Abstract
This study aimed to investigate the suitability of lentil protein and emulsions thereof for the formulation of a milk substitute. The effect of high-pressure homogenisation and heat treatments on functional and physico-chemical properties of lentil protein solutions (3.3% w/w) and the emulsions, containing fat contents similar to commercial cow’s milk, was studied. Dynamic high-pressure treatments of 180 and 900 bar greatly affected physical and structural properties of the lentil proteins: the particle size was reduced by 100-fold to 129.00 nm for samples homogenised at 900 bar, leading to an almost complete solubilisation. Surface properties of lentil protein changed, as shown in an increase of hydrophobicity and decrease of free sulfhydryl groups, while changes in secondary structure and aggregation did not develop. Little impact was observed of the heat-treatment at 65 or 85 °C, however, colour changed from a faint pink hue to be more white in appearance. The obtained emulsions exhibited good colloidal stability at both homogenisation pressures, while overall product quality was best when treated at 900 bar. Sensory analyses showed the formulated lentil-based milk substitute had textural and organoleptic profiles comparable to commercial plant-based milk substitutes, including soya-based products. Lentil protein isolates showed great potential to be used formulating milk substitutes with a high-protein content, similar to cow’s milk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cow’s milk is one of the most complete nutrient sources in the human diet and dairy products are widely consumed around the world. Since it is designed to be the sole food source for calves, it is not surprising that it provides all the major nutrients like fat, carbohydrates and proteins to our diet, while also being a good source of minerals and vitamins [1]. Yet, the consumption of cow’s milk is decreasing. In fact, sales of cow’s milk have fallen by 15% in the US since 2012, while the demand for plant-based milk substitutes (PBMSs) has grown by 61% within the same time period, reaching 2.11 billion US$ in 2017 [2]. The trend is driven by the desire of consumers for new flavours and taste, followed by health reasons and concerns about animal welfare and environment [3]. Especially the latter is gaining attention recently; knowing that agriculture is a major contributor to the global greenhouse gas output and that livestock farming is the main contributor to these emissions is a major concern for many consumers [4]. However, the market for PBMSs still in its infancy and currently only soya-based milk substitutes (BMSs) are catering as balanced alternatives to cow’s milk based on nutrients [5,6,7]. However, soya may not be the most sustainable alternative, as it does not grow in cold climates and relies on long supply chains [8, 9]. Similar to soya beans, lentils belong to the legume family (fabaceae) and are a traditional staple food in many temperate climates, like Canada or Europe [10]. Lentils contain high amounts of protein between 20.6 and 31.4% with promising properties, as it has high-digestibility (~ 83%) with high-nutritional value (good Leu/Ile and Leu/Lys ratios) and provides a wide variety of functional properties [11].
The isolation und application of proteins inherits some advantages: anti-nutrients and compounds causing off-flavours can be removed and the protein isolate can be specifically applied to administer techno-functional properties. Lentil proteins have been investigated for their functionality and exhibit promising properties as emulsifiers [12,13,14]. Proteins can stabilise emulsions as they are able to interact with both oil and water, due to their amphiphilic properties: their polar and nonpolar regions (i.e., hydrophilic and hydrophobic amino acid residues) align at the oil–water interface to form stabilising films by providing electrostatic and steric repulsion between droplets, and by reducing interfacial tension between the two phases [15]. In the process of creating emulsions homogenisation is one crucial step. With increasing pressure, the oil droplet size decreases. Furthermore, this treatment may also alter specific features and the spatial structure of proteins, which may affect their emulsifying properties, since these strongly depend on the proteins structure and surface properties [16]. The impact of dynamic high-pressure treatment on proteins has still scarcely been studied. However, as shown for lupin and whey protein isolates high-pressure homogenisation is likely to improve interfacial and other functional properties [17, 18]. While the mechanism of emulsion formation has been studied intensively by many researchers [16, 19], the newly gained knowledge about protein modification may help to design improved emulsion or also protein ingredients with specific altered properties for other applications.
Recent work from Primozic et al. [20, 21] examined the emulsifying property of lentil protein isolates at pH 3 and demonstrated its potential as an emulsifier in nano-emulsions. However, at higher protein concentrations the emulsion was unstable, and aggregates were formed leading to emulsion gels. Further, most research focuses on high oil load emulsions [12, 13, 22], but few studies have looked at applicable systems with the aim to develop milk alternatives, comprising stable high-protein contents. In this regard, there is still a missing body of research bringing the knowledge together.
This study aims to evaluate the formation of emulsions for the development of a novel lentil-BMS, containing protein (3.3% w/w) and fat contents (3.3 or 1.5% w/w) similar to commercial cow’s milk. The impact of dynamic pressure and pasteurisation treatments on lentil proteins, as on the prepared emulsion thereof was studied in terms of functional properties and product properties, respectively. This provides a thorough understanding of the process and final product.
Materials and methods
Lentil protein isolate (LPI) was provided by Fraunhofer (Institute for Process Engineering and Packaging, Freising, Germany). In short, brown lentils of commercial quality (Lens culinaris cv. Itaca), provided by Agroservice Spa (San Severino Marche, Italy) were used as raw material and extracted using ultrafiltration. Protein content of the final LPI was 93.7 g/100 g. Chemicals were purchased from Sigma-Aldrich (St Louis, Missouri, USA) unless otherwise stated.
Preparation of protein stabilised emulsions
Emulsions were prepared as outlined by Jacobs et al. [23]: solutions of LPI were prepared using a stirring and an ultraturrax device (Janke & Kunkel IKA Labortechnik). The solution was heated to 50 °C and pH was adjusted to 7 and hydrated for an hour. The solution was stirred at 70 rpm and utraturraxed at 4600 rpm for 10 min. Then, sunflower oil was mixed with the protein suspension for 10 min using a stirrer and ultraturrax. The final emulsion contained 3.3% or 1.5% (w/w) oil, and in both cases 3.3% (w/w) protein. To further reduce the particle size, the emulsions were homogenised with a two-stage high-pressure homogeniser at 180 bar (150 bar and 30 bar), or 900 bar (750 bar and 150 bar) (APV-2000, SPX FLOW Inc., Charlotte, USA). To ensure microbial stability, samples were subjected to pasteurisation. A low temperature at 65 °C for 30 min and a higher temperature at 85 °C for 2 min (to simulate high-temperature short-time processing) were chosen and applied in a stirring water bath (Lochner mashing device LP electronic, Berching, Germany). Samples were refrigerated (4 °C) and measured on the same day of preparation. Further, samples were stored for 21 days to assess storage stability, supplemented with sodium azide [0.02% (w/w)] to evade microbial spoilage.
Preparation of protein solutions
To study the impact of processing on the proteins, solutions of 3.3% (w/w) protein were prepared in the same manner without the addition of oil.
Surface hydrophobicity
Surface hydrophobicity was measured according to Hayakawa and Nakai [24] measuring the fluorescence intensity using 1-anilino-8-naphthalenesulfonate (ANS) with some modifications. Protein solutions were serially diluted with 0.01 M phosphate buffer (pH 7) ranging from 0.0006 to 0.015% (w/v). Ten microlitre ANS (8.0 mM in 0.1 M phosphate buffer, pH 7) were mixed with 2 mL diluted sample and left in darkness for 15 min. Fluorescence was measured (λexcitation = 390 nm, λemission = 470 nm) and corrected by a blank measured without ANS. The values represent the slopes (R2 ≥ 0.98) calculated by linear regression analysis and used as an index of the protein surface hydrophobicity.
Total sulfhydryl groups
Total sulfhydryl groups were determined with Ellman’s reagent [5,5′-dithiobis-(2-nitrobenzoic acid)] according to the method of Van der Plancken et al. [25]. The protein samples were diluted to 2 mg mL−1 with 0.01 M phosphate buffer (pH 7) containing 6 M urea and 0.5 M SDS. Eighty microlitre of Ellman’s reagent were added to 2.5 mL of diluted samples. The absorbance was measured at 412 nm after 15 min. For the reagent blank, the protein samples were replaced by the sodium phosphate buffer mixed with 80 µL of Ellman’s reagent. SH contents were calculated as follows:
where A412 is the absorbance at 412 nm, A412B is the absorbance at 412 nm for the blank, ε is the extinction coefficient, which was taken as 13,600 M−1 cm−1, and C is the sample concentration in mg mL−1.
Protein solubility
Protein solubility was adapted from the method of the International Dairy Federation [24]. Protein contents of whole samples and supernatants (centrifuged at 3000g for 10 min) were determined using the Kjeldahl method (MEBAK 1.5.2.1) and nitrogen content was converted into protein using the factor 6.25. Protein solubility was expressed as percentage of protein content in the supernatant of total protein content:
Secondary structure
Far-UV circular dichroism (CD) measurements of protein solutions were obtained using a circular dichroism spectrophotometer (Chirascan, Applied Photophysics, Leatherhead, UK). Protein solutions of 1 mg mL−1 were prepared in ultrapure water. CD spectra were measured with a path length of 0.1 mm in the range 180–260 nm, bandwidth 1 nm, spectral resolution 1 nm, 1 s/point. The average of three spectra was obtained and a five-point smoothing algorithm was applied after correction for the water baseline [27].
Particle size distribution
Particle size distribution (PSD) of untreated protein dispersions was measured using a laser light diffraction unit (Mastersizer 3000, Malvern Instruments Ltd, Malvern, UK). A polydisperse model with particle and dispersant refractive index of 1.336 and 1.33, respectively, and absorption of 0.1 were selected for data analysis. Samples were introduced to the mixing chamber and dispersed in ultrapure water until a laser obscuration of 12% was reached and three readings were taken. Size measurements are presented as volume weighted mean particle diameter (d4,3). Due to the shift to smaller sizes with homogenisation particle size and oil droplet distribution of protein solutions and emulsions were determined by dynamic light scattering using a Malvern Zetasizer Nano ZS system (Malvern Instruments Ltd., Malvern, Worcestershire, UK), equipped with a 633 nm laser. All solutions and emulsions were diluted with ultrapure water (1:250) before analysis.
Accelerated physical stability
Stability was measured using an analytical centrifuge (LUMiSizer®, LUM GmbH, Berlin, Germany). The samples were treated at 1000 rpm for 30 min and subsequently at 3000 rpm for 60 min at 24 °C [6]. The separation rate represents the slope in % h−1 and was determined by plotting the % of transmission over time. The creaming, sediment height and bottom clearance (mm) were observed by subtracting the position with ≤ 20% (or ≥ 20% for the bottom clearance) light transmission of the last profile from the meniscus or cell bottom.
Rheological behaviour
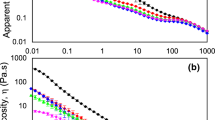
The rheological behaviour of the products was characterised using a controlled stress rheometer (MCR301, Anton Paar GmbH, Austria) equipped with a sensor system of coaxial cylinders (C-CC27-T200/SS, Anton Paar GmbH, Austria). The shear stress was measured as a function of shear rate ranging from 0.5 to 100 s−1 within 500 s [6]. The measurements were carried out at 10 °C. The power law model was fitted to the experimental points to determine the flow behaviour index (n) and the apparent viscosity measured at 10 s−1 is referred to as viscosity.
Heat stability
For the determination of heat stability 2.5 mL of samples were placed in glass tubes (10 mm × 130 mm, AGB Scientific, Dublin, Ireland), sealed with silicone bungs, immersed in an oil bath thermostatically controlled at 140 °C (Elbanton BV, Kerkdriel, The Netherlands), with continuous rocking at motor speed setting 3 [28]. The heat coagulation time (HCT) was examined visually and taken as the time in minutes that elapsed between placing the sample in the oil bath and the onset of coagulation.
Confocal laser scanning microscopy
The microstructural analysis of emulsions was performed using a confocal laser scanning microscope (CLSM) (Olympus FV1000, incorporating an IX81 inverted microscope Germany). A saturated solution of Nile blue (Sigma-Aldrich, Wicklow, Ireland; 500 μL) was used to label both protein and lipid in 1 mL of sample. Samples were observed using a 100 × oil immersion objectives, an Ar laser operating at an excitation wavelength of 488 nm with emission detected between 500 and 530 nm and a He–Ne laser operating at an excitation wavelength of 633 nm with emission detected between 565 and 615 nm for oil and protein observation, respectively [29]. At least three specimens of each sample were observed to obtain representative micrographs of samples.
Colour
The colour values were measured using the CIE L*a*b* colour system and obtained using illuminant D65. The instrument used was a colorimeter (CR-400, Konica Minolta, Osaka, Japan). Colour of samples was characterised according to whiteness index (WI) [30].
Sensory affective testing
Sensory acceptance testing (SAT) was performed on the emulsion containing 3.3% sunflower oil, treated at 900 bar and 85 °C, and five commercial PBMSs based on soya, almond, oat, rice, and hemp, purchased from a local health store. This lentil-based emulsion was chosen, since it performed best in the physico-chemical tests completed beforehand, however, sucrose (2.5% w/w), salt (0.08% w/w) were added to the formulation, to improve the taste. SAT was conducted according to the methods of Stone et al. [26] using a total of 30 untrained assessors (n = 30, 53% female, ages ranged from 20 to 55). SAT took place over two separate days, evaluating the samples in duplicates, and was carried out in the panel booths of the sensory science laboratory, food science building, University College Cork according to international standards (ISO 11136 2014). The refrigerated samples (20 mL) were assigned a randomised three-digit code and presented to the panellists under white light. Participants used a nine-point hedonic scale (appearance, aroma, mouth feel, flavour, overall; like extremely—dislike extremely) to indicate their degree of liking of the six PBMSs in each session. After the sensory assessment, panellists were asked to fill out a questionnaire on demographic information, habits and motivation for milk and milk substitute consumption.
Statistics
All analyses were carried out at least in triplicate, unless otherwise stated. Means were compared using one-way analysis of variance (ANOVA) and Tukey’s post hoc test using Minitab release 16 (Minitab Inc. State College, PA, USA). The level of significance was determined at p < 0.05. Linear correlation measurements of results were performed using Pearson’s correlation.
Results and discussion
Effect of homogenisation and heat treatment on protein solubility, surface properties and secondary structure
The effect of homogenisation pressures and heat treatments on solubility, surface hydrophobicity and total sulfhydryl groups of lentil protein dispersions (3.3% w/w) is shown in Fig. 1. The protein solubility increased due to homogenisation considerably from 54.34 to 96.40% and 98.85% at homogenisation pressures of 180 bar and 900 bar, respectively. The increase in protein solubility might be due to the improved dispersion and hydration of the smaller protein particles [17]. This can also be seen in the reduced particle size and CLSM pictures. Subsequent thermal treatment did not change the solubilities considerably. However, thermal treatments lead to an increase of surface hydrophobicity and decrease in total sulfhydryl groups, revealing changes in the protein conformation. Surface hydrophobicity increased significantly with homogenisation pressure from 1959.8 to 2831.3 and 3124.3 for 180 bar and 900 bar treatment, respectively. However, heat treatments increased the values further to 3355.6 and 3811.2 for 65 and 85 °C treated samples, respectively, for protein dispersions homogenised at 180 bar. A similar trend was observed for 900 bar treated samples. Surface hydrophobicity is the predominant factor assessed by many studies as it is important for functionality of food proteins. Correlations have been found to interfacial tension and emulsifying activity. It enables the protein to interact between phases, may it be water and oil for emulsions, or water and air for foams. Indeed, this study also showed this correlation between hydrophobicity and the stability index (− 0.772, < 0.001). The exposure of hydrophobic groups to the surface by partially unfolding of protein molecules, can make them more flexible and facilitate their faster adsorption into the oil-interfaces [32].
Total sulfhydryl groups were found to be highest for untreated protein dispersions. No significant differences were found for samples homogenised at 180 bar, while 900 bar homogenisation showed a considerable decrease from 34.02 down to 31.08 µM g−1 protein. However, temperature showed to have a big impact and significantly decreased the total sulfhydryl groups to 27.60, and 24.22 µM g−1 protein for protein solutions homogenised at 180, and 900 bar, respectively, after a heat treatment of 85 °C for 2 min. The decrease of sulfhydryl groups suggests that new disulphide bridges were formed post-heating. Disulphide bonds play an important role in the formation of aggregates and gel network structures. For instance, the formation of self-standing gels upon heating of soya or whey proteins in the manufacture of tofu, or ricotta is mainly driven by the formation of disulphide bonds [33, 34]. However, also emulsion stability may be improved; once formed, film stability may be enhanced by disulphide bond formation as shown by Dickinson [30]. Further, the decrease of disulphide bonds also indicated a flexibility of LP to alter its structure, which is also relevant in the formation of stable interfacial layers.
The secondary structure of the proteins as analysed by far-UV CD spectroscopy is shown in Fig. 2. The homogenised and pasteurised protein solutions all exhibit a similar profile with a respective negative and positive band above and below 200 nm. The observed patterns are characteristic of α-helical structures. While only slight shifts across the spectra appeared, structural changes may have occurred but suggested no major difference arose. In regard to the untreated sample, these spectra did differ. However, it needs to be noted, that only 54% of the protein were soluble. This might also be the reason why less pronounced bands were obtained; nevertheless, the same pattern of bands can be observed, indicating the same secondary structure of the untreated protein dispersion. Aryee and Boye [36] observed similar spectra for lentil protein. However, they observed after cooking lentils for 30 min at 95 °C, the spectra changed and a loss in secondary structure conformational was shown. Heat treatments in this study did not show such changes, neither did the homogenisation pressures.
Particle and droplet size distribution
The initial particle size of lentil protein dispersions was measured using a static light-scattering system. The dispersion consisted predominantly of large and poorly dispersible particles being about 28 µm big with a broad distribution with a span of 1.7 mm. With the application of homogenisation, the protein changed drastically, as already seen in the protein solubility. The particle size was reduced by more than 100-fold. Therefore, samples were analysed using the dynamic light-scattering system and values using this method are reported as z-average presented in Table 1. The particle size was shifted down to 219.19 nm for protein solutions homogenised at 180 bar, and further down to 129.00 nm for samples homogenised at 900 bar. As indicated by the polydispersity index, a narrow and monomodal distribution was achieved by both pressure treatments. Further, no significant changes (p < 0.05) in these parameters were found when applying the different heat treatments. This decrease in particle size may be the main reason for improved solubility: increasing the surface of protein particles improved the hydration possibilities [37]. Both pressure and heat treatments are known to cause denaturing of proteins. As apparent from the values of surface hydrophobicity and sulfhydryl groups, changes in the structure of proteins occurred. However, as evident from the reduction of particle size and high-protein solubility, this did not lead to extensive protein–protein interactions, and aggregation. In contrary, Joshi et al. [13] assumed LPI aggregated upon heat treatment at 80 °C for 10 min, which lead to inferior emulsifying properties. Particle size does not deduce such in this study, which may be due to the lower protein concentration. Similarly, the effect of high-pressure homogenisation on lupin protein isolates did not show the formation of large aggregates at pressures up to 1000 bar, but a reduction of the particle size distribution to 0.1–10 µm [17].
With the addition of sunflower oil, emulsions were prepared by applying the same homogenisation pressure, and pasteurisation treatments. At a homogenisation pressure of 180 bar, mean particle/droplet size of 371.78 nm and 447.82 nm using 1.5% and 3.3% of sunflower oil respectively were achieved. The polydispersity index was rather high for these samples (< 0.3), indicating a polydisperse distribution. From the PSD of the mere protein solutions it can be concluded that the smaller particles are mainly proteins, whereas fat droplets constitute the biggest particles. The size of oil droplets was successfully decreased with the homogenisation processing at 900 bar. Mean particle/droplet sizes of 205.12 and 223.36 nm were achieved for 1.5% and 3.3% oil emulsions, respectively. Samples treated at 900 bar showed a narrower distribution due to the size reduction of the oil droplets, as expressed in the decrease of the polydispersity index (≤ 0.15). Additionally, it may also be concluded that more of LPI was absorbed in the interface of oil/water, due to the increased surface area of the oil droplets.
No significant differences in the PSD were found when applying the different heat treatments. Further, during storage, no considerable differences were found, and in all but one case the differences were not significant. Oil droplets were successfully stabilised by electrostatic repulsive forces of the protein film, which protected droplets against flocculation and coalescence during storage and upon heating.
Primozic et al. [20] studied the characteristics of 5% oil-in-water emulsions stabilised with LPI at pH 3 as a function of protein concentration, using a high-pressure homogeniser at 1378 bar. Similar to the present study, nano-emulsions with an oil droplet size of 0.163 µm at a protein concentration of 3% (w/w) were achieved. However, these emulsions were considerably unstable during storage, and protein aggregates were formed with particle sizes of approximately 10 µm. At a protein concentration of 5% even a strong gel was formed. Joshi et al. [13] studied the emulsion characteristics of LPI-stabilized emulsions at pH 7, but with higher oil-loads. With a pressure of 500 bar, they achieved similar droplet sizes of 398 nm containing 10% (v/v) oil and 3% (w/v) protein. These results show that processing conditions are vital, and especially the pH within a food system is impacting the characteristics and functionality.
Rheology
The influence of homogenisation and pasteurisation on the rheological parameters (K, n and η) is shown in Table 2. With the application of homogenisation the viscosity of protein dispersion decreased as smaller and more homogenous particles were produced. The untreated protein dispersion showed a viscosity of 3.35 mPa·s, with a newton-like behaviour (n = 0.92), which was reduced to about 2 mPa·s for all protein solutions, as changing homogenisation pressure, and pasteurisation temperature showed no significant impact on the viscosity. The flow behaviour index (n) was slightly shifted to values above 1, indicating a slight shear thickening behaviour. Emulsions exhibited no differences in flow behaviour, while viscosity was slightly increased. The incorporation of 3.3% oil at low homogenisation pressure of 180 resulted in the highest viscosity of 3.07 mPa s. Cow’s milk shows a similar viscosity with 3.15 mPa s [6], suggesting a comparable mouth feel when swallowing. Pasteurisation treatments did not cause significant changes (p < 0.05) in the consistency index, or apparent viscosity of samples which indicates that no changes in the component arrangement were induced by thermal treatment. Further, no changes were observed after a storage time of 21 days.
Heat stability of protein solutions and emulsions
Suspensions of LPI exhibited a heat coagulation time (HCT) of 8.28 min (Table 2) in an oil bath at 140 °C. The homogenisation pressure and pasteurisation temperature seemed not to affect heat stability considerably. Heat coagulation is caused by hydrophobic interactions, and covalent cross-linking (disulphides), induced by the denaturation of protein [38]. Even the pasteurised protein solutions, which already had more exposed hydrophobic groups and underwent a reduction of total sulfhydryl groups seemed to be relatively insusceptible to coagulation. Thermal stability of soya protein isolate, at pH 7 and 3.6% (w/v) was reported to be around 20 min [28], while high-concentrated milk protein, exhibited a low HCT of < 2 min at pH 7 and a suspension concentration of 3.50% (w/w) [39]. The prepared emulsion showed higher heat stabilities than the mere protein solutions itself. Emulsions with 3.3% fat, homogenised at 180 bar and pasteurised at 65 °C were the most stable and coagulated after 17.41 min. The other samples showed similar HCT, being around 14 min. Generally, it can be concluded that proteins were stabilised and protected against heat induced coagulation to a certain extent. While proteins are in the interface of oil, their hydrophobic groups are aligned into the oil phase, thus, no interactions are forced between the exposed hydrophobic sites of protein molecules. However, with increasing degree of denaturation, proteins may lose their ability to stabilise the interface and eventually the system gets unstable, leading to the exposure of hydrophobic groups and aggregation occurs.
With storage the heat stability decreased, only not significantly for 900 bar treated 3.3% oil emulsions. This could be an indication of an onset of instability of the protein in solution or in the oil interface. However, particle size measurements indicated no aggregation, therefore, it can be assumed, that little changes in the protein conformation affected the heat stability.
Confocal laser scanning microscopy
Selected micrographs, as obtained by CLSM are displayed in Fig. 3. Untreated LPI dispersions were constituted by a heterogeneous mixture of small and larger rounded, or broken pieces of particles with freckled, smooth surfaces. As observed in the particle size, much smaller particles are found in the homogenised samples. The proteins were distributed very homogeneously, without any kind of formation. As indicated by particle size distribution and viscosity already, the heat treatment did not affect the component arrangement, also when observing the emulsions. Oil droplets were distributed homogeneously and did get reduced with increased homogenisation pressure.
Colour
As it was isolated from brown lentils, the LPI contained some pigments, exhibiting a faint light pink colour also in the solutions. Pictures of protein dispersions and emulsion are shown in Fig. 4 and values of instrumental colour analysis are presented in Table 2. This light pink colour of the LPI powder is expressed in the a* value (4.12 for the untreated LPI dispersion). Most interestingly, with heat treatment at 85 °C, these values were reduced and shifted away from red, appearing more neutral (− 0.25 for LPI homogenised at 180 bar, pasteurised at 85 °C). The colour compounds in lentils, such as anthocyanins, are sensitive to heat treatments [40]. This pigment degradation is clearly an advantage for the preparation of emulsions, aiming to imitate the white appearance of cow’s milk. Further, with the incorporation of oil, the lightness of the samples was increased to more than 70 from initially 46.46 for the untreated LPI dispersion. The dynamic high-pressure treatment at 900 bar increased the lightness the most, with values reaching 77, as the number and size of oil droplets is increasing the scattering of light. Both the degradation of colour compounds and light scattering is presented in the WI. A value of 76.78 was reached for the emulsion containing 3.3% (w/w) fat treated at 900 bar and 85 °C, which is relatively comparable to cow’s milk with a whiteness index of 81.89 [6].
Accelerated physical stability
Physical stability was analysed with an analytical centrifuge, measuring light transmission across the entire length of the sample over time. Figure 5 shows exemplary typical stability graphs, and numerical data of the separation rate of protein dispersions and emulsions obtained as affected by homogenisation and thermal treatments. It can be seen that the untreated LPI dispersion was highly unstable (compare transmission profile A). The transmission increased quickly throughout the whole length of the sample during the measurement (red profile being the initial transmission to green the last profile) and a thick sediment layer of 6.08 mm at the bottom of the cuvette was formed. Due to homogenisation, the LPI became more stable in solution; Transmission profiles (B) of 180 bar treated LPI dispersions changed slowly and only during the second step of centrifugation at higher g forces a higher transmission, with a slight slope from top to bottom can be seen. This indicates a particle movement to the bottom of the cuvette, which formed a sediment layer of 0.18 mm. Homogenisation of 900 bar treatments lead to an increase of the initial transmission. This is due to a decrease of opacity, since the protein particles were reduced in size and solubilised. During accelerated gravitation, the transmission profiles increased slowly throughout the samples, showing the same trend as the 180 bar treated samples. Temperature treatments showed no significant effect on the stability. Emulsions were characterised by an initial transmission very close to zero throughout the cuvette, meaning no light was passing through the sample due to homogeneous distribution of oil droplets and protein particles (compare profile C and D). During accelerated gravitation, the transmission at the bottom of the cuvette progressively increased, indicating a movement of particles and droplets to the top, in the opposite direction as the protein particles moved. The addition of oil and formation of oil droplets improved the stability of the proteins in the solution, by diffusing at the interface of the oil droplets. Essentially, the protein as the oil stabilised each other. Overall, both homogenisation pressures produced stable emulsions, but 3.3% fat emulsions homogenised at 900 bar were the most stable, having the lowest separation rate of about 2% h−1. Colloidal stability is one of the main issues for PBMSs. A wide range of commercial PBMSs analysed in a previous study [6] showed high-separation rates for almost all products due to insoluble plant material, flocculation and fat separation. Cow’s milk on the other hand, as a stable emulsion, showed a separation rate of 3.87% h−1. For all LPI stabilised emulsion comparable separations rates were found while the 900 bar treated sample heated to 85 °C was even more stable (2% h−1), exhibiting a lower separation rate than cow’s milk.
Separation rate (black) and bottom clearance (grey) of lentil protein (LP) dispersions and lentil protein stabilised emulsion (LPE) containing 1.5 or 3.3% fat, measured on day 0 and after 21 days of storage as effected by of homogenisation pressure (180 or 900 bar) and heat treatment (65 or 85 °C) with selected transmission profiles (A) untreated lentil protein dispersions, (B) lentil protein dispersions homogenised at 180 bar, lentil stabilised emulsion, containing 3.3% fat, pasteurised at 65 °C, homogenised at (C) 180 bar and (D) 900 bar
After a storage time of 21 days, emulsions were still as stable. Only changes were found regarding the bottom clearance for emulsions homogenised at 180 bar. These samples showed to have slightly less particles moving to the top of the cuvette during accelerated gravitation. However, the separation rate did not change over storage, indicating the same colloidal stability overall.
Sensory affective testing
The sensory ratings of selected commercial PBMSs and the lentil emulsion, containing 3.3% oil and additionally 2.5% (w/w) sucrose and 0.08% (w/w) salt, are presented in Table 3. Bovine milk was deliberately not included, and consumers were informed that only PBMSs were displayed, to avoid a comparison to bovine milk, and concentrate on “liking”. The hedonic ratings were similar for all the samples and “slightly liked” overall, accept for the hemp-BMS. This is probably due to the untrained assessors, of which just 36.7% consumed PBMSs at least weekly, while 20% of them claimed to never drink it. While the appearance of hemp-BMS was liked most, possibly due to the bright white colour similar to cow’s milk, it scored the lowest for all the other attributes and was overall “disliked slightly”. No significant differences were found between the lentil-BMS and the other commercial PBMSs. Only in terms of appearance, the lentil sample scored lowest together with soya and oat, probably due to the off-white colour. Some of the commercial PBMSs contained additives, like stabilisers, and flavours, which improve the sensory. Already, this simple formulation of a lentil-BMS showed promising results and further incorporating of some ingredients could improve the sensory perception of this lentil-BMS. While taste is one of the most important reasons for consumers to buy a product (60% of the panellists claimed they would consume more often PBMSs, if the taste improved), also 40% look for proven health benefits. For instance, the produced lentil-BMS could be advertised as “high in protein”, according to the European law, since the protein provides 25% of the energy [41], while most of the PBMS contain < 0.5% protein [6].
Conclusion
Lentil proteins have been studied for their functional properties and ability to stabilise emulsions. With the application of high-pressure homogenisation lentil proteins were solubilised to a major extent and sunflower oil was successfully emulsified. With a homogenisation pressure of 900 bar and a heat treatment of 85 °C highly stable nano-emulsion were generated with great colloidal stability, and appearance and viscosity similar to cow’s milk. Sensory testing also proved the great potential of lentil protein-based emulsions as novel products, since the textural and organoleptic attributes compared well to commercial PBMSs, including soya-based products. The produced lentil-BMS possessed great functional and nutritional properties, providing valuable protein to the diet. Further work on the formulation and processing of related products like yoghurt will be performed to meet the growing demand of consumers of such dairy alternatives.
References
FAO (2013) Milk and dairy products in human nutrition, 1st edn. FAO, Rome
Mintel Group Ltd. (2018) US non-dairy milk sales grow 61% over the last 5 years|Mintel.com. http://www.mintel.com/press-centre/food-and-drink/us-non-dairy-milk-sales-grow-61-over-the-last-five-years. Accessed 17 Aug 2018
Mintel Group Ltd. (2018) Taste is top reason Americans eat plant-based proteins|Mintel.com. http://www.mintel.com/press-centre/food-and-drink/taste-is-the-top-reason-us-consumers-eat-plant-based-proteins. Accessed 17 Aug 2018
Aleksandrowicz L, Green R, Joy EJM et al (2016) The impacts of dietary change on greenhouse gas emissions, land use, water use, and health: a systematic review. PLoS One. https://doi.org/10.1371/journal.pone.0165797
Vanga SK, Raghavan V (2018) How well do plant based alternatives fare nutritionally compared to cow’s milk? J Food Sci Technol 55:10–20. https://doi.org/10.1007/s13197-017-2915-y
Jeske S, Zannini E, Arendt EK (2017) Evaluation of physicochemical and glycaemic properties of commercial plant-based milk substitutes. Plant Foods Hum Nutr 72:26–33. https://doi.org/10.1007/s11130-016-0583-0
Sousa A, Kopf-Bolanz KA, Kopf-Bolanz K (2017) Nutritional implications of an increasing consumption of non-dairy plant-based beverages instead of cow’s milk in Switzerland. Adv Dairy Res. https://doi.org/10.4172/2329-888X.1000197
Riaz MN (2006) Processing of Soybeans into Ingredients. In: Riaz MN (ed) Soy application in food, 1st edn. Taylor & Francis Group, Boca Raton
Nadathur SR, Wanasundra JPD, Scanlin L (2017) Sustainable protein sources, 1st edn. Academic Press, London
Joshi M, Timilsena Y, Adhikari B (2017) Global production, processing and utilization of lentil: a review. J Integr Agric 16:2898–2913. https://doi.org/10.1016/S2095-3119(17)61793-3
Jarpa-Parra M (2018) Lentil protein: a review of functional properties and food application. An overview of lentil protein functionality. Int J Food Sci Technol 53:892–903. https://doi.org/10.1111/ijfs.13685
Ma Z, Boye JI, Simpson BK (2016) Preparation of salad dressing emulsions using lentil, chickpea and pea protein isolates: a response surface methodology study. J Food Qual 39:274–291. https://doi.org/10.1111/jfq.12190
Joshi M, Adhikari B, Aldred P et al (2012) Interfacial and emulsifying properties of lentil protein isolate. Food Chem 134:1343–1353. https://doi.org/10.1016/j.foodchem.2012.03.029
Johnston SP, Nickerson MT, Low NH (2015) The physicochemical properties of legume protein isolates and their ability to stabilize oil-in-water emulsions with and without genipin. J Food Sci Technol 52:4135–4145. https://doi.org/10.1007/s13197-014-1523-3
Dickinson E (1994) Protein-stabilized emulsions. Water in Foods 1:59–74
Kinsella JE (1982) Relationships between structure and functional properties of food proteins. In: Fox PF, Condon JJ (eds) food proteins. Applied Science Publishers, London
Bader S, Bez J, Eisner P (2011) Can protein functionalities be enhanced by high-pressure homogenization?—a study on functional properties of lupin proteins. Proced Food Sci 1:1359–1366. https://doi.org/10.1016/J.PROFOO.2011.09.201
Bouaouina H, Desrumaux A, Loisel C, Legrand J (2006) Functional properties of whey proteins as affected by dynamic high-pressure treatment. Int Dairy J 16:275–284. https://doi.org/10.1016/j.idairyj.2005.05.004
Dickinson E, Miller R (2001) Food colloids, fundamentals of formulation. The Royal Society of Chemistry, Cambridge
Primozic M, Duchek A, Nickerson M, Ghosh S (2017) Effect of lentil proteins isolate concentration on the formation, stability and rheological behavior of oil-in-water nanoemulsions. Food Chem 237:65–74. https://doi.org/10.1016/J.FOODCHEM.2017.05.079
Primozic M, Duchek A, Nickerson M, Ghosh S (2018) Formation, stability and in vitro digestibility of nanoemulsions stabilized by high-pressure homogenized lentil proteins isolate. Food Hydrocoll 77:126–141. https://doi.org/10.1016/j.foodhyd.2017.09.028
Can KA, Nickerson MT, Low NH (2011) Lentil and chickpea protein-stabilized emulsions: optimization of emulsion formulation. J Agric Food Chem 59:13203–13211. https://doi.org/10.1021/jf203028n
Jacobs D, Stephany M, Eisner P, Toestede S (2016) Sensory quality of lupin protein based milk alternatives. Agro Food Ind Hi Tech 27:XII–XV
Hayakawa SS, Nakai S (1985) Relationships of hydrophobicity and net charge to the solubility of milk and soy proteins. J Food Eng 50:486–491. https://doi.org/10.1111/j.1365-2621.1985.tb13433.x
Van der Plancken I, Van Loey A, Hendrickx MEG (2005) Changes in sulfhydryl content of egg white proteins due to heat and pressure treatment. J Agric Food Chem 53:5726–5733. https://doi.org/10.1021/jf050289+
International Dairy Federation (1995) 173:1995 Dried milk protein products—determination of nitrogen solubility index. International Dairy Federation, Brussels
Greenfield N (2007) Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc 1:2876–2890. https://doi.org/10.1038/nprot.2006.202
Ryan M, McEvoy E, Duignan S et al (2008) Thermal stability of soy protein isolate and hydrolysate ingredients. Food Chem 108:503–510. https://doi.org/10.1016/j.foodchem.2007.11.001
Auty MAE, Twomey M, Guinee TP, Mulvihill DM (2018) Development and application of confocal scanning laser microscopy methods for studying the distribution of fat and protein in selected dairy products. J Dairy Res 68:417–427. https://doi.org/10.1017/S0022029901004873
Bernat N, Cháfer M, Rodríguez-García J et al (2015) Effect of high pressure homogenisation and heat treatment on physical properties and stability of almond and hazelnut milks. LWT Food Sci Technol 62:488–496. https://doi.org/10.1016/j.lwt.2014.10.045
Stone H, Bleibaum RN, Thomas HA (2012) Sensory evaluation practices. Academic Press, London
Beverung CJ, Radke CJ, Blanch HW (1999) Protein adsorption at the oil/water interface: characterization of adsorption kinetics by dynamic interfacial tension measurements. Biophys Chem 81:59–80. https://doi.org/10.1016/S0301-4622(99)00082-4
Saio K, Kajikawa M, Watanabe T (1971) Food processing characteristics of soybean proteins. Agric Biol Chem 35:890–898. https://doi.org/10.1080/00021369.1971.10860005
Berghout JAM, Boom RM, van der Goot AJ (2015) Understanding the differences in gelling properties between lupin protein isolate and soy protein isolate. Food Hydrocoll 43:465–472. https://doi.org/10.1016/j.foodhyd.2014.07.003
Dickinson E (1986) Mixed proteinaceous emulsifiers: review of competitive protein adsorption and the relationship to food colloid stabilization. Top Catal 1:3–23. https://doi.org/10.1016/S0268-005X(86)80003-0
Aryee ANA, Boye JI (2015) Comparative study of the effects of processing on the nutritional, physicochemical and functional properties of lentil. J Food Process Preserv. https://doi.org/10.1111/jfpp.12824
Sathe SK, Zaffran VD, Gupta S, Li T (2018) Protein solubilization. J Am Oil Chem Soc 95:883–901. https://doi.org/10.1002/aocs.12058
Nakai S (1983) Structure–function relationships of food proteins with an emphasis on the importance of protein hydrophobicity. J Agric Food Chem 3:676–683. https://doi.org/10.1021/jf00118a001
Crowley SV, Megemont M, Gazi I et al (2014) Heat stability of reconstituted milk protein concentrate powders. Int Dairy J 37:104–110. https://doi.org/10.1016/J.IDAIRYJ.2014.03.005
Sadilova E, Carle R, Stintzing FC (2007) Thermal degradation of anthocyanins and its impact on color and in vitro antioxidant capacity. Mol Nutr Food Res 51:1461–1471. https://doi.org/10.1002/mnfr.200700179
European Parliament and Council of the European Union (2006) Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods
Acknowledgements
The work for this publication has been undertaken as part of the PROTEIN2FOOD project. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 635727. We thank our colleagues Martin Vogelsang O’Dwyer, Lilit Ispiryan and Michael Cronin for technical assistance, and James A. O’Mahony, Martina Hickisch and Dave Waldron, who provided insight and expertise that greatly assisted the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with animal subjects or humans, besides the sensory evaluation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeske, S., Bez, J., Arendt, E.K. et al. Formation, stability, and sensory characteristics of a lentil-based milk substitute as affected by homogenisation and pasteurisation. Eur Food Res Technol 245, 1519–1531 (2019). https://doi.org/10.1007/s00217-019-03286-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03286-0