Abstract
This study investigated the technological properties of barley hydrocolloids to produce a kinetically stable plant-based non-dairy alternative milk. The impact of mechanical extraction assisted by electrolysis treatments (0, 5, and 15 V) on the barley protein content was examined. The protein-rich barley aqueous extract was used to emulsify canola oil enriched with vitamin D at different pH conditions (5, 5.6, 6, and 7) and temperatures (30 and 60 °C). The homogenization treatment performed at 60 °C allowed higher protein solubilization and better kinetic stabilization than the one obtained at 30 °C. This temperature probably promoted water retention in barley’s hydrocolloids and protein agglomeration, producing samples with larger Sauter mean diameters of ~ 14 µm and higher apparent viscosities from 13 to 16 mPa s. The rise in pH from 5 to 7 increased the electrostatic charges of the samples. In contrast, the pH decrease allowed a higher reduction in the interfacial tension between barley aqueous extract and canola oil up to 3.2 mN/m at pH 5. Despite these electrostatic and steric forces provided by the barley hydrocolloids, the beverage only showed better kinetic stability after the homogenization treatment at 60 °C. Therefore, the barley hydrocolloids acted as structuring agents in the production of barley-based non-dairy alternative milk, contributing to its kinetic stability by high internal phase Pickering emulsions.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The market for plant-based non-dairy alternative milk is growing due to the rise consumers who are lactose intolerant, allergic to milk proteins, or are turning to more plant-based lifestyles (Domke, 2018; Flom & Sicherer, 2019). More than 250 new plant-based products were added to the shelves in the US market in 2021 (Good Food Institute, 2021). Furthermore, the dairy alternative market is predicted to reach a value of US$ 47.1 billion in 2033, growing at an estimated 8.3% compound annual growth rate in 10 years from 2023 to 2033 (Dairy Alternatives Market, 2023).
Milk substitutes produced from plant raw materials have been highlighted since they can carry and deliver bioactive compounds, such as oil-soluble vitamins, ω-3 oils, carotenoids, curcuminoids, and polyphenols (Tan & McClements, 2021; Mekala et al., 2022). However, plant-based milk alternatives have shown lower kinetic stability due to their complex system formed by a colloidal system of large dispersed particles of proteins, fat globules, and starch granules (Silva et al., 2020). These solid particles are responsible for unwanted sensory characteristics such as the sandy, gritty, or chalky mouthfeel (Silva et al., 2020). Therefore, the current industrial challenge is to develop a stable product to be transported and stored for a long time without solid particle settling.
The manufacturing of plant-based non-dairy alternative milk includes the following steps of soaking, extraction, filtration, homogenization, and pasteurization (Bocker & Silva, 2022). The aqueous extraction stage is commonly carried out through wet milling using mechanical energy. However, the in-line coupling of other extraction techniques may be advantageous, allowing higher protein extraction yields. In this regard, electrolysis processes have efficiently assisted the extraction of proteins from different raw materials (Ekaette, 2020; Roselló-Soto et al., 2015; Sankaran, et al., 2018; Xiao & Zhou, 2020). These processes are performed by applying an electrical voltage through metal electrodes into the extraction media, generating ionic species, propagating shock waves, and causing cavitation bubbles (Sankaran, et al., 2018; Xiao et al., 2020). The electrical discharges, in its turn, have disrupted cell structures, releasing intracellular compounds like proteins for the liquid medium. Moreover, electrolysis voltage is one of the most significant variables for protein extraction (Ekaette, 2020).
After the aqueous extraction, separation processes such as filtration and sieving remove the plant solids from the suspensions. However, the plant extracts still present large insoluble particles that may favor kinetic instabilities (Aydar et al., 2020). Thus, plant-based emulsions similar to dairy colloidal system are prepared by adding oils, emulsifying and gelling agents to the aqueous plant extracts to overcome this challenge (McClements, 2020). Therefore, different plant-based ingredients have been studied for the kinetic stabilization of plant-based non-dairy alternative milk (Sarkar & Dickinson, 2020). Different processes x have also been carried out to improve the homogenization of the beverage, avoiding phase separation phenomena (Boukid et al., 2021; McClements, 2020; Sethi et al., 2016; Strieder et al., 2021). Recently, barley ingredients, such as barley flour, starch, β-glucans, hordeins, and antioxidants, have been studied and added to plant products to improve nutritional and kinetic stability (Boostani et al., 2020; Ekaette & Saldaña, 2021; Karp et al., 2019; Kaushik et al., 2017; Mishra et al., 2020). Barley grains contain glutelin that demonstrated superior oil-binding property and emulsifying stability; hordein that exhibited good foaming capacity; β-glucans that can act as emulsifiers and gelling agents; and starch that is a hydrocolloid that can stabilize emulsion systems by coating oil droplets with formed film, reducing interfacial tension (Karp et al., 2019; Kumari et al., 2022; Li et al., 2021; Wang et al., 2010). Recently, Strieder et al. (2022) studied the impact of pulsed high-pressure processing on the quality parameters of β-carotene enriched barley-based milk. However, there are no studies evidencing barley as a source of all the necessary ingredients to produce a kinetically stable beverage with added vegetable oils enriched with vitamin.
Among the homogenization process conditions, the pH strongly influences emulsion stability by affecting electrostatic and steric interactions in the continuous phase, thus promoting dispersion stability or droplet aggregation (Abdullah et al., 2020; Manassero et al., 2018). The temperature, on the other hand, can indirectly affect emulsions by altering the interfacial tension, adsorption of the emulsifier, and viscosity (Chen & Tao, 2005). Therefore, this study evaluated the barley hydrocolloids as emulsifying and stabilizing ingredients to produce barley-based non-dairy alternative milk by emulsifying canola oil enriched with vitamin D. First, the effect of the electrolysis process (5 and 15 V) in the extraction step to obtain an aqueous barley extract rich in proteins was examined. Then, the impact of the pH (5, 5.6, 6, and 7) and homogenization temperature (30 and 60 °C) on the stabilization mechanism, physicochemical and rheological properties, and kinetic stability of the barley-based milk was assessed.
Material and Methods
Barley and Barley Preparation
Whole barley grains were measured for their moisture, protein, and fat contents by gravimetric determination, Dumas’ combustion method, and the test tube measurement, respectively (Bradley, 2010; Gajzago & Czukor, 1993). The fat content was determined by extracting the crude fat from the ground barley grains with the addition of hexane. The samples were shaken with hexane and left in contact with the solvent for 24 h. After the extraction time, the sample was centrifuged, and the hexane part was separated and subsequently evaporated using a stream of nitrogen. Thus, we weighted the test tubes containing the crude fat and determined the content by calculating the mass difference. The protein content was quantified using Leco TruSpec® C/N Analyzer with a nitrogen conversion factor of 6.25 (Vo & Saldaña, 2023). The grains presented 4.3 ± 0.1 g/100 g, 13.69 ± 0.02 g/100 g (dried basis), and 1.4 ± 0.2 g/100 g (dried basis) of moisture, protein, and crude fat contents, respectively.
Barley grains were soaked in water at a ratio of 10% (25 g of barley for 250 mL of water) for 20 h at room temperature (24 ± 2 °C). After the soaking, the water was drained, and the hydrated barley grains were weighted then the barley hydration observed was 39.6 ± 0.2%. Subsequently after barley hydration, the operational procedures followed the flow diagram presented in Fig. 1.
Aqueous Extraction of Barley Hydrocolloids
Hydrated barley grains (72 g) were immediately placed in a Cuisinart ® Velocity Ultra 1 HP 1.5 L blender (Conair Corporation, Hong Kong, China), and 216 g of distilled water was added to the grains. The first extraction step was carried out for 2 min using the blender at the maximum nominal power of the equipment. This condition was selected based on preliminary tests, where extraction times of 2 or 3 min did not significantly (p value ≥ 0.05) affect the protein extraction yield. Moreover, a higher extraction time heated the system to more than 10 °C. The extract acquired in this first step was named as the control extract.
After that, this suspension was placed in a 400 mL glass beaker with a magnetic stirrer, and this recipient was positioned on top of magnetic stirrer equipment. Platinum rods (99.99% pure, 0.15 cm diameter × 15 cm length) from Surepure Chemetals (Florham Park, NJ, USA), considered as electrodes were fitted in the beaker through rubber hoses and held 2.5 cm apart on a plastic lid following the procedure described in detail by Ekaette and Saldaña (2021). The electrolysis chamber consisted of the plastic lid placed on the beaker while inserting the platinum rods into the slurry at rod lengths of 6 cm (Ekaette & Saldaña, 2021). The upper ends of the rods were connected to a direct current supply. The studied voltages were 5 and 15 V based on previous results obtained in our laboratory (Ekaette, 2020). Thus, the electrolysis extraction was performed in an open electrolysis chamber at constant stirring (9600 rpm) for 30 min (Ekaette & Saldaña, 2021). After each extraction, the extract was separated from the solids by sieving using the 75 µm diameter size for 2 min. Temperature and pH values were measured immediately before and after electrolysis extraction as process control. The pH measurements were performed using a pH and conductivity meter (Accumet, Fisher Scientific, Ottawa, ON, Canada) at 30 ± 1 °C. The extraction experiments were performed in triplicate.
Aqueous Barley Extract Characterization
The extraction yield was determined using Eq. 1. The aqueous barley extracts were characterized according to their solid and protein contents and visual appearance. The solid and protein contents were determined in the same way as for barley grains as described in the “Barley and Barley Preparation” section.
Homogenization Process
The aqueous barley extract with the highest protein content was used in the following stages of the study. Thus, the barley extract was acquired from four batches of mechanical extraction followed by electrolysis at 15 V, according to the experimental procedure described in the “Aqueous Extraction of Barley Hydrocolloids” section. After the extractions, the aqueous extracts were homogenized and separated into four portions. The emulsions were prepared with barley extract at different pH (5.6, 5, 6, and 7) and 0.5 g/100 g of canola oil enriched with vitamin D. The pH adjustment of the aqueous barley extract was carried out adding droplets of 0.5 M of NaOH or HCl solutions under constant magnetically stirring. The oil with vitamin was prepared by the addition of 3.75 mg of cholecalciferol (≥ 98%, Sigma) in 100 g of canola oil (Compliments, Mississauga, Canada). The complete dissolution of the vitamin in the oil was promoted by magnetic homogenization at 600 rpm overnight. Falcon tubes of 50 mL were used to process 30 g of the sample prepared with 0.15 g of oil enriched with vitamin D and 29.85 g of barley extract. An Ultra Turrax® homogenizer (Heidolph Diax900, Schwabach, Germany) coupled with a water bath was used to process the samples. The emulsification processes were performed at 21,500 rpm for 10 min. Thus, the pH (5, 5.6, 6, and 7) and bath temperature (30 and 60 °C) were evaluated on the barley-based non-dairy alternative milk characteristics. Preliminary tests were carried out to select the temperature levels evaluated. The initial proposal was to study temperatures of 30, 60, and 90 °C. However, samples with a cream appearance were obtained with a homogenization temperature of 90 °C. The pH values were selected considering those that can be reached by adding ingredients to a plant-based milk.
Characterization of Barley-Based Non-dairy Alternative Milk
The effects of the pH and homogenization temperature on the characteristics of the barley-based non-dairy alternative milk were analyzed according to its soluble protein content, dynamic interfacial tension, zeta (ζ)-potential, droplet size distribution, microstructure, rheological properties, creaming stability, suspension rate, and color parameters.
Soluble Proteins
The soluble proteins were quantified according to the Lowry method. Immediately after processing, 1.5 mL of each sample was transferred to a 2-mL Eppendorf tube. The tubes were centrifuged for 15 min at 13,000 rpm, and the supernatant was separated and filtered using Whatman filter paper 1 (25 mm). The samples were diluted twice and then proceeded with the described methodology according to Waterborg (2009) Vo and Saldaña (2023). A calibration curve (R2 = 0.99) using a protein standard from 50 to 2000 µg/mL was used to quantify the protein content.
Stabilization Mechanism
The effects of the pH on the kinetic stabilization mechanism of the barley-based emulsions were analyzed from the dynamic interfacial tension and surface charges. The homogenization temperature effects were also evaluated on the surface charges. The measurement of the dynamic interfacial tension between the canola oil and the barley aqueous extract was performed by the pendant drop method using a Theta optical tensiometer (Attension Theta, Biolin Scientifc Inc., Gothenburg, Sweden). Approximately 4 mL of sample diluted 20 × in deionized water was placed in a cuvette mounted onto a bench. The oil droplet (volume is ~ 10 µL) was deposited on the suspension by a J-shaped hook brought into focus to estimate the interfacial tension. The inverted droplet images were recorded at 12 fps for 10 min at room temperature (24 °C). The interfacial tension was determined by digitizing the drop and fit to the Young–Laplace equation (Eq. 2).
where, γ is the interfacial tension, \(\Delta \rho\) is the density difference between the fluids (canola oil and water), g is the gravitational acceleration constant, \({R}_{O}\) is the radius of drop curvature at the apex, and β is the shape factor. The oil droplet was injected into a syringe and released into the cuvette containing diluted barley extract at different pH values (5, 5.6, 6, and 7).
The surface charges of the barley-based milk emulsion were determined by measuring the ζ-potential using a Zetasizer Nano-ZS instrument (Malvern Instruments Ltd., Worcestershire, UK). The samples were diluted to 1.0% (v/v) in deionized water for the measurements and performed in duplicate.
Droplet Size Distribution
The droplet size distribution of the samples was measured using the laser diffraction analyzer (Mastersizer 2000, Malvern Instruments, Worcestershire, UK). The samples were dispersed in distilled water to obtain laser obscuration of 15% at a refractive index of 1.52 and pump speed at 2250 rpm. The Sauter mean diameter (\({D}_{\mathrm{3,2}}\)) was determined according to Eq. 3.
where \({d}_{i}\) is the mean diameter of the droplets and \({n}_{i}\) is the number of droplets.
Microstructure
The sample microstructures were observed using a Leica DM RXA optical microscope (Leica microsystems, Concord, ON, Canada). The images were captured by the QI click camera using immersion oil with 40 × and 100 × objective lenses. We took images of the different phases formed in the samples produced at 30 °C. After that, we homogenized the emulsions to observe the microstructures of the mixture.
Rheological Properties
The viscosity and rheological profile of the barley-based milk emulsions were measured using a rheometer (Discovery HR-1, TA Instruments, Mississauga, ON, Canada) with DIN rotor type probe with radius of 14 mm (Peltier Steel – 106,351) as described earlier by Mekala et al. (2021). Approximately 25 mL of the emulsion was placed in the cylindrical tube, and the spindle was lowered, maintaining a gap of 1500 μm. Flow curves (Shear rate: 0–300/s; 300–0/s; and 0–300/s) were obtained using an up-down-up program to eliminate the thixotropic behavior. The third flow curve data were fitted to the model for shear-thinning fluids which follows the power-law model according to Eq. 4. The parameters k and n were estimated by non-linear regression using the Quasi-Newton method using the Minitab 18® software (Minitab Inc., State College, PA, USA). The model fit was evaluated based on the coefficient of determination (R2) and the mean relative percentage deviation modulus (E), according to Eq. 5.
where σ is the shear stress (Pa), \(\dot{\gamma }\) is the shear rate (s−1), k is the consistency index (Pa sn), n is the flow behavior index (dimensionless), mi is the experimental value, mp is the predicted value, and N is the population of the experimental data.
Kinetic Stability
The kinetic stability of the samples was measured through creaming stability by visual appearance and suspension rate. The creaming stability was visually observed in samples (15 mL), placed in a bottle with a lid, and photographed right after processing (0 h) and after 24 h of cold storage (6 ± 2 °C). The suspension rate was determined using a 250-mL cylinder tube following the China national standard GB/T14825-2006, according to Mekala et al. (2021).
Color
The color parameters of the beverage were measured in 10 mL of sample in a white container (65 mm in diameter) using a Hunter Lab colorimeter (CR-400, Konica Minolta, Ramsey, NJ, USA). The results were expressed in the values of L (luminosity), a * (red-green), and b * (blue-yellow).
Statistical analysis
The effects of the pH and homogenization temperature on the barley-based non-dairy alternative milk characteristics were verified by analysis of variance (ANOVA) using the Minitab 18® software with a 95% confidence level (p value ≤ 0.05). Tukey’s test of means was performed.
Results and Discussion
Extraction Results
The barley-based non-dairy alternative milk was produced using the barley aqueous extract obtained through mechanical extraction assisted by electrolysis. The effect of electrolysis voltage on the recovery of barley proteins to obtain an extract rich in protein was examined. Therefore, two different voltages were studied to obtain the barley suspension. The temperatures before and after electrolysis were 31 ± 1 °C and 29 ± 1 °C, respectively. Thereby, non-thermal extraction processes were carried out.
Figure 2 presents the effect of electrolysis voltage on extraction yield, barley solid content, protein extraction, and pH value. The electrolysis voltage did not affect the total solid content (p value = 0.2). However, the processing employing 15 V promoted the highest extraction of proteins compared to the control. Sankaran et al. (2018) also observed an increase in microalgae protein extraction increasing the electrolysis voltage from 5 to 20 V as higher voltages promoted better cell disruption of microalgae, enhancing the extraction process by releasing more protein from the plant cells to the system. Our results showed that the electrolysis voltages evaluated did not significantly affect the pH value (p value = 0.17) of the aqueous extracts and the extraction yield (p value = 0.08) (Fig. 2). All processes presented an extraction yield of 51 ± 1 gextract/100 graw material and produced an extract with pH 5.6 ± 0.1.
The electrolysis voltage did not affect the barley extract visual appearance (Fig. 3). Immediately after extraction, the samples visually showed good homogeneity. However, they presented visual phase separation after 1 h of storage at room temperature of 24 ± 1 °C. This kinetic instability is a major problem of plant milk alternatives since they are colloidal systems formed by large-sized dispersed particles such as protein and starch granules (Wu & Inglett, 1974). Thus, we selected the experimental procedure that produced the extract with the highest protein content to proceed with the homogenization stage of the study.
Homogenization Results
The temperature profile of each homogenization treatment is presented in Fig. S1 in the Supporting Information. The initial temperature of homogenization of the samples was standardized at 5 ± 2 °C (refrigerator temperature). The processing time of 10 min started when we turned on the homogenizer after coupling the sample into the water bath system at 30 or 60 °C. Thus, the samples reached 30 and 60 °C after 4.5 and 7 min of homogenization, respectively. Furthermore, the maximum temperatures reached by the samples were 33 ± 1 and 62 ± 1 °C, respectively.
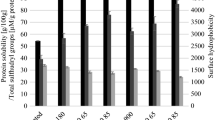
Protein Solubility
Table 1 presents the effects of the pH (5, 5.6, 6, and 7) and the homogenization temperature (30 and 60 °C) on soluble protein content and ζ-potential of the barley-based milk.
Homogenization treatments at 60 °C promoted higher protein solubilization than treatments at 30 °C, regardless of the sample pH value (Table 1). The treatments performed at 30 °C maintained the same soluble protein content as the extract. Therefore, the heat applied at 60 °C combined with the homogenization treatment was essential to increase the protein solubility. Yu et al. (2022) also observed an increase from about 0.2 to 0.7 mg/mL in the soluble protein content of their high-denatured peanut protein samples treated by shearing at 12,000 rpm for 10 min. They attributed this result to the homogenization treatment which combined strong shear force, cavitation effect, and collision, resulting in the depolymerization, fragmentation, and separation of the protein aggregates. Then, the molecular structure of the protein might be unfolded and exposed to more polar and non-polar groups in aqueous solutions, increasing the interaction of the hydrophilic group with water molecules. However, they did not consider the effects of heat on their results but reported that the treatments evaluated reached up to 60 °C. An increase in the soluble protein content was also observed by Krentz et al. (2022) for samples at 90:10 and 50:50 w/w of casein micelle:pea protein blend after homogenization and pasteurization treatment at 60 °C for 30 min. According to them, the process of homogenization disrupted the 3-dimensional globular protein bodies into smaller, more soluble aggregates suspended in the aqueous phase. Despite the heat probably contributing to the results achieved, they also did not consider the effect of pasteurization on their results. The heat provided by the treatments can favor the diffusion, interfacial tension reduction, and homogenization, favoring the solubilization of proteins (Chen and Tao 2005). The pH of the extracts only affected the soluble protein content in the sample produced at pH 7 and 60 °C (Table 1). This lower soluble protein content may be associated with the lowest medium protonation provided by pH 7.
Stabilization Mechanism
An emulsion can be stabilized, depending on the surface characteristics of the material by electrostatic or steric stabilization (Robins, 2000). Thus, we evaluated the dynamic interfacial tension and the surface charges of the samples. Figure 4 presents the pH effects on the interfacial tension between the canola oil enriched with vitamin D and the barley extracts. The contact between oil and water presented the highest value of interfacial tension up to 19.8 mN/m at 10 min of contact, as expected, considering the immiscibility of these two fluids. Therefore, barley components acted as emulsifying agents, reducing the interfacial tension between the water and oil. The interfacial tension between the extracts and the oil at different pH values decreased with time but at different rates. The pH value of 5 resulted in the lowest value of interfacial tension (3.2 mN/m at 10 min of contact). Moreover, the barley extract compounds were quickly adsorbed in the oil/water interface, reducing the interfacial tension. The equilibrium was also rapidly attained, indicating a rapid saturation of the adsorption surface (Silva et al., 2015). We assumed that the pH reduction affected the microenvironment of the samples, influencing the emulsifying action of the barley hydrocolloids. Therefore, barley proteins at pH 5 might have presented a better surface activity to act as emulsifiers in our plant-based milk.
Furthermore, all samples presented negative charges measured by the ζ-potential (Table 1). These negative charges can be associated with the barley proteins and starch, which present negative charges in aqueous solutions (Boostani et al., 2020; Kumari et al., 2022). On the other hand, β-glucan’s ζ-potential is slightly negative, but very close to zero (Sarantis et al., 2021). We observed a similar pH effect on the ζ-potential of the samples at the two homogenization temperatures. Extracts with low pH of 5 and 5.6 produced barley-based milk samples with low surface charges. Boostani et al. (2020) observed a different behavior for their Pickering emulsions produced with barley hordein and canola. Their samples produced at pH 6 and 7 presented charges around 0 mV, while the sample produced at pH 5 had a charge value higher than 10 mV. They attributed the lower charges (0 mV) to the protein isoelectric point. However, they evaluated purified barley hordein, which presents an isoelectric point between pH 6 and 7. But our samples are complex with unknown mixture of barley proteins (albumin, globulin, gliadin (hordein), and glutelin), starch, β-glucan, and other components extracted from barley (Bishop, 1928; Henry, 1988). Gumus et al. (2017) observed similar ζ-potential results for their emulsions produced from lentil, pea, and faba bean protein concentrates and ω-3 microalgae oil. The coarse 10:20 w/w oil-in-water emulsions prepared using a high-shear mixer for 2 min at 10,000 rpm and a high-pressure homogenizer operating at 68.9 kPa showed particles with lower charges at a pH value of 5. The protein concentrates evaluated by them presented more globulins and albumins that had isoelectric points of 4.5 and 6, respectively. Our barley-based milk may have these protein groups, and we also verified lower ζ-potential values for the samples produced at lower pH values. Şirin and Yalçın (2019) observed that their hull-less barley protein concentrates had an isoelectric point close to 6. Therefore, the studied pHs were close to the isoelectric point of the barley proteins. Moreover, we observed higher negative charges for samples treated at 30 °C than at 60 °C. This difference can be attributed to the effect of heat on barley hydrocolloids.
The 30 °C and pH values of 6 and 7 favored the barley-based milk stabilization by electrostatic forces. On the other hand, the pH 5 modified the microenvironment of the extract, promoting a better action of barley’s hydrocolloids as emulsifying agents, improving stabilization by steric forces. The equipment dis not allow us to assess the temperature effects on reducing the interfacial tension between the aqueous and oil phases. However, we observed that the proteins better solubilized at 60 °C (Table 1), which may favor their action as an emulsifying agent in the samples treated at this temperature.
Mean Droplet Diameter and Microstructure
Table 2 presents the pH and temperature effects on the mean size diameter (\({D}_{\mathrm{3,2}}\)), rheological properties, and suspension rate of the barley-based milk. The higher homogenization temperature (60 °C) produced droplets of a larger mean diameter (\({D}_{\mathrm{3,2}}\)). The heat provided by this treatment promoted the solubilization of proteins and may have promoted the particles’ agglomeration. Figure 5 presents the microstructures and the droplet size distribution of the extracts and the barley-based milk acquired at different pH values and homogenization temperatures. The microstructures of the samples were similar to those acquired for barley starch by Kumari et al. (2022). According to them, the onset, peak, and conclusion temperatures of barley starch gelatinization were at 58.76, 62.74, and 69.72 °C, respectively. Thus, the larger diameter droplets observed for samples processed at 60 °C may be related to onset starch gelatinization since the water retaining starch molecules have larger diameters. Moreover, Berggren (2018) observed an increase in β-glucan adsorption of water, increasing the processing temperature to 95 °C. Thus, the adsorption of water by these hydrocolloids at 60 °C may have generated the largest sample droplets diameters. Previous studies performed by our research group have shown similar behavior. Strieder et al. (2021) observed higher mean Sauter diameters for plant-based colorants produced from oat, rice, peanut, and almond aqueous extract after a thermosonication treatment associating ultrasound and heat at 70 °C. The thermosonicated oat colorant presented a higher \({D}_{\mathrm{3,2}}\) and lower span value than the aqueous oat extract. Then, the oil droplets in the aqueous plant-based extracts were reduced by acoustic cavitation but the water-retaining starch molecules had larger diameters increasing the \({D}_{\mathrm{3,2}}\) value.
The samples treated at 30 °C showed larger droplets than the extract, except for the acquired at the pH value of 7 (Table 2 and Fig. 5). In this case, the particle’s higher mean diameter may be associated with particle agglomeration. Oliete et al. (2019) observed that combining shear, turbulence, and collisions by microfluidization during emulsion homogenization increased the medium temperature, probably causing an association of protein molecules. Thus, forming high-sized aggregated structures in their pea globulin-based emulsions prepared at different homogenization pressures (50, 70, and 130 MPa). Silva et al. (2016) also observed an increase in the mean Sauter diameter of their oil-in-water emulsions stabilized with modified starch after a process intensification using acoustic energy. According to them, a re-coalescence phenomenon occurred due to the “overprocessing,” resulting in higher \({D}_{\mathrm{3,2}}\) value.
The pH of the samples treated at 30 °C affected the mean diameter of the particles in different ways. The treatments at the pH 7 produced droplets with smaller diameters, while pH 6 produced intermediate mean diameters, and pH 5.6 and 5 produced the highest mean diameters. These results may be attributed to the effects of pH on barley hydrocolloids protonation. According to Dai et al. (2020), the proteins tend to form agglomerates due to the lowest repulsion charges in the isoelectric point values. However, all our samples were prepared in a pH value close to the isoelectric point (pI = 6) of barley proteins (Şirin & Yalçın, 2019). Nevertheless, we observed higher repulsion charges measured by ζ-potential in samples produced at pH values of 7 and 6 (Table 1). Thus, the higher pH values (6 and 7) probably promoted greater repulsion of the molecules, avoiding agglomeration and thus producing droplets of smaller mean diameter. Therefore, homogenization treatments did not reduce the particles’ size but may have promoted water retention in molecules and agglomeration.
Furthermore, samples processed at 30 °C showed a less homogeneous size distribution than those obtained at 60 °C. This behavior was observed in the particle size distribution presented in Fig. 5. Thus, the treatment at 60 °C promoted a better homogeneity in size distribution, increasing droplet size diameter; the same was observed by Strieder et al. (2021).
Rheological Properties
The larger droplet diameters of the samples obtained at 60 °C also provided a higher apparent viscosity of 98 s−1 to the beverage (Table 2). This higher viscosity, as previously discussed, may be associated with water retention in molecules and protein agglomeration, considering the isoelectric points of the barley proteins (Boostani et al., 2020). Otherwise, although the samples obtained at 30 °C had larger droplet diameters than the extract, they did not show higher apparent viscosities. Thus, we can infer that the viscosity increased in samples processed at 60 °C due to water retention by barley hydrocolloids at this temperature (Kumari et al., 2022).
The parameters obtained by the regression using the power-law model (Eq. 2) were also reported in Table 2. This model has been used to demonstrate the fluid viscosity behavior by observing the shear rate promoted by shear stress (Silva et al., 2015). We observed a good adjustment of the experimental values to the model through the coefficient of determination (R2) and the mean relative percentage deviation modulus (E). The values of R2 were very close to 1, indicating a good correlation between predicted and observed values. The consistency index (k) indicates the viscosity of a fluid by relating the shear stress required to cause a shear rate of 1 s−1. Thus, higher values of k indicate more ticker fluids. Therefore, samples produced at 30 °C are thicker than the barley extract since they presented higher k values. Additionally, the pH of the samples did not affect this index at 30 °C. However, the k index observed for the samples processed at 60 °C presented a different behavior than the observed at 30 °C. The barley extract and samples processed at 60 °C and pH values of 6 and 7 showed statistically the same and lowest values for k. This result can also be observed in the flow curves presented in Fig. 6. At low shear rates, the profile of the samples obtained at 60 °C and pH 6 and 7 showed a profile more similar to the extract. This result may be associated with the repulsive charges observed in Table 1. Lower repulsive charges were observed at pH values of 5 and 5.6. Thus, the barley molecules may have agglomerated more, promoting the higher viscosity demonstrated by the k parameter. On the other hand, the barley molecules had higher repulsive charges at pH values of 6 and 7. Therefore, fewer agglomerations of proteins can occur, generating lower viscosity values.
The n, otherwise, indicates the type of fluid: n = 1 indicates a Newtonian, n > 1 Dilatant, and 0 < n < 1 a Pseudoplastic fluid. Our samples presented a pseudoplastic behavior since all n values were lower than 1. The same behavior was observed in the flow curves presented in Fig. 6. All the samples presented a shear-thinning behavior, characteristic of pseudoplastic fluids. We observed a decrease in the viscosity of the samples by increasing the shear rate. The samples obtained at 30 °C showed the same n and flow behavior as the barley extract regardless of the evaluated pH value. However, the samples prepared at 60 °C had higher n values and different flow curves than the other samples. The heat provided at 60 °C probably was responsible for these rheological differences as our samples are complex mixtures of proteins and polysaccharides such as starch and β-glucan. As previously discussed, the heat may have promoted the water retention by barley hydrocolloids and their agglomeration, promoting the increase in the apparent viscosity of the beverage. The rheological characteristics observed in our samples were similar to others reported by plant-based milk (Silva et al., 2019). Furthermore, pseudoplastic fluids are industrially advantageous as less energy is necessary to transport them through tubes and pipes.
Kinetic Stability
The kinetic stability of plant-based milk alternatives produced by oil in water emulsions is an important parameter to be evaluated, considering the number of insoluble particles present in these products. Figure 7 presents the visual appearance of the samples right after the homogenization treatments (0 h) and after 24 h of cold storage at 5 ± 1 °C. The homogenization treatments performed at 60 °C visually improved the kinetic stability of the barley-based milk, regardless of the pH value. Small phase separation can be observed at the top of the tube after 24 h of cold storage. However, the treatments at 30 °C did not visually improve the kinetic stability of the beverage. Therefore, when heated at 60 °C, the barley hydrocolloids may have acted as Pickering stabilizers.
The same behavior was observed for the suspension rate values presented in Table 2. This value indicated the kinetic stability of samples considering the particle’s sedimentation rate in a diluted medium. Therefore, high rates indicated lower sedimentation and better kinetic stability of the sample. Thus, the heat provided at 60 °C was essential to promote higher kinetic stabilization of the barley-based milk. Besides the temperature, the pH value also affected the kinetic stability of the sample. The pH values of 5 and 5.6 allowed the production of more kinetically stable beverages at 60 °C, according to the suspension rate. The pH 5 promoted the lower interfacial tension between the water and oil phase (Fig. 4), probably favoring the emulsification and kinetic stabilization of the beverage. The samples processed at 30 °C presented lower kinetic stability than the barley extract. Thus, once again, we observed that the energy provided by processing at 30 °C was not enough to promote a good kinetic stabilization of the samples.
We believe that barley hydrocolloids homogenized at 60 °C acted as Pickering stabilizers, producing a stable kinetic emulsion. According to Xia et al. (2021), Pickering emulsions were stabilized by insoluble solid particles that localize at the interface between oil and water, reducing the surface of this high-energy interface. The solids adsorption at the interface occurred through partial wetting by both phases, forming an obstacle to limiting the merging (coalescence) between droplets (Gonzalez Ortiz et al., 2020). These solids, named Pickering stabilizers, are proteins; modified proteins; complex particles formed by protein–polysaccharide, polysaccharide–polysaccharide, protein–protein, protein–polyphenol, polysaccharide–polyphenol, and ternary (protein–polyphenol–polysaccharide); flavonoid; food-grade wax; and fat crystals (Xia et al., 2021). Therefore, we believe that the homogenization at 60 °C modified the barley hydrocolloids, allowing the formation of Pickering stabilizers that produced kinetic stable emulsions in our systems.
After oil-in-water emulsion production, different phenomena of phase separation can occur due to the interactions of the particles. Thus, to better understand these phenomena, Fig. 8 presents the microstructures of the particles present in each phase formed in the barley extract and beverage samples obtained at 30 °C. Larger particles were observed in the bottom part of the samples, while small ones can be observed in the top part. These indicate particles can aggregate by coalescence and flocculation that form sediments and creaming layer. The sedimentation and creaming phenomenon were also visually observed by the turbidity at the top and bottom parts of samples obtained at 30 °C (Fig. 7). Besides that, samples obtained at pH 6 and 7 presented smaller particles on the top part than those produced at pH values of 5 and 5.6. These larger particles in the top part can indicate creaming phenomena, which can be associated with the protein agglomerations.
We conclude that only the electrostatic and steric forces provided by barley hydrocolloids were not enough to promote good emulsification and kinetic stabilization of the plant-based milk. Therefore, the energy supplied through homogenization at 60 °C favored the kinetic stabilization, by allowing Pickering emulsification. Then, the barley hydrocolloids acted as nonadsorbing “structuring agents” and promoted “high internal phase Pickering emulsions” (Sarkar & Dickinson, 2020). In this type of emulsion, the hydrocolloids promoted changes in the viscosity of the system, improving the emulsion stability (Silva et al., 2015). The steric stabilization is more stable to pH variations than the electrostatic forces (Ozturk & McClements, 2016). Because of that, we could not observe the visual pH effect on the kinetic stability of the samples treated at 60 °C (Table 2 and Fig. 7).
Color
Table 3 presents the extract pH and the homogenization temperature effects on the color parameters of the barley-based milk. The samples prepared at 60 °C had darker color (lower L values) than the barley extract and samples prepared at 30 °C. The heat provided by the 60 °C treatment may have favored browning reactions by the Maillard reaction since our samples contain proteins and probably reducing sugars, as observed earlier by Lazaridou et al. (2008). Da Silva et al. (2023) also observed darker (lower L values) rice plant-based milk beverages acquired after pasteurization due to browning reactions. Moreover, the samples produced at 60 °C had the most reddish color (higher a* values), and all the beverage samples had a less yellow color (lower b* values) than the barley extract. These color changes can be attributed to the addition of canola oil and homogenization treatments. Variations in the color parameters a* and b* were also observed by Da Silva et al. (2023) for rice-based milk beverages. The color change was related to the degradation of compounds in the raw material. Sivabalan and Sablani (2022) observed a remarkable decline in red (a*) and yellow (b*) colors parameters of their chitosan emulsions loaded with β-carotene in the oil phase after thermal treatment at 121 °C for 1 h. They attributed this difference to β-carotene degradation. We did not observe major differences among the samples due to the lower temperature and processing time employed.
Conclusion
Our results have demonstrated that the barley hydrocolloids were promising as emulsifiers and stabilizers for the production of kinetically stable non-dairy alternative milk. The electrolysis step applying 15 V allowed a higher yield, producing an aqueous barley extract richer in proteins (2.86 g/200 g liquid extract). Although beverages produced at 30 °C had higher electrostatic repulsion forces and lower pH values allowed the reduction of the interfacial tension between the extract and the oil, these forces were not sufficient to promote a beverage with good kinetic stability. On the other hand, the treatment at 60 °C promoted greater kinetic stability of the beverage. Thus, we believe that barley hydrocolloids acted as structuring agents, allowing greater kinetic stability and emulsification by high internal phase Pickering emulsions. However, these macromolecules need higher thermal energy to promote their technological functions. Therefore, we suggest the performance of subsequent steps to reduce the mean diameter of particles, promoting finer emulsions and better kinetic stability to the beverage. Overall, this study demonstrated the possibility of using barley more integrally, supplying ingredients to produce a plant-based non-dairy alternative milk.
Data Availability
The data are available from the corresponding author upon suitable request.
References
Abdullah, Weiss, J., Ahmad, T., Zhang, C., & Zhang, H. (2020). A review of recent progress on high internal-phase Pickering emulsions in food science. Trends in Food Science & Technology, 106, 91–103. https://doi.org/10.1016/j.tifs.2020.10.016
Aydar, E. F., Tutuncu, S., & Ozcelik, B. (2020). Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects. Journal of Functional Foods, 70. https://doi.org/10.1016/j.jff.2020.103975
Berggren, S. (2018). Water holding capacity and viscosity of ingredients from oats: The effect of beta-glucan and starch content, particle size, pH and temperature. Bachelor thesis (Chemistry), Linnaeus University Sweden, Sweden.
Bishop, L. (1928). First report on barley proteins: The composition and quantitative estimation of barley proteins. Journal of the Institute of Brewing, 34(2), 101–118.
Bocker, R., & Silva, E. K. (2022). Innovative technologies for manufacturing plant-based non-dairy alternative milk and their impact on nutritional, sensory and safety aspects. Future Foods, 5, 100098. https://doi.org/10.1016/j.fufo.2021.100098
Boostani, S., Hosseini, S. M. H., Golmakani, M. -T., Marefati, A., Hadi, N. B. A., & Rayner, M. (2020). The influence of emulsion parameters on physical stability and rheological properties of Pickering emulsions stabilized by hordein nanoparticles. Food Hydrocolloids, 101, 105520. https://doi.org/10.1016/j.foodhyd.2019.105520
Boukid, F., Rosell, C. M., & Castellari, M. (2021). Pea protein ingredients: A mainstream ingredient to (re)formulate innovative foods and beverages. Trends in Food Science & Technology, 110, 729–742. https://doi.org/10.1016/j.tifs.2021.02.040
Bradley, R. L. (2010). Moisture and total solids analysis. In Food analysis (pp. 85–104): Springer. https://doi.org/10.1016/j.fuproc.2004.03.010
Chen, G., & Tao, D. (2005). An experimental study of stability of oil–water emulsion. Fuel Processing Technology, 86(5), 499–508. https://doi.org/10.1016/j.fuproc.2004.03.010
Da Silva, L. R., Velasco, J. I., & Fakhouri, F. M. (2023). Use of rice on the development of plant-based milk with antioxidant properties: From raw material to residue. LWT, 173. https://doi.org/10.1016/j.lwt.2022.114271
Dai, T., Li, T., Li, R., Zhou, H., Liu, C., Chen, J., & McClements, D. J. (2020). Utilization of plant-based protein-polyphenol complexes to form and stabilize emulsions: Pea proteins and grape seed proanthocyanidins. Food Chemistry, 329, 127219. https://doi.org/10.1016/j.foodchem.2020.127219
Dairy Alternatives Market. (2023). Dairy alternatives market outlook. [Internet]. Available from: https://www.futuremarketinsights.com/reports/dairy-alternatives-market. Accessed 13 January 2023.
Domke, F. (2018). Vegetarian and vegan products-labelling and definitions. European Food and Feed Law Review, 13(2), 102–107. https://www.jstor.org/stable/90021310
Ekaette, I. U. (2020). Rutin and barley starch modification using subcritical water, ultrasonication and electrolysis technologies. PhD thesis (Bioresource and Food Engineering), 263 pp, Department of Agricultural, Food, and Nutritional Science, University of Alberta, Canada.
Ekaette, I. U., & Saldaña, M. D. A. (2021). The effect of rutin on starch hydrogels/aerogels made from electrolyzed barley flour. Starch, 73, 2000099. https://doi.org/10.1002/star.202000099
Flom, J. D., & Sicherer, S. H. (2019). Epidemiology of cow’s milk allergy. Nutrients, 11(5), 1051. https://doi.org/10.3390/nu11051051
Gajzago, I., & Czukor, B. (1993). Rapid protein determination with LECO FP-428 protein detector. Elelmezesi Ipar (hungary), 47(8), 229–232.
Gonzalez Ortiz, D., Pochat-Bohatier, C., Cambedouzou, J., Bechelany, M., & Miele, P. (2020). Current trends in Pickering emulsions: Particle morphology and applications. Engineering, 6(4), 468–482. https://doi.org/10.1016/j.eng.2019.08.017
Good Food Institute (GFI). (2021). State of the Industry report: plant-based meat, eggs and dairy. [Internet]. Accessed on January 13 2023. Available from: https://gfi.org/resource/plant-based-meat-eggs-and-dairy-state-of-the-industry-report/
Gumus, C. E., Decker, E. A., & McClements, D. J. (2017). Formation and stability of ω-3 oil emulsion-based delivery systems using plant proteins as emulsifiers: Lentil, pea, and faba bean proteins. Food Biophysics, 12(2), 186-197. https://doi.org/10.1007/s11483-017-9475-6
Henry, R. J. (1988). The carbohydrates of barley grains - A review. Journal of the Institute of Brewing, 94(2), 71–78.
Karp, S., Wyrwisz, J., & Kurek, M. A. (2019). Comparative analysis of the physical properties of o/w emulsions stabilised by cereal β-glucan and other stabilisers. International Journal of Biological Macromolecules, 132, 236–243. https://doi.org/10.1016/j.ijbiomac.2019.03.212
Kaushik, I., Singh, R., & Bhisnoi, J. P. (2017). Effect of barley malt, chickpea and peanut on quality of barley based beverage. Journal of Applied and Natural Science, 9(2), 1182–1186. https://doi.org/10.31018/jans.v9i2.1563
Krentz, A., García-Cano, I., Ortega-Anaya, J., & Jiménez-Flores, R. (2022). Use of casein micelles to improve the solubility of hydrophobic pea proteins in aqueous solutions via low-temperature homogenization. Journal of Dairy Science, 105(1), 22–31. https://doi.org/10.3168/jds.2021-20902
Kumari, S., Yadav, B. S., & Yadav, R. B. (2022). Effect of nano-conversion on morphological, rheological and thermal properties of barley starch. Journal of Food Science and Technology, 59(2), 467–477. https://doi.org/10.1007/s13197-021-05029-0
Lazaridou, A., Chornick, T., Biliaderis, C. G., & Izydorczyk, M. S. (2008). Composition and molecular structure of polysaccharides released from barley endosperm cell walls by sequential extraction with water, malt enzymes, and alkali. Journal of Cereal Science, 48(2), 304–318. https://doi.org/10.1016/j.jcs.2007.09.011
Li, Y., You, M., Liu, H., & Liu, X. (2021). Comparison of distribution and physicochemical properties of β-glucan extracted from different fractions of highland barley grains. International Journal of Biological Macromolecules, 189, 91–99. https://doi.org/10.1016/j.ijbiomac.2021.08.094
McClements, D. J. (2020). Development of next-generation nutritionally fortified plant-based milk substitutes: Structural design principles. Foods, 9(4), 421. https://doi.org/10.3390/foods9040421
Manassero, C. A., Beaumal, V., Vaudagna, S. R., Speroni, F., & Anton, M. (2018). Calcium addition, pH and high hydrostatic pressure effects on soybean protein isolates—Part 2: Emulsifying properties. Food and Bioprocess Technology, 11(11), 2079–2093. https://doi.org/10.1007/s11947-018-2164-8
Mekala, S., Silva, E. K., & Saldaña, M. D. A. (2021). Mechanism, kinetics, and physicochemical properties of ultrasound-produced emulsions stabilized by lentil protein: A non-dairy alternative in food systems. European Food Research and Technology, 248, 185–196. https://doi.org/10.1007/s00217-021-03871-2
Mekala, S., Silva, E. K., & Saldaña, M. D. A. (2022). Ultrasound-assisted production of emulsion-filled pectin hydrogels to encapsulate vitamin complex: Impact of the addition of xylooligosaccharides, ascorbic acid and supercritical CO2 drying. Innovative Food Science & Emerging Technologies, 76, 102907. https://doi.org/10.1016/j.ifset.2021.102907
Mishra, N., Gupta, E., Prasad, R., & Singh, P. (2020). Formulation and optimization of malted barley based symbiotic beverage by response surface methodology. Journal of Advances in Food Science & Technology, 7(1), 12–20.
Oliete, B., Potin, F., Cases, E., & Saurel, R. (2019). Microfluidization as homogenization technique in pea globulin-based emulsions. Food and Bioprocess Technology, 12(5), 877–882. https://doi.org/10.1007/s11947-019-02265-3
Ozturk, B., & McClements, D. J. (2016). Progress in natural emulsifiers for utilization in food emulsions. Current Opinion in Food Science, 7, 1–6. https://doi.org/10.1016/j.cofs.2015.07.008
Robins, M. M. (2000). Emulsions — creaming phenomena. Current Opinion in Colloid & Interface Science, 5(5), 265–272. https://doi.org/10.1016/S1359-0294(00)00065-0
Roselló-Soto, E., Barba, F. J., Parniakov, O., Galanakis, C. M., Lebovka, N., Grimi, N., & Vorobiev, E. (2015). High voltage electrical discharges, pulsed electric field, and ultrasound assisted extraction of protein and phenolic compounds from olive kernel. Food and Bioprocess Technology, 8(4), 885–894. https://doi.org/10.1007/s11947-014-1456-x
Sankaran, R., Show, P. L., Cheng, Y. -S., Tao, Y., Ao, X., Nguyen, T. D. P., & Van Quyen, D. (2018). Integration process for protein extraction from microalgae using liquid biphasic electric flotation (LBEF) system. Molecular Biotechnology, 60(10), 749–761. https://doi.org/10.1007/s12033-018-0111-6
Sarantis, S. D., Eren, N. M., Kowalcyk, B., Jimenez–Flores, R., & Alvarez, V. B. (2021). Thermodynamic interactions of micellar casein and oat β-glucan in a model food system. Food Hydrocolloids, 115. https://doi.org/10.1016/j.foodhyd.2020.106559
Sarkar, A., & Dickinson, E. (2020). Sustainable food-grade Pickering emulsions stabilized by plant-based particles. Current Opinion in Colloid & Interface Science, 49, 69–81. https://doi.org/10.1016/j.cocis.2020.04.004
Sethi, S., Tyagi, S. K., & Anurag, R. K. (2016). Plant-based milk alternatives an emerging segment of functional beverages: A review. Journal of Food Science and Technology, 53(9), 3408–3423. https://doi.org/10.1007/s13197-016-2328-3
Silva, A. R. A., Silva, M. M. N., & Ribeiro, B. D. (2020). Health issues and technological aspects of plant-based alternative milk. Food Research International, 131, 108972. https://doi.org/10.1016/j.foodres.2019.108972
Silva, E. K., Azevedo, V. M., Cunha, R. L., Hubinger, M. D., & Meireles, M. A. A. (2016). Ultrasound-assisted encapsulation of annatto seed oil: Whey protein isolate versus modified starch. Food Hydrocolloids, 56, 71–83. https://doi.org/10.1016/j.foodhyd.2015.12.006
Silva, E. K., Gomes, M. T. M., Hubinger, M. D., Cunha, R. L., & Meireles, M. A. A. (2015). Ultrasound-assisted formation of annatto seed oil emulsions stabilized by biopolymers. Food Hydrocolloids, 47, 1–13. https://doi.org/10.1016/j.foodhyd.2015.01.001
Silva, K., Machado, A., Cardoso, C., Silva, F., & Freitas, F. (2019). Rheological behavior of plant-based beverages. Food Science and Technology, 40, 258–263. https://doi.org/10.1590/fst.09219
Şirin, E., & Yalçın, E. (2019). Effects of concentration and partial hydrolysis on functional properties of hull-less barley protein concentrates. Quality Assurance and Safety of Crops & Foods, 11(2), 201–210. https://doi.org/10.3920/QAS2018.1390
Strieder, M. M., Neves, M. I. L., Silva, E. K., & Meireles, M. A. A. (2021). Impact of thermosonication pretreatment on the production of plant protein-based natural blue colorants. Journal of Food Engineering, 299, 110512. https://doi.org/10.1016/j.jfoodeng.2021.110512
Strieder, M. M., Silva, E. K., Mekala, S., Meireles, M. A. A., & Saldaña, M. D. A. (2022). Pulsed high-pressure processing of barley-based non-dairy alternative milk: β-carotene retention, protein solubility and antioxidant activity. Innovative Food Science & Emerging Technologies, 82, 103212. https://doi.org/10.1016/j.ifset.2022.103212
Sivabalan, S., & Sablani, S. (2022). Design of β-carotene encapsulated emulsions for thermal processing and storage. Food and Bioprocess Technology, 15(2), 338–351. https://doi.org/10.1007/s11947-021-02754-4
Tan, Y., & McClements, D. J. (2021). Plant-based colloidal delivery systems for bioactives. Molecules, 26(22), 6895. https://doi.org/10.3390/molecules26226895
Vo, H. H. P., & Saldaña, M. D. A. (2023). Hydrolysis of pea protein concentrate in subcritical water media with addition of citrus pectin and citric acid, The Journal of Supercritical Fluids, 195, 105866.
Wang, C., Tian, Z., Chen, L., Temelli, F., Liu, H., & Wang, Y. (2010). Functionality of barley proteins extracted and fractionated by alkaline and alcohol methods. Cereal Chemistry, 87(6), 597–606. https://doi.org/10.1094/CCHEM-06-10-0097
Waterborg, J. H. (2009). The Lowry method for protein quantitation. In The protein protocols handbook (pp. 7–10). Springer. https://doi.org/10.1007/978-1-59745-198-7_2
Wu, Y. V., & Inglett, G. E. (1974). Denaturation of plant proteins related to functionality and food applications. A Review. Journal of Food Science, 39(2), 218–225. https://doi.org/10.1111/j.1365-2621.1974.tb02861.x
Xia, T., Xue, C., & Wei, Z. (2021). Physicochemical characteristics, applications and research trends of edible Pickering emulsions. Trends in Food Science & Technology, 107, 1–15. https://doi.org/10.1016/j.tifs.2020.11.019
Xiao, K., & Zhou, Y. (2020). Protein recovery from sludge: A review. Journal of Cleaner Production, 249, 119373. https://doi.org/10.1016/j.jclepro.2019.119373
Yu, J. J., Li, N., Li, S., & Chen, Y. (2022). Solubilization mechanism and structural properties of high‐denatured peanut protein treated by shearing. Journal of Food Process Engineering, 45(2), e13951. https://doi.org/10.1111/jfpe.13951
Funding
We acknowledge the Natural Sciences and Engineering Research Council of Canada (NSERC, #04371–2019, Discovery Grant of Dr. Saldaña) for providing funds to conduct this research; Monique Martins Strieder thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant number 141110/2018–0) for her doctorate assistantship; and Maria Angela A. Meireles thanks CNPq (grant number 309825/2020–2) for her productivity grant.
Author information
Authors and Affiliations
Contributions
Monique Martins Strieder: Conceptualization, investigation, formal analysis, writing — original draft. Eric Keven Silva: Conceptualization, visualization, supervision, writing — review and editing. Srujana Mekala: Investigation, methodology. Maria Angela A. Meireles: Supervision, resources. Marleny D.A. Saldaña: Conceptualization, supervision, resources, funding acquisition, project administration, writing — review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Strieder, M.M., Silva, E.K., Mekala, S. et al. Barley-Based Non-dairy Alternative Milk: Stabilization Mechanism, Protein Solubility, Physicochemical Properties, and Kinetic Stability. Food Bioprocess Technol 16, 2231–2246 (2023). https://doi.org/10.1007/s11947-023-03037-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03037-w