anti-proliferative properties against breast cancer cells. Separation and Purification Technology, 251, 117341. https://doi.org/10.1016/j.seppur.2020.117341
Favas, A. R. R. (2021). Anti-ageing potential of cyanobacteria: Effect on matrix metalloproteinases and oxidative stress.
Ferreira, L. A., Uversky, V. N., & Zaslavsky, B. Y. (2018). Phase equilibria, solvent properties, and protein partitioning in aqueous polyethylene glycol-600-trimethylamine N-oxide and polyethylene glycol-600-choline chloride two-phase systems. Journal of Chromatography A, 1535, 154–161.
Grilo, A. L., Raquel Aires-Barros, M., & Azevedo, A. M. (2016). Partitioning in aqueous two-phase systems: Fundamentals, applications and trends. Separation & Purification Reviews, 45(1), 68–80. https://doi.org/10.1080/15422119.2014.983128
Haraguchi, L. H., Mohamed, R. S., Loh, W., & Pessôa Filho, Pd. A. (2004). Phase equilibrium and insulin partitioning in aqueous two-phase systems containing block copolymers and potassium phosphate. Fluid Phase Equilibria, 215(1), 1–15. https://doi.org/10.1016/S0378-3812(03)00368-6
Hou, D., & Cao, X. (2014). Synthesis of two thermo-responsive copolymers forming recyclable aqueous two-phase systems and its application in cefprozil partition. Journal of Chromatography A, 1349, 30–36.
Huisman, J., Codd, G. A., Paerl, H. W., Ibelings, B. W., Verspagen, J. M., & Visser, P. M. (2018). Cyanobacterial blooms. Nature Reviews Microbiology, 16(8), 471–483.
Jamshidi, S., & Pazuki, G. (2018). Effect of hybrane hyperbranched polymer additive on partitioning of cephalexin antibiotic in aqueous biphasic systems. Journal of Molecular Liquids, 259, 48–54. https://doi.org/10.1016/j.molliq.2018.03.011
Kee, P. E., Yim, H. S., Kondo, A., Lan, J. C.-W., & Ng, H.S. (2022). Extractive fermentation of Kytococcus sedentarius TWHKC01 using the aqueous biphasic system for direct recovery of keratinase: ABS extractive fermentation for keratinase production and recovery. Journal of the Taiwan Institute of Chemical Engineers, 104232. https://doi.org/10.1016/j.jtice.2022.104232
Khan, Z., Bhadouria, P., & Bisen, P. (2005). Nutritional and therapeutic potential of Spirulina. Current Pharmaceutical Biotechnology, 6(5), 373–379. https://doi.org/10.2174/138920105774370607
Khayati, G., & Alizadeh, S. (2013). Extraction of lipase from Rhodotorula glutinis fermentation culture by aqueous two-phase partitioning. Fluid Phase Equilibria, 353, 132–134. https://doi.org/10.1016/j.fluid.2013.05.037
Kulshreshtha, A., Jarouliya, U., Bhadauriya, P., Prasad, G., & Bisen, P. (2008). Spirulina in health care management. Current Pharmaceutical Biotechnology, 9(5), 400–405.
Lauceri, R., Cavone, C., Chini Zittelli, G., Kamburska, L., Musazzi, S., & Torzillo, G. (2023). High purity grade phycocyanin recovery by decupling cell lysis from the pigment extraction: An innovative approach. Food and Bioprocess Technology, 16(1), 111–121.
Liao, G., Gao, B., Gao, Y., Yang, X., Cheng, X., & Ou, Y. (2016). Phycocyanin inhibits tumorigenic potential of pancreatic cancer cells: Role of apoptosis and autophagy. Scientific Reports, 6(1), 1–12.
Merchuk, J. C., Andrews, B. A., & Asenjo, J. A. (1998). Aqueous two-phase systems for protein separation: Studies on phase inversion. Journal of Chromatography B: Biomedical Sciences and Applications, 711(1–2), 285–293. https://doi.org/10.1016/S0378-4347(97)00594-X
Mittal, R., Sharma, R., & Raghavarao, K. (2019). Aqueous two-phase extraction of R-phycoerythrin from marine macro-algae. Gelidium Pusillum. Bioresource Technology, 280, 277–286.
Moraes, C. C., De Medeiros Burkert, J. F., & Kalil, S. J. (2010). C-phycocyanin extraction process for large-scale use. Journal of Food Biochemistry, 34, 133–148. https://doi.org/10.1111/j.1745-4514.2009.00317.x
Nandini, K., & Rastogi, N. (2011). Liquid–liquid extraction of lipase using aqueous two-phase system. Food and Bioprocess Technology, 4(2), 295–303.
Narayan, A., & Raghavarao, K. (2007). Extraction and purification of C-phycocyanin from Spirulina platensis employing aqueous two phase systems. International Journal of Food Engineering, 3(4). https://doi.org/10.2202/1556-3758.1105
Nascimento, S. S., Santos, V. S. V., Watanabe, E. O., & de Souza, F. J. (2020). Assessment of the purification of phycobiliproteins in cyanobacteria through aqueous two-phase systems with different proportions of PEG/salt. Food and Bioproducts Processing, 119, 345–349.
Nowruzi, B., Konur, O., & Anvar, S. A. A. (2022). The stability of the phycobiliproteins in the adverse environmental conditions relevant to the food storage. Food and Bioprocess Technology, 1–18
Patel, H. M., Rastogi, R. P., Trivedi, U., & Madamwar, D. (2018). Structural characterization and antioxidant potential of phycocyanin from the cyanobacterium Geitlerinema sp. H8DM. Algal Research, 32, 372–383. https://doi.org/10.1016/j.algal.2018.04.024
Patil, G., & Raghavarao, K. (2007). Aqueous two phase extraction for purification of C-phycocyanin. Biochemical Engineering Journal, 34(2), 156–164. https://doi.org/10.1016/j.bej.2006.11.026
Patil, G., Chethana, S., Sridevi, A., & Raghavarao, K. (2006). Method to obtain C-phycocyanin of high purity. Journal of Chromatography a, 1127(1–2), 76–81. https://doi.org/10.1016/j.chroma.2006.05.073
Pei, Y., Li, Z., Liu, L., & Wang, J. (2012). Partitioning behavior of amino acids in aqueous two-phase systems formed by imidazolium ionic liquid and dipotassium hydrogen phosphate. Journal of Chromatography A, 1231, 2–7.
Peng, Q., Li, Z., & Li, Y. (1994). Thermodynamics of potassium hydrogen phosphate-potassium dihydrogen phosphate-polyethylene glycol aqueous two-phase systems. Fluid Phase Equilibria, 95, 341–357. https://doi.org/10.1016/0378-3812(94)80078-2
Phong, W. N., Show, P. L., Chow, Y. H., & Ling, T. C. (2018). Recovery of biotechnological products using aqueous two phase systems. Journal of Bioscience and Bioengineering, 126(3), 273–281.
Pimentel, J. G., Bicalho, S. F., Gandolfi, O. R. R., Verissimo, L. A. A., de Sousa, C. S., Souza, E. A., Veloso, C. M., Fontan, Rd. C. I., Sampaio, V. S., & Bonomo, R. C. F. (2017). Evaluation of salting-out effect in the liquid–liquid equilibrium of aqueous two-phase systems composed of 2-propanol and Na2SO4/MgSO4 at different temperatures. Fluid Phase Equilibria, 450, 184–193. https://doi.org/10.1016/j.fluid.2017.08.001
Pitto-Barry, A., & Barry, N. P. (2014). Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polymer Chemistry, 5(10), 3291–3297.
Prandi, B., Di Massimo, M., Tedeschi, T., Rodríguez-Turienzo, L., & Rodríguez, Ó. (2022). Ultrasound and microwave-assisted extraction of proteins from coffee green beans: Effects of process variables on the protein integrity. Food and Bioprocess Technology, 15(12), 2712–2722.
Rito-Palomares, M., & Benavides, J. (2017). Aqueous two-phase systems for bioprocess development for the recovery of biological products. Springer.
Rito-Palomares, M., & Hernandez, M. (1998). Influence of system and process parameters on partitioning of cheese whey proteins in aqueous two-phase systems. Journal of Chromatography B: Biomedical Sciences and Applications, 711(1–2), 81–90. https://doi.org/10.1016/S0378-4347(98)00011-5
Saini, M. K., & Sanyal, S. N. (2015). Cell cycle regulation and apoptotic cell death in experimental colon carcinogenesis: Intervening with cyclooxygenase-2 inhibitors. Nutrition and Cancer, 67(4), 620–636.
Shiran, H. S., Baghbanbashi, M., Ahsaie, F. G., & Pazuki, G. (2020). Study of curcumin partitioning in polymer-salt aqueous two phase systems. Journal of Molecular Liquids, 303, 112629. https://doi.org/10.1016/j.molliq.2020.112629
Silvério, S. C., Rodríguez, O., Teixeira, J. A., & EnA, M. (2013). The effect of salts on the liquid–liquid phase equilibria of PEG600+ salt aqueous two-phase systems. Journal of Chemical & Engineering Data, 58(12), 3528–3535. https://doi.org/10.1021/je400825w
Sintra, T. E., Bagagem, S. S., Ahsaie, F. G., Fernandes, A., Martins, M., Macário, I. P., Pereira, J. L., Gonçalves, F. J., Pazuki, G., & Coutinho, J. A. (2021). Sequential recovery of C-phycocyanin and chlorophylls from Anabaena cylindrica. Separation and Purification Technology, 255, 117538.
Suarez Ruiz, C. A., Emmery, D. P., Wijffels, R. H., Eppink, M. H., & van den Berg, C. (2018). Selective and mild fractionation of microalgal proteins and pigments using aqueous two-phase systems. Journal of Chemical Technology & Biotechnology, 93(9), 2774–2783. https://doi.org/10.1002/jctb.5711
Taragjini, E., Ciardi, M., Musari, E., Villaró, S., Morillas-España, A., Alarcón, F. J., & Lafarga, T. (2022). Pilot-scale production of A. platensis: Protein isolation following an ultrasound-assisted strategy and assessment of techno-functional properties. Food and Bioprocess Technology, 15(6), 1299–1310
Vicente, F. A., Santos, J. H., Pereira, I. M., Gonçalves, C. V., Dias, A. C., Coutinho, J. A., & Ventura, S. P. (2019). Integration of aqueous (micellar) two-phase systems on the proteins separation. BMC Chemical Engineering, 1(1), 1–12. https://doi.org/10.1186/s42480-019-0004-x
Wang, L., Wang, Y., Qin, Y., Liu, B., & Zhou, Y. (2022). Extraction and determination of protein from edible oil using aqueous biphasic systems of ionic liquids and salts. Food and Bioprocess Technology, 15(1), 190–202.
Wang, Y., Li, Y., Han, J., Xia, J., Tang, X., Chen, T., & Ni, L. (2016). Cloudy behavior and equilibrium phase behavior of triblock copolymer L64+ salt+ water two-phase systems. Fluid Phase Equilibria, 409, 439–446. https://doi.org/10.1016/j.fluid.2015.10.046
Wang, Y., Mao, Y., Han, J., Liu, Y., & Yan, Y. (2010). Liquid− liquid equilibrium of potassium phosphate/potassium citrate/sodium citrate+ ethanol aqueous two-phase systems at (298.15 and 313.15) K and correlation. Journal of Chemical & Engineering Data, 55(12), 5621–5626. https://doi.org/10.1021/je100501f
Wu, J., Xu, Y., Dabros, T., & Hamza, H. (2005). Effect of EO and PO positions in nonionic surfactants on surfactant properties and demulsification performance. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 252(1), 79–85. https://doi.org/10.1016/j.colsurfa.2004.09.034
Zhao, X., Xie, X., & Yan, Y. (2011). Liquid–liquid equilibrium of aqueous two-phase systems containing poly (propylene glycol) and salt ((NH4) 2SO4, MgSO4, KCl, and KAc): Experiment and correlation. Thermochimica Acta, 516(1–2), 46–51. https://doi.org/10.1016/j.tca.2011.01.010
Funding
The study was financially supported by the Iran National Science Foundation (INSF) with grant number 98020043.
Author information
Authors and Affiliations
Contributions
Alireza Ebrahimi: conceptualization, methodology, investigation, formal analysis, writing–original draft. Gholamreza Pazuki: investigation, writing–review and editing, supervision. Mehrdad Mozaffarian: writing–review and editing, supervision. Farzaneh Ghazizadeh Ahsaie: methodology, investigation, writing–review and editing. Hamed Abedini: investigation, supervision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ebrahimi, A., Pazuki, G., Mozaffarian, M. et al. Separation and Purification of C-Phycocyanin from Spirulina platensis Using Aqueous Two-Phase Systems Based on Triblock Thermosensitive Copolymers. Food Bioprocess Technol 16, 2582–2597 (2023). https://doi.org/10.1007/s11947-023-03057-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03057-6