Abstract
Light-emitting diode (LED) irradiation is a promising way of food non-thermal preservation in recent years, and modified atmosphere packaging (MAP) is a traditional preservation method that can realize long-term storage. To maximize the shelf life of pakchoi and maintain its quality, pakchoi was packaged and stored under modified atmosphere with and without red and white LED illumination. The initial gas partial pressure (kPa) of O2/CO2 in the three modified atmospheres was 5/10, 5/5, and 10/5. At the end of 30-day refrigerated storage period, LED illumination + 5 kPa O2 + 5 kPa CO2 showed better effects than other treatments in delaying losses of mass, chlorophylls, soluble sugars, and ascorbic acid and ultimately maintained the package gas partial pressure at O2/CO2 = 5.11/5.09. Furthermore, the treatment alleviated the accumulation of malondialdehyde, reduced the activity of the polyphenol oxidase and peroxidase, and better maintained DPPH radical scavenging activity. In addition, the combined treatment significantly reduced the amount of soluble quinone accumulated in pakchoi at the end of the storage period. The optimal treatment was effectively distinguished from other treatments by principal component analysis (PCA). The analysis confirmed that the freshness of the jointly treated samples was least different from the start day samples. Through correlation analysis, respiratory rate was highly positively correlated with soluble sugar content, color index, and DPPH radical scavenging activity. In conclusion, the combined treatment of LED irradiation and MAP led to the cumulative effect of different indicators, effectively alleviated the occurrence of browning of pakchoi, enhanced the preservation effect, and extended the shelf life of pakchoi to 30 days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brassicaceae is a plant family with significant economic values; more than 300 genera and as much as 3200 species of these plants have been identified (Zhang et al., 2019). Cruciferous vegetables differ from other vegetables in that they are a major source of glucosinolates (Goelge, 2009). Pakchoi (Brassica campestris L. ssp. chinensis) is an important member of Brassica in Cruciferae; the vegetable is native to China and has been cultivated for nearly six thousand years (Huang et al., 2021). Pakchoi is preferred because of its desirable eating quality, short growth cycle, high yield, and high nutritive value. It can provide essential vitamins, minerals, and phenolics and, like other cruciferous plants, has been repeatedly shown to be rich in glucosinolates with potential anti-cancer effects (Sidransky et al., 1966; Lewis & Fenwick, 1987, 1988). Therefore, regular consumption of pakchoi is very beneficial to maintain human health (Verhoeven et al., 1997; Goelge, 2009). Young pakchoi freshly harvested from a greenhouse nevertheless exhibits high respiration rate and large leaf areas, resulting therefore in significant water loss and wilting, which in turn shorten its shelf life (Yang et al., 2009). Appropriate ways to preserve freshness of post-harvest pakchoi has always been a research hotspot that people are concerned about.
Modified atmosphere packaging (MAP) has increasingly been used in the preservation of fruit and vegetables (Wilson et al., 2019). MAP has been proven to have positive effect on maintaining the original quality of such produce as banana, broccoli, cabbage (Yang et al., 2018), and Brussels sprouts (Kobori et al., 2011), among many others. Although the shelf life of most crops can be extended via the use of MAP, MAP alone can easily cause metabolic disorder of the enzyme system related to the respiratory chain, destroying then the dynamic balance between the generation and scavenging of free radicals in the plant body (Peng et al., 2020; Zhang et al., 2019). Therefore, it is not adequate to maintain the quality of postharvest vegetables by using only the modified atmosphere.
At present, light-emitting diode (LED) illumination is one of the most popular emerging alternative technologies that can be used for the preservation of agricultural products. Such a postharvest treatment is effective as it is known that many postharvest fruit and vegetables can still respond to part of the visible light (Nassarawa et al., 2021). Zhou et al. (2019), for example, reported that continuous white LED irradiation could help increase the contents of chlorophylls and vitamin C during room temperature storage of pakchoi, thereby effectively delaying the postharvest senescence of the product. It has also been demonstrated that white and blue LED light irradiation increases the amount of phenolic compounds and associated synthetic amino acids in okra (Wilawan et al., 2019). In an exemplifying study on fruit, strawberries exposed to 40 μmol m−2 s−1 blue light were noted to not only exhibit higher levels of ascorbic acid and sugars, but also increased antioxidant and enzyme activities during the 12-day cold storage (Xu et al., 2014). Research on photodynamic inactivation has also found that LED illumination at a wavelength of 405 ± 5 nm could inactivate photodynamic activity of Salmonella on the surface of fresh-cut papaya, making the fruit less susceptible to spoilage (Kim et al., 2017). However, when only LED treatment is applied, there is a risk of excessive stomatal opening and transpiration, resulting in water (and quality) loss of a treated product (Bhavya et al., 2021).
To alleviate limitations of single postharvest treatments, several combined treatment strategies have been proposed and tested; these include the combination of MAP with ozone treatment (Chen et al., 2016), UV treatment (Vunnam et al., 2014), ultrasound (Zhang et al., 2019), chitosan carbon-dot coating (Fan et al., 2021), as well as with the use of sodium hypochlorite (Waghmare & Annapure, 2015). Interestingly, although there exist a number of studies on separate use of MAP and LED illumination as a method of postharvest plant preservation, a combination of these two attractive treatment technologies is less common. Among the limited studies, Luo et al. (2021) reported that red and blue LED when used in combination with MAP could effectively decelerate mass loss of flowering Chinese cabbage and inhibit the activities of chlorophyllase, pheophytinase, and chlorophyll-degrading peroxidase. The color quality of flowering Chinese cabbage could then be maintained for four days at room temperature. Suitable LED illumination can up regulate the synthesis genes of ascorbic acid and phenolics, induce the production of amino acids from excited free radicals upon photo-oxidation, and inactivate browning-related enzymes. LED illumination can also help inhibit the activities of microorganisms on the surface of vegetables through photodynamic inactivation. We speculate that these advantages of LED illumination can compensate for the shortcomings of MAP when the two treatment technologies are used in combination. The photosynthetic reaction of postharvest vegetables can make up for the loss of organic matter caused by aerobic respiration and well maintain the stability of the gas partial pressure in the package. Conversely, accelerated mass loss of agricultural produce upon LED irradiation may also be alleviated by the application of MAP.
Poorly studied combination of red and white (RW) LED was selected as the illumination source. Red LED light with wavelength in the range of 610–720 nm has been shown to be capable of stimulating plants to maintain their short-term photosynthesis as well as to promote chlorophylls synthesis and carbohydrate accumulation (Muneer et al., 2014; Castillejo et al., 2021). White LED light, on the other hand, is the most commonly used light; white light was indeed noted to inhibit degradation of chlorophylls in irradiated pakchoi (Yan et al., 2020; Kasim & Kasim, 2017).
Based on the aforementioned arguments, in order to extend the edible period of pakchoi to 30 days, this study explored the effect and mechanism of MAP combined with RW LED illumination on the nutritional value, anti-oxidation, apparent index, and enzyme activity of pakchoi during storage. Finally, the correlation between 12 indexes affecting the quality of postharvest pakchoi was analyzed, and the principal component analysis (PCA) of five treatments was carried out based on the indexes to verify the difference between the preservation effect of the best combination at 30 days and the other treatments.
Materials and Analysis Method
Materials
The preliminary preparations such as the selection, procurement (Xuelang veggie market in Wuxi, Jiangsu, China), transportation, and standby of fresh pakchoi (Brassica campestris L. ssp. Chinensis) were completed within 1 h. Any vegetables with uneven color, yellowing, and mechanical damage were not allowed to be purchased as samples.
All chemicals are analytical pure reagents purchased from Beijing Inokai Technology Co., Ltd. (Beijing, China) and Shanghai TITAN Technology Co., Ltd. (Shanghai, China).
Postharvest Treatments and Storage
Soil and other foreign materials were shaken off the pakchoi; the vegetable was then stored without any further pretreatment. Every two samples (160 ± 5 g each) were packed in an 80-μm polyethylene terephthalate (PET) bag (Xiamen Emison New Material Technology Co., Ltd., Fujian, China) with the dimensions of 28 × 40 cm. The packaging material has an oxygen permeability of 0.39 mL m−2 day−1 atm−1 and a carbon dioxide permeability of 0.125 mL m−2 day−1 atm−1 at 25 °C (Zhang et al., 2019; Fan et al., 2021).
To preliminarily study the effect of different LED irradiation treatments, pakchoi was wrapped with polyethylene films (Foshan Baixian Environmental Packaging Co., Ltd., Guangdong, China) to prevent excessive water loss (Hasperué et al., 2016) and treated with either red light (660 nm), white light (440 nm, 540 nm), and red-white light (RW LED, R:W ratio is 1:1). The light intensity of all LEDs can be adjusted through the own knob of the lamp bar, with a light intensity of up to 300 μmol m−2 s−1. Mass loss, soluble sugars content, and ascorbic acid content were determined. This preliminary study revealed that red-white LED illumination yielded the best effect. Similarly, in the pre-experiment to determine the optimal gas ratio, the four indexes showed that the quality of pakchoi stored in 5 kPa O2 + 5 kPa CO2 + 90 kPa N2 modified atmosphere was better than that in 5 kPa O2 + 10 kPa CO2 + 85 kPa N2 and 10 kPa O2 + 5 kPa CO2 + 85 kPa N2 modified atmosphere. The inflation mode of MAP was vacuum filling replacement. The gas was mixed in a preset proportion in the modified atmosphere packaging machine (LQ-370, Zhejiang Liqiang Packaging Technology Co., Ltd., Zhejiang, China) and then filled into the vacuum packaging (vacuumizing for 5 s, filling and flushing for 10 s). The selected optimal LED illumination color and optimal gas ratio are used as the control group for the follow-up formal experiment.
Refrigeration equipment was placed with sixteen vegetables on each floor, and each two intact whole plants of pakchoi were packed horizontally in one package without leaves overlap. In the formal experimental operation, RW LEDs with an intensity of 30 μmol m−2 s−1 (inside the bag) and a period of 12 h/day was used to irradiate pakchoi samples packed with different gas components during the entire storage period. Packages were placed on a shelf at a fixed distance from the lamp board, and a handheld high-precision luminometer (SPIC-300BW, Yuanfang Optoelectronics Information Co., Ltd., Hangzhou, China) was measured inside the unsealed bag to yield the light intensity inside the package. During this period, the bags were turned upside down at every 4-h interval to avoid the influence of water vapor that accumulated on the inner wall of the bags on the illumination (Dhakal & Baek, 2014; Huyskens-Keil et al., 2020; Shen et al., 2019). The LED lighting device was installed in the upper panel of the refrigeration space; the set-up consisted of two adjustable lamp bars (18.0 W, Guangyin Lighting Technology Co., Ltd., Shandong, China). Distance between the two lamp bars was 43 cm, which allowed each vegetable sample directly below the lamp bars to receive a light intensity of 30 μmoL m−2 s−1. All samples were stored at a temperature of 4 ± 1 °C and relative humidity of 90 ± 5% for 30 days.
The samples were divided into five groups: (1) control 1, sole RW LED irradiation; (2) control 2, sole 5 kPa O2 + 5 kPa CO2 + 90 kPa N2 modified atmosphere (light-proof treatment); (3) LED + MAP1, pakchoi under RW LED irradiation combined with 5 kPa O2 + 10 kPa CO2 + 85 kPa N2 gas ratio for modified atmosphere storage; (4) LED + MAP2, pakchoi under RW LED irradiation combined with 5 kPa O2 + 5 kPa CO2 + 90 kPa N2 gas ratio for modified atmosphere storage; and (5) LED + MAP3, pakchoi under RW LED irradiation combined with 10 kPa O2 + 5 kPa CO2 + 85 kPa N2 gas ratio for modified atmosphere storage. The samples of control 1 were wrapped with perforated polyethylene film during the whole storage process to prevent the acceleration of water loss caused by LED irradiation. The film was evenly punched with 18 holes (9 on each side) to ensure low water vapor transmittance without affecting the normal transmittance of oxygen and carbon dioxide, and the light intensity passing through the film was consistent with that as described above.
The interval between samples collection was set to 5 days (0, 5, 10, 15, 20, 25, and 30 d); three bags were randomly selected at each time. Only the green leaves of pakchoi were taken for index measurement, and the sampling amount and position of inner and outer leaves were the same to avoid errors. All samples were frozen in liquid nitrogen on the sampling day and then stored in a refrigerator at -80 °C until further analyses.
Respiration Rate Determination
Respiration rate of the sample was determined according to the improved method of Meng et al., (2013). About 100 g of pakchoi samples were placed in an independent 1000-mL sealed container and stored in a 4 °C refrigerator for two hours. 5 ml gas was extracted from the container and injected into a gas chromatograph (Shimadzu, GC-2010, Japan). Both the input port temperature and the thermal conductivity detector (TCD) temperature were set to 80 °C; Poropak N column was used, and the column temperature was 50 °C; the gas flow rate of high-purity nitrogen as carrier gas was 35 mL/min. Each measurement was performed in triplicate. The results of respiratory rate were characterized by mg CO2 kg−1 h−1.
Gas Partial Pressures Determination
The gas partial pressure in each package was measured with a headspace gas analyzer (Checkmate 9900, PBI, Danish). Fully shaken package before measurement for uniform gas distribution. Three packages of the same treatment were taken for measurements each time, and the results were expressed as kPa.
Color and Chlorophylls Content Determination
Surface color of pakchoi was determined in terms of CIE L*, a*, and b* using a colorimeter (Konica-Minolta, CR200, Tokyo, Japan). Three points were measured inside the outermost green leaf of each pakchoi and the results were characterized as -a*/b* value.
Chlorophylls content in each sample was determined using the method suggested by Mustafa et al. (2013), with slight modifications. About 1 g (fresh weight, FW) of the outermost leaf with veins remove was ground with 80% (v/v) acetone until the vegetable tissues turned white; the sample was placed in a dark place for 4 min for chlorophylls to be fully extracted. The content was filtered into a 25-mL brown volumetric flask, washed with acetone until the filter paper, mortar, and glass stick had no green attachment; light was avoided as much as possible. The absorbance of the extract was then measured at 645 nm and 663 nm using a UV–vis spectrophotometer (Techcomp, UV2600, Beijing, China). Three randomly selected samples of the same treatment were determined, and the average value is reported. The chlorophylls content is computed as
where V represents to the total volume of the filtered liquid (mL) and M represents to the mass of the sample used (g). The absorbance of the sample extract at 645 nm and 663 nm are denoted by A645 and A663, respectively.
Mass Loss Determination
Initial mass of a vegetable sample was immediately determined after the purchase. Masses at different sampling dates were also recorded. The vegetables were weighed together with the packages to avoid possible error caused by condensation in the packages. Mass loss is then calculated as
where M1 is the initial mass of the individual sample prior to treatment (g) and M2 is the mass of the sample at the moment of measurement (g).
Soluble Sugars and Ascorbic Acid Contents Determination
The method for the determination of soluble sugar content was suggested by Leng et al. (2016) with a slight modification. About 1 g of fresh sample was ground into a homogenate in a mortar, and 10 mL of distilled water was then added. The diluted homogenate was transferred to a test tube and extracted with boiling water for 30 min. The cooled extraction solution was directly filtered into a 100 mL volumetric flask, and the part below the scale was supplemented with distilled water. The extracted sample solution was allowed to react with anthrone-sulfuric acid; the absorbance was then determined at 630 nm. The corresponding sucrose mass was determined via the sucrose standard curve. Three randomly selected samples of the same treatment were measured each time, and the average value is reported in terms of mass fraction of soluble sugar in the sample (%).
Ascorbic acid content of a sample was explored by high performance liquid chromatography (Kumar et al., 2020). Five mL of sample homogenate thoroughly ground by mortar was placed in a 50-mL beaker, mixed with 20 g/L metaphosphoric acid solution and transferred to a volumetric flask, shaken and diluted to 40 mL with 25 g/L metaphosphoric acid solution. After the diluted solution was extracted by ultrasound for 5 min (frequency, 30 kHz; power intensity, 2.4 W/g), the supernatant was placed in a centrifuge for 4000 × g centrifugation for 5 min (Sigma, Osterode am Harz, 2-16PK, Germany). The supernatant was filtered through 0.45-μm aqueous phase membrane and then injected into Agilent 1100 liquid chromatograph (Agilent Technologies, Santa Clara, CA, USA). The separation was performed on a C18 column (250 × 4.6 mm i.d., and particle size of 5 μm). The elution phase consists of liquid A (6.8 g potassium dihydrogen phosphate and 0.91 g cetyltrimethylammonium bromide dissolved in water and dilute to 1 L) and liquid B (100% methanol). The parameter settings were as follows: liquid volume ratio, A:B = 98:2; column temperature, 25 °C; and flow rate, 0.65 mL/min. The concentration of the sample was determined at 245 nm against a standard curve of ascorbic acid. Three randomly selected samples were measured at a time, and the average value is reported. The unit of measurement result was g kg−1 (FW).
Malondialdehyde Content Determination
The method for the determination of malondialdehyde (MDA) content was suggested by Xu and Liu (2017) with a slight modification. Samples were homogenized with trichloroacetic acid (TCA) and centrifuged for 20 min (10000 × g, 4 °C). Supernatant was added into 0.67% (w/w) thiobarbituric acid solution; TCA was added into the control blank tube instead of the extract. All the samples were boiled in the water bath for 30 min. After cooling to ambient temperature, centrifugation was repeated. Absorbance of the supernatant was measured at 450, 532, and 600 nm. Three optional samples of the same treatment were measured at a time, and the average value is reported. MDA content is calculated as per Eq. (3):
where V represents the volume of homogenate (mL), Vs represents the volume of extracted liquid (mL) of the extracted sample during the determination, and M is the mass of the sample (g FW). A450, A532, and A600 are used to represent the absorbance at 450 nm, 532 nm, and 600 nm, respectively.
DPPH Radical Scavenging Activity Determination
The method suggested by Luo (2016) was slightly modified to measure the DPPH radical scavenging activity of the sample. After homogenization, the sample was centrifuged for 20 min (10,000 × g, 4 °C). Supernatant (0.1 mL) was added into a mixture of 3.9 mL of 25 mg/L methanolic DPPH solution and 0.1 mL of distilled water. After the reaction liquid was placed in a dark environment for 30 min, its absorbance was measured at 517 nm; equivalent volume of methanol substituted supernatant was used as the blank control. Three randomly selected samples of the same treatment were measured at a time, and the average value is reported. DPPH radical scavenging activity is calculated as per Eq. (4):
where Asample refers to the absorbance of the sample, while A0 refers to the absorbance of the blank control.
PPO and POD Activities Determination
Extraction and activities determination of PPO and POD were conducted as per the methods of Fang et al. (2007) and Hägele et al. (2016) with some modification. The outer leaves of pakchoi were homogenized in an extraction buffer consisted of 1 mmol polyethylene glycol 6000, 4% polyvinylpyrrolidone, 1% Trition X-100, and acetate-sodium acetate buffer solution (pH 5.5) in ice bath. The content was then centrifuged (8000 × g, 4 °C) for 20 min. Supernatant obtained by centrifugation was retained as an enzyme extract. A mixture of 100 μL of enzyme extract and 1 mL of 50 mmol/L catechol solution was added to 4 mL of acid-sodium acetate buffer. Distilled water was used as the reference. The absorbance at 420 nm (in the case of PPO activity determination) was determined at the beginning of the reaction and then at every 15-s interval. Three mL of 25 mmol/L guaiacol and 200 μL 0.5 mol/L hydrogen peroxide solution were added into 3 mL of the extract to start the reaction. Distilled water was used as the reference. The absorbance at 470 nm (in the case of POD activity determination) was determined at the beginning of the reaction and then at every 15-s interval.
Three randomly selected samples were determined, and the average value is reported. Units of enzyme activity per kg of sample are used to express the results (U kg−1 FW).
Soluble Quinone Content Determination
Soluble quinone content was determined as per the methods of Zhan et al. (2013). The outer leaf tissues were extracted by grinding them with methanol; the homogenate supernatant filtered with a double-layer quantitative filter paper was centrifuged for 20 min (10000 × g). The results of light absorption value measured at 437 nm are expressed as the absorbance per kg fresh sample (ODA437 kg−1 FW). Three randomly selected samples of the same treatment were determined, and the average value is reported.
Statistical Analysis
All experimental data were analyzed using one-way analysis of variance (ANOVA) with SPSS software (version 11.5.1; SPSS Inc., Chicago, IL, USA) and are presented as means with standard deviations. Tukey multiple range test was established using in this experiment to determine the significance of the difference among the mean values, and the significance level was P < 0.05. To determine the effect of the different treatments, a PCA analysis was performed with all treatment indicators as independent variables and different treatments as dependent variables (Vollmer et al., 2020). Origin Pro 2021 software was used to draw the heat map of index correlation. The correlation and difference between different indexes following Pearson’s correlation analysis via color change trend feedback. Differences were due to storage temperature, light intensity, individual differences in vegetables, and experimental treatment.
Results and Discussion
Respiration Rate and Partial Pressure of \({\mathrm{O}}_{2}\) and \({\mathrm{CO}}_{2}\)
Strong respiration will cause the nutrients in the harvested vegetables to be quickly consumed, which is not conducive to long-term storage (Hasperué et al., 2016). The traditional storage method does not involve the photosynthesis of plants and can only maintain the quality of agricultural products by adjusting the respiration rate. In the early postharvest period, green vegetables can still undergo photosynthesis to produce organic matter to maintain the consumption caused by aerobic respiration. The sample of pakchoi used in this experiment is stored as whole plants, so the maintenance of quality is the joint effect of respiration and photosynthesis.
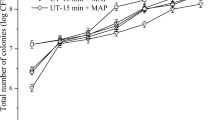
The different changing trends of the respiration rate of the five groups of pakchoi are shown in Fig. 1A. The initial CO2 partial pressure in the sample storage environment treated with LED + MAP1 was relatively high, which resulted in the inhibition of aerobic respiration in the initial phase, manifested by a rapid increase in O2 partial pressure (Fig. 1B) (Zhang et al., 2008). As expected, due to the existence of photosynthesis, sugar and oxygen accumulate rapidly, bringing the oxygen concentration in the package above the osmotic equilibrium maximum, thus triggering a respiratory peak occurring on day 15, similar to the findings of previous studies (Charles et al., 2008). Other processed samples differ from the above trend, showing that the respiratory rate first increased and then decreased, but the time of their respiratory peak was significantly different. The peak of the respiratory rate of samples treated with LED + MAP2 was significantly delayed, and the peak was not high. Lower respiratory rates are key to maintaining the quality of vegetables in storage (Chitravathi et al., 2015). This probably because the initial gas ratio of 5 kPa O2 + 5 kPa CO2 in the packaging had a strong inhibition on respiration of pakchoi, and the light compensation intensity of pakchoi at this stage was lower than the light intensity chosen in our experiment, so the oxygen accumulated for a long time until respiration began to dominate. However, because the initial set gas ratio of MAP3 was conducive to the respiration of pakchoi, so different from LED + MAP2 treatment, the CO2 partial pressure tended to increase (Fig. 1C) (Garrido et al., 2016). With the subsequent degradation of chloroplasts, the lack of inorganic salts, and the reduction of molecular enzyme activity, the quality of vegetables began to decline, photosynthesis gradually stopped, and the concentration of carbon dioxide gradually stabilized, leading to a continuous decreasing respiratory rate (Meng et al., 2012).
As the quality of pakchoi continues to decline in the later stage of storage, the light compensation point is not stable. But it was certain that photosynthesis changed the gas partial pressure in the packaging, thus realizing the regulation of the pakchoi breathing rate. On the 30th day, the ratio of gas already balanced in the package treated by LED + MAP2 was O2:CO2 = 5.09 kPa:5.11 kPa. The combination of light intensity of 30 μmol m−2 s−1 and 5 kPa O2 + 5 kPa CO2 packaging was the best combination parameter to maintain the quality of pakchoi.
Color and Chlorophylls Content
In this study, the -a*/b* value was used to express the color of stored pakchoi. The higher the value, the greener the outermost leaves of the sample retained and the higher the edible value. Notably, chlorophyll breakdown during storage causes leaves discoloration and yellowing (Zhan et al., 2014). In all cases, -a*/b* values showed an overall downward trend and recovered somewhat after 10 days of storage; the chlorophylls content showed the same trend (Fig. 2A and B). After 15 days of storage, chlorophylls contents of pakchoi treated with control 1 or control 2 were lower than that of the combined treatments (P < 0.05). At the 30th day, the chlorophylls content of the sample treated with LED + MAP2 was 29.07% and 19.05% higher than those of the sole MAP group and sole illumination group, respectively.
Visual observation revealed that yellowing of the outer leaves of pakchoi was not extensive, with most of the leaves showing bright green color. This might be due to the fact that RW LED reduced the activity of chlorophylls degradation enzymes and even promoted the synthesis of chlorophyll; chlorosis in the vegetables might have also been inhibited (Luo et al., 2021). One study show that after continuous white light irradiation, the relative expression levels of chlorophyll degradation related genes such as BrChlase1, BrChlase2, and BrPPH remained low during storage, while no similar phenomenon occurred in control samples (Zhou et al., 2019). In this experiment, samples treated with control 1 were well colored in early storage but had the lowest chlorophyll content in the late stages. This might be due to the higher water loss, aggravating the leaf wilting and yellowing. Kasim and Kasim (2017) also reported similar results. Compared with other wavelengths of light, the chlorophylls content of lettuce stored for 21 days was higher under red and white LED irradiation. Lamb’s lettuces treated with warm-white LEDs also exhibited similar behavior (Braidot et al., 2014).
Mass Loss
Mass loss is directly related to the loss of quality, adversely affecting the commercial and edible qualities of the vegetables in all aspects (Nassarawa et al., 2021). An increased rate of transpiration often leads to weight loss, the shrinking appearance of leaves, and hastening wilting. Figure 3 shows the mass loss of pakchoi undergone different treatments in 30 days. The mass loss rate of all samples increased during storage; however, among the four groups with modified atmosphere, the value was within 1%. LED + MAP2 exhibited the lowest loss of mass. A higher mass loss in the case of sample undergone LED + MAP3 than in the case of the control 2 (0.97 ± 0.01% and 0.93 ± 0.01%, respectively) might be due both to the higher oxygen concentration accelerating the respiration rate of the vegetable, as well as to the fact that light led to larger stomatal opening, which accelerated transpiration (Ma et al., 2014).
Mass loss of the sample subjected to sole illumination was as high as 2.53 ± 0.07% at 30 days, which was beyond the maximum acceptable limit of 2%. A number of previous studies also showed that LED illumination indeed accelerated the weight loss rate of stored produce. Mass loss of kale stored under 21.8 μmol m−2 s−1 fluorescent lamp at 1 °C for 10 days was 3.9%, compared with 1.8% in the control group (Noichinda et al., 2007). The above data showed that MAP made up for the shortcoming of LED illumination. In other words, the combination of the two treatments could achieve the expected effect of delaying the mass loss of pakchoi.
Soluble Sugar and Ascorbic Acid Contents
As the energy source of plant growth and metabolism, sugar are the substrates of a number of chemical reactions and play an important role in maintaining the postharvest quality of agricultural produce (Hasperué et al., 2016). As expected, the combination of LED illumination and MAP had a positive effect on maintaining the soluble sugars content of pakchoi (Fig. 4A). At the 30th day, soluble sugars content in the case of LED + MAP2 was significantly higher than other treatments (P < 0.05), which were 18.75%, 23.62%, 48.69%, and 68.16% higher than those in the cases of LED + MAP1, LED + MAP3, control 1, and control 2, respectively.
It is well known that glucose is the main product of photosynthesis, so the final storage value of sugars depends on a comprehensive balance of respiratory consumption and photosynthetic synthesis (Ruiz de Larrinaga et al., 2019). Each group of samples exposed to light showed a trend to increase the soluble sugar content during the first 15 days of storage, while the control 2-treated samples remained decreased throughout the experiment. The soluble sugar content of the samples treated with LED + MAP3 was slightly recovered on day 15. This may be due to the high initial respiration rate of the sample, but with increasing CO2 concentration, photosynthesis played a major role on day 10, followed by a response to soluble sugar content and oxygen concentration, respectively, with a new small peak on days 15 and 20. Total sugars content in strawberries irradiated with 40 μmol m−2 s−1 blue light at 5 °C for 6 days was also noted to be higher than that of control (Xu et al., 2014). Similar conclusions were made in banana treated with blue light, where eight-fold higher soluble sugars content was noted compared with that of the control (Huang et al., 2018).
Soluble sugars such as hexose precursors likely serve as a carbon source in the ascorbic acid synthesis pathway. It is therefore of interest to determine of the ascorbic acid content has any positive correlation with the soluble sugars content (Shi et al., 2016). Figure 4B shows the change in the ascorbic acid content of pakchoi during storage. Ascorbic acid content of all the samples decreased upon prolonged storage, with LED + MAP2 treated pakchoi having higher content than the samples treated by other treatments during the first 15 days; the downward trend was slower afterward. At the end of 30-day storage, the ascorbic acid content of this treated sample was 0.79 ± 0.05 g kg−1 FW, which was 2 times and 2.38 times higher than the samples undergone the other two combined treatments and 52.97% lower than the initial value (P < 0.05). On the other hand, the samples that have undergone control 1 and control 2 exhibited the ascorbic acid contents of 0.018 ± 0.033 g kg−1 FW and 0.2188 ± 0.022 g kg−1 FW, 98.91% and 86.68% lower when compared with the initial values. This clearly illustrates that combined LED illumination and MAP could maintain the contents of soluble sugars and ascorbic acid by reducing the respiration and maintaining the photosynthesis, making the nutritional quality of pakchoi better than that of the control even after 30 days of storage.
Malondialdehyde (MDA) Content
Plants generate a large amount of reactive oxygen species (ROS) under abiotic stress conditions, thereby generating toxic products (Foyer & Shigeoka, 2011). MDA is a product of the peroxidation reaction of cell membrane lipids in agricultural products. Its content is indeed negatively correlated with the integrity of cell membrane, therefore reflecting the degree of damage of cell membrane in the aging process of fruits and vegetables (Chen et al., 2020). As shown in Fig. 5A, MDA contents of all treated samples increased during the storage. The sample subjected to LED + MAP2 exhibited the MDA content increase of 6.82 ± 0.22 μmol kg−1, significantly lower than those undergone other treatments (P < 0.05). MDA content in the case of sole MAP was increased by 9.60 ± 0.54 μmol kg−1, the highest of all treatments.
In the other two combined processes, LED + MAP1 slowed the MDA increase better than LED + MAP3. The efficacy of the latter treatment was similar to that of the control 1 treatment, which could not prevent the accumulation of MDA. It could be inferred then that LED illumination might have reduced membrane lipid peroxidation during the storage process of pakchoi, therefore reducing the accumulation of MDA and hence the delayed decomposition and senescence of the vegetable. Similarly, there was a study using a 20-μm-thick bag to store litchi at 5 ± 1 °C, and the results proved that the MDA content of the control group placed in the air atmosphere was higher than that of the MAP treatment during 28 days (Ali et al., 2019).
DPPH Radical Scavenging Activity
The antioxidant activity of low molecular-weight metabolites and the content of antioxidants such as phenols, flavonoids, and ascorbic acid are closely related to the free radical scavenging activity of DPPH (1,1-diphenyl-2-picrylhydrazyl) (Ilić & Fallik, 2017). It is seen in Fig. 5B that when LED illumination treatment was applied, the DPPH free radical scavenging activity exhibited an upward trend during an initial stage of storage. The three combined treatments resulted in higher activities, while that of the control 2-treated pakchoi gradually decreased within 30 days.
Compared with other experimental groups with illumination, pakchoi treated by LED + MAP2 maintained the upward trend of DPPH radical scavenging activity for 10 days, with a maximum of 90.93%, reduced only by 17.20% at the end of storage (P < 0.05); the other treatments resulted in the decline of 20.38%, 22.44%, 36.94%, and 35.82%. The DPPH radical scavenging activity of pitaya peel also showed an increased trend after red LED irradiation; this was negatively correlated with the fruit decay rate (Wu et al., 2020).
The higher free radical scavenging ability of pakchoi treated with combined LED illumination and MAP might be caused by the higher contents of such antioxidant substances such as ascorbic acid and even phenolics and anthocyanins. In the early stage of photosynthesis, the light reaction generates energetic molecules such as ATP and NADPH to stimulate the electron transport chain and generate ROS (Chen et al., 2016). The initial accumulation of ROS may activate the antioxidant system and increase the antioxidant capacity of plants. Some oxygen free radical detoxification enzymes, such as catalase (CAT) and superoxide dismutase (SOD), might also produce active responses, thereby reducing the content of H2O2 and O2− radicals, which is beneficial to the improvement of the stress resistance and quality of stored pakchoi (Song et al., 2020).
Activities of POD and PPO
During the preservation of fruits and vegetables, enzymatic browning is one of the most common and important undesirable phenomena that must be avoided. Storage of pakchoi for an extended period of time results in large areas of yellowing and browning, which adversely affects the appearance and consumer’s desire to purchase the vegetable (Zhan et al., 2013). POD and PPO activities are the major indicators of fruit and vegetable browning; both enzymes catalyze the oxidation of phenolic compounds by H2O2 to produce quinones and form brown-black pigments (Edwards, 2001).
As can be seen from Fig. 6A, POD activity increased throughout the course of storage. Four groups of treated samples with light participation showed a small increase in enzyme activity during the first five days of storage, compared with the rapid increase of control 2-treated samples during the same period. POD activity of pakchoi samples treated with LED illumination in combination with MAP at 30 days were 6.87 ± 0.18, 6.64 ± 0.15, and 6.79 ± 0.12 U kg−1 FW, respectively. LED + MAP2 was noted to yield the most desirable result, more desirable than the other two treatments (P < 0.05). PPO activity evolution is shown in Fig. 6B. The changes in response to the PPO after light irradiation were similar to the POD. It was apparent that LED irradiation stabilized the PPO activity of the sample at later stages of storage, while the control 2-treated samples exhibited high enzymatic activity. The enzymatic activity of the control 2-treated samples was 16.039%, 23.154%, 17.902%, and 13.690% higher than the other samples, respectively. These data suggested that LED irradiation may be an effective strategy to inhibit PPO activity in postharvest pakchoi, although differences between treatments were not significant when different gas partial pressures were combined with it.
Studies have shown that LED irradiation significantly affects the activities of PPO and POD, but the opposite results appear due to different sample varieties and light intensity. Zhan et al. found that high intensity visible light at 2500 lx promoted ascorbic acid accumulation and effectively inhibited PPO and POD activity, while low intensity light at 500 lx caused the exact opposite result (Zhan et al., 2012). Manzocco et al. subjected aqueous solutions of PPO without additional photosensitizers to UV-C and different intensities of visible light irradiation respectively and found a physiological decay in PPO activity after visible light irradiation on the order of hours. The decreasing trend of PPO activity under high radiation illumination (12.7 W m−2) was similar to that of UV-C irradiation, but lower intensity visible light irradiation (11.7 and 9.4 W m−2) activated the enzyme at the initial stage of storage, and only after 6 h did the enzyme activity start to gradually decrease (Manzocco et al., 2009). These phenomena are similar to the results obtained in this study using intermittent LED irradiation treatments, i.e., not only the UV spectrum but also visible light may have caused changes in enzyme structure leading to enzyme inactivation. The light intensity used in our experiments may have exceeded the range of promoting enzyme activity, which has an inhibitory effect on PPO activity. It has also been found that white, red, and blue LED irradiation inhibited POD activity in basal spear sections of post-harvest white asparagus, while UV-C treatment increased it (Huyskens-Keil et al., 2020).
The exact principle responsible for enzyme inactivation is not known for sure, but there are several possibilities: (1) direct photo-oxidation of amino acid residues generates free radicals and excited state species that reduce enzyme activity (Manzocco et al., 2009); (2) visible light irradiation increases the content of soluble carbohydrates, which regulates the increase in the size of the ascorbic acid pool, effectively maintaining a high concentration of ascorbic acid in the tissue and thus keeping cytoplasmic pH below the optimal range for enzymatic activity (Vamos-Vigyazo, 1981; Toledo et al., 2003; Degl’Innocenti et al., 2007); (3) light as an external environmental stimulus may lead to an increase in hydrogen peroxide content and phenolic compounds in pakchoi, which increases the amount of peroxidase through feedback and may therefore lead to excessive spatial resistance, thus making it difficult for the substrate to bind precisely to the active site of the enzyme, resulting in reduced enzyme activity; and (4) visible light irradiation of appropriate intensity was able to reduce the expression of PPO and POD genes in the samples, leading to a reduction in enzyme activity and a decrease in browning (Liu et al., 2015). Our results are consistent with the previous research results of Zhan et al. A 30 ± 1 μmoL m−2 s−1 of visible light irradiated continuously for 7 days reduced the PPO and POD activities of spinach samples by 36.69% and 10.77% (Zhan et al., 2020), and 2000 ± 2 Lux visible light irradiation also inhibited the PPO and POD activities of fresh-cut celery stored at 7 °C and increased the phenylalanine aminolytic enzyme activity, thus increasing the total phenolic content (Zhan et al., 2013).
Soluble Quinones Content
As discussed in the introduction, soluble quinones are products of enzymatic browning reactions. Evolution of soluble quinones content in pakchoi during storage is shown in Fig. 6C. The content of soluble quinone in the treated samples tended to increase regardless of the presence of light irradiation. The basic elements of enzymatic browning reaction are substrate, oxygen, and related enzymes (Degl’Innocenti et al., 2007). Therefore, different gas partial pressure and photosynthesis will affect the oxygen concentration and the production of soluble quinone. The initial oxygen partial pressure of the LED + MAP3 treatment setting was high, while in the early stage of storage, light exposure may not cause substantial photo-oxidation of amino acid residues, so the generated free radical content and the structural changes of the enzyme were not enough to inactivate PPO and POD (Zhan et al., 2014). Hence, the samples from this treatment group showed a significantly high soluble quinone content in the first 10 days as compared to the other treated samples (P < 0.05). In contrast, the gas partial pressure of 5 kPa O2 + 5 kPa CO2 effectively inhibited aerobic respiration. Combined with photosynthesis, the O2 concentration fluctuated slightly in the whole storage process. Due to the inhibitory effect of light on the enzyme activity described above, the quinone content of LED + MAP2-treated samples were low, with only 39.27 ± 0.21 ODA437 kg−1 FW at 30 days, up only 23.76% over the initial value.
Higher ascorbate concentrations are known to help inhibited PPO catalytic activity and are beneficial in reducing quinone accumulation (Peng et al., 2013). Therefore, LED illumination in combination with MAP could retard enzymatic browning and maintain pakchoi appearance quality by reducing melanin accumulation through increased ascorbate concentration. A previous study found a similar phenomenon to this experiment and confirmed the conjecture. Zhan et al. (2013), for example, reported that celery stored at 7 °C had lower soluble quinone content than dark treated samples after 8 days of white light irradiation with intensity of 2000 ± 2 Lux. The combination of LED illumination and MAP may achieve the goal of reducing the pakchoi browning and maintaining its post-harvest quality by inhibiting the key enzymatic activity during the enzymatic browning reaction and controlling the oxygen partial pressure.
Figure 7 shows the quality changes of all samples at different stages of storage. The appearance of pakchoi treated with LED + MAP2 were superior to that undergone control 1 and control 2. The degree of yellowing of leaves was the lowest. The values of most quality indicators were also preferable than those belonging to the sample undergone the other combined treatments.
Principal Component Analysis and Correlation Analysis
Principal component analysis (PCA) is a common multivariate statistical analysis method simplified by using dimension reduction techniques and by substituting a few representative comprehensive indicators instead of multiple original variables based on the size of the contribution rate. This analysis method is able to objectively determine the weights of each indicator, avoiding subjective arbitrariness (Yang et al., 2019). At present, the principal component analysis method has been widely used in food products (Echeverría et al., 2008).
After the analysis, there were two principal component eigenvalues greater than 1, while the total variance contribution of these two principal components reached 89.48, successively 76.08%, and 13.40% (Fig. 8A). Therefore, multiple quality indicators of pakchoi were successfully reduced to two unrelated main components. Separation was evident between the various treatments. LED + MAP2 treated samples clustered together and were closest to the horizontal direction of the fresh sample, indicating that the quality of cabbage in this treated group was closest to “fresh”.
Correlations between the twelve quality assessment indicators were discussed by relevant heatmap analysis (Fig. 8B). It can be seen that respiratory rate was highly positively correlated with soluble sugar content, color index, and DPPH radical scavenging activity, negatively with weight loss and MDA content, while soluble quinone content was highly positively associated with PPO and POD activity and negatively with color index. It has been shown that the appropriate gas partial pressure can limit the respiratory rate of pakchoi and thus regulate the soluble sugar content and the degree of weight loss, whereas LED light irradiation can achieve the purpose of inhibiting pakchoi browning by limiting the activity of browning-related enzymes and the accumulation of tissue quinone.
Conclusion
LED illumination and MAP were combined to alleviate the disadvantages of each individual treatment technology; the combined treatment was applied to maintain postharvest quality of pakchoi during 30-day refrigerated storage. Based on the color, chlorophylls content, respiration rate, gas partial pressure, mass loss, soluble sugars, ascorbic acid and MDA contents, DPPH radical scavenging activity, PPO and POD activities, and soluble quinone content, combination of red-white LED illumination and MAP could maintain the quality of pakchoi better than sole illumination or sole MAP. LED illumination + 5 kPa O2 + 5 kPa CO2 exhibited the best effect and could delay the browning of outer pakchoi leaves and inhibit the occurrence of membrane lipid peroxidation and browning reaction. Such a combination also helped reduce PPO and POD activities, while maintaining the antioxidant activity of the vegetable. Compared with traditional refrigeration, LED irradiation, and MAP, the shelf life of pakchoi has been extended by at least 23 days, 15 days, and 5 days (Fig. 7). A follow-up study is underway to make a more precise proportional determination of the gas atmosphere and eliminate possible negative effects of the combined treatment. More research on the interaction between gas and light specific to the molecular level will be carried out.
Data Availability
The data are available from the corresponding author upon suitable request.
References
Ali, S., Sattar Khan, A., Ullah Malik, A., Anjum, M. A., Nawaz, A., & Shoaib Shah, H. M. (2019). Modified atmosphere packaging delays enzymatic browning and maintains quality of harvested litchi fruit during low temperature storage. Scientia Horticulturae, 254, 14–20.
Edwards, A. M., & E. S. (2001). Effect of visible light on selected enzymes, vitamins and amino acids. Journal of Photochemistry and Photobiology B: Biology, 63, 126–131.
Bhavya, M. L., Shewale, S. R., Rajoriya, D., & Hebbar, H. U. (2021). Impact of blue LED illumination and natural photosensitizer on bacterial pathogens, enzyme activity and quality attributes of fresh-cut pineapple slices. Food and Bioprocess Technology, 14(2), 362–372.
Braidot, E., Petrussa, E., Peresson, C., & Patui, S. (2014). Low-intensity light cycles improve the quality lamb’s lettuce (Valerianella olitorio L.Pollich) during storageat low temperature. Postharvest Biology and Technology, 90, 15–23.
Castillejo, N., Martinez-Zamora, L., Gomez, P. A., Pennisi, G., Crepaldi, A., Fernandez, J. A., Orsini, F., & Artes-Hernandez, F. (2021). Postharvest LED lighting: Effect of red, blue and far red on quality of minimally processed broccoli sprouts. Journal of the Science of Food and Agriculture, 101(1), 44–53.
Charles, F., Guillaume, C., & Gontard, N. (2008). Effect of passive and active modified atmosphere packaging on quality changes of fresh endives. Postharvest Biology and Technology, 48(1), 22–29.
Chen, F., Zhang, M., & Yang, C. H. (2020). Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason Sonochem, 63, 104953.
Chen, J., Hu, Y., Wang, J., Hu, H., & Cui, H. (2016). Combined effect of ozone treatment and modified atmosphere packaging on antioxidant defense system of fresh-cut green peppers. Journal of Food Processing and Preservation, 40(5), 1145–1150.
Chitravathi, K., Chauhan, O. P., Raju, P. S., & Madhukar, N. (2015). Efficacy of aqueous ozone and chlorine in combination with passive modified atmosphere packaging on the postharvest shelf-life extension of green chillies (Capsicum annuum L.). Food and Bioprocess Technology, 8(6), 1386–1392.
Degl’Innocenti, E., Pardossi, A., Tognoni, F., & Guidi, L. (2007). Physiological basis of sensitivity to enzymatic browning in ‘lettuce’, ‘escarole’ and ‘rocket salad’ when stored as fresh-cut products. Food Chemistry, 104(1), 209–215.
Dhakal, R., & Baek, K. H. (2014). Metabolic alternation in the accumulation of free amino acids and γ-aminobutyric acid in postharvest mature green tomatoes following irradiation with blue light. Horticulture, Environment, and Biotechnology, 55(1), 36–41.
Echeverría, G., Graell, J., Lara, I., & López, M. L. (2008). Physicochemical measurements in ‘Mondial Gala®’ apples stored at different atmospheres: Influence on consumer acceptability. Postharvest Biology and Technology, 50(2–3), 135–144.
Fan, K., Zhang, M., Guo, C., Dan, W., & Devahastin, S. (2021). Laser-induced microporous modified atmosphere packaging and chitosan carbon-dot coating as a novel combined preservation method for fresh-cut cucumber. Food and Bioprocess Technology, 14(5), 968–983.
Fang, Z., Zhang, M., & Sun, Y. (2007). Polyphenol oxidase from baybenry (Myrica rubra Sieb. et Zucc.) and its role in anthocyanin degradation. Food Chemistry, 103(2), 268–273.
Foyer, C. H., & Shigeoka, S. (2011). Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiology, 155, 93–100.
Garrido, Y., Tudela, J. A., Hernández, J. A., & Gil, M. I. (2016). Modified atmosphere generated during storage under light conditions is the main factor responsible for the quality changes of baby spinach. Postharvest Biology and Technology, 114, 45–53.
Goelge, S. (2009). Glucosinolates in crucifers and their potential effects against cancer: Review. Canadian Journal of Plant Science, 89, 953–959.
Hägele, F., Nübling, S., Schweiggert, R. M., Baur, S., Weiss, A., Schmidt, H., Menegat, A., Gerhards, R., & Carle, R. (2016). Quality improvement of fresh-cut endive (Cichorium endivia L.) and recycling of washing water by low-dose UV-C irradiation. Food and Bioprocess Technology, 9(12), 1979–1990.
Hasperué, J. H., Guardianelli, L., Rodoni, L. M., Chaves, A. R., & Martínez, G. A. (2016). Continuous white–blue LED light exposition delays postharvest senescence of broccoli. LWT - Food Science and Technology, 65, 495–502.
Hasperué, J. H., Rodoni, L. M., Guardianelli, L. M., Chaves, A. R., & Martínez, G. A. (2016). Use of LED light for Brussels sprouts postharvest conservation. Scientia Horticulturae, 213, 281–286.
Huang, J. Y., Xu, F., & Zhou, W. (2018). Effect of LED irradiation on the ripening and nutritional quality of postharvest banana fruit. Journal of the Science of Food and Agriculture, 98(14), 5486–5493.
Huang, Y. C., Yang, Y. H., Sridhar, K., & Tsai, P. J. (2021). Synergies of modified atmosphere packaging and high-voltage electrostatic field to extend the shelf-life of fresh-cut cabbage and baby corn. LWT, 138.
Huyskens-Keil, S., Eichholz-Dündar, I., Hassenberg, K., & Herppich, W. B. (2020). Impact of light quality (white, red, blue light and UV-C irradiation) on changes in anthocyanin content and dynamics of PAL and POD activities in apical and basal spear sections of white asparagus after harvest. Postharvest Biology and Technology, 161.
Ilić, Z. S., & Fallik, E. (2017). Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environmental and Experimental Botany, 139, 79–90.
Kasim, M. U., & Kasim, R. (2017). While continuous white LED lighting increases chlorophyll content (SPAD), green LED light reduces the infection rate of lettuce during storage and shelf-life conditions. Journal of Food Processing and Preservation, 41(6).
Kim, M. J., Bang, W. S., & Yuk, H. G. (2017). 405 +/- 5 nm light emitting diode illumination causes photodynamic inactivation of Salmonella spp. on fresh-cut papaya without deterioration. Food Microbiology, 62, 124–132.
Kobori, C. N., Huber, L. S., Sarantopoulos, C. I., & Rodriguez-Amaya, D. B. (2011). Behavior of flavonols and carotenoids of minimally processed kale leaves during storage in passive modified atmosphere packaging. Journal of Food Science, 76(2), H31-37.
Kumar, S. S., Arya, M., Nagbhushan, P., Giridhar, P., Shetty, N. P., Yannam, S. K., & Mahadevappa, P. (2020). Evaluation of various drying methods on bioactives, ascorbic acid and antioxidant potentials of Talinum triangulare L., foliage. Plant Foods Human Nutrition, 75(2), 283–291.
Leng, F., Sun, S., & Jing, Y. (2016). A rapid and sensitive method for determination of trace amounts of glucose by anthrone-sulfuric acid method. Bulgarian Chemical Communications, 48(1), 109–113.
Lewis, J., & Fenwick, G. R. (1987). Glucosinolate content of Brassica vegetables: Analysis of twenty-four cultivars of calabrese (green sprouting broccoli, Brassica oleracea L. var. botrytis subvar, cymosa Lain.). 25, 259–268.
Lewis, J., & Fenwick, G. R. (1988). Glucosinolate content of Brassica vegetables -Chinese cabbages pe-tsai (Brassica pekinensis) and pak-choi (Brassica chinensis). Journal of the Science of Food and Agriculture, 45, 379–386.
Liu, R., Yang, G., & Wu, Y. (2015). Effects of light intensity on associated enzyme activity and gene expression during callus formation of Vitis vinifera. Chinese Journal of Biotechnology, 31(8), 1219–1229.
Luo, Q. (2016). Chemical properties and antioxidant activity of a water-soluble polysaccharide from Dendrobium officinale. International Joumal of Biological Macromolecules, 89, 219–227.
Luo, Z., Xu, C., & Chen, F. (2021). Effects of LED light on chlorophyll synthesis and metabolism of modified atmosphere cabbage. Storage and Process, 21(7), 8–16.
Ma, G., Zhang, L., Setiawan, C. K., Yamawaki, K., Asai, T., Nishikawa, F., Maezawa, S., Sato, H., Kanemitsu, N., & Kato, M. (2014). Effect of red and blue LED light irradiation on ascorbate content and expression of genes related to ascorbate metabolism in postharvest broccoli. Postharvest Biology and Technology, 94, 97–103.
Manzocco, L., Quarta, B., & Dri, A. (2009). Polyphenoloxidase inactivation by light exposure in model systems and apple derivatives. Innovative Food Science & Emerging Technologies, 10(4), 506–511.
Meng, X., Zhang, M., Zhan, Z., & Adhikari, B. (2013). Changes in quality characteristics of fresh-cut cucumbers as affected by pressurized argon treatment. Food and Bioprocess Technology, 7(3), 693–701.
Meng, X., Zhang, M., & Adhikari, B. (2012). Extending shelf-life of fresh-cut green peppers using pressurized argon treatment. Postharvest Biology and Technology, 71, 13–20.
Muneer, S., Kim, E. J., Park, J. S., & Lee, J. H. (2014). Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). International Journal Molecular Sciences, 15(3), 4657–4670.
Mustafa, M. A., Ali, A., Manickam, S., & Siddiqui, Y. (2013). Ultrasound-assisted chitosan–surfactant nanostructure assemblies: Towards maintaining postharvest quality of tomatoes. Food and Bioprocess Technology, 7(7), 2102–2111.
Nassarawa, S. S., Abdelshafy, A. M., Xu, Y., Li, L., & Luo, Z. (2021). Effect of light-emitting diodes (LEDs) on the quality of fruits and vegetables during postharvest period: A review. Food and Bioprocess Technology, 14(3), 388–414.
Noichinda, S., Bodhipadma, K., Mahamontri, C., Narongruk, T., & Ketsa, S. (2007). Light during storage prevents loss of ascorbic acid, and increases glucose and fructose levels in Chinese kale (Brassica oleracea var. alboglabra). Postharvest Biology and Technology, 44(3), 312–315.
Peng, H., Sthapit Kandel, J., Michelmore, R. W., & Simko, I. (2020). Identification of factors affecting the deterioration rate of fresh-cut lettuce in modified atmosphere packaging. Food and Bioprocess Technology, 13(11), 1997–2011.
Peng, X., Li, R., Zou, R., Chen, J., Zhang, Q., Cui, P., Chen, F., Fu, Y., Yang, J., & Xia, X. (2013). Allicin inhibits microbial growth and oxidative browning of fresh-cut lettuce (Lactuca sativa) during refrigerated storage. Food and Bioprocess Technology, 7(6), 1597–1605.
Ruiz de Larrinaga, L., Resco de Dios, V., Fabrikov, D., Guil-Guerrero, J. L., Becerril, J. M., Garcia-Plazaola, J. I., & Esteban, R. (2019). Life after harvest: Circadian regulation in photosynthetic pigments of rocket leaves during supermarket storage affects the nutritional quality. Nutrients, 11(7).
Shen, X., Zhang, M., Fan, K., & Guo, Z. (2019). Effects of ε-polylysine/chitosan composite coating and pressurized argon in combination with MAP on quality and microorganisms of fresh-cut potatoes. Food and Bioprocess Technology, 13(1), 145–158.
Shi, L., Cao, S., Shao, J., Chen, W., Yang, Z., & Zheng, Y. (2016). Chinese bayberry fruit treated with blue light after harvest exhibit enhanced sugar production and expression of cryptochrome genes. Postharvest Biology and Technology, 111, 197–204.
Sidransky, H., Ito, N., & Verney, E. (1966). Influence of alpha-naphthyl-isothiocyanate on liver tumorigenesis in rats ingesting ethionine and N-2-fluorenylacetamide. Journal of the National Cancer Institute, 37(5), 677.
Song, Y., Qiu, K., Gao, J., & Kuai, B. (2020) Molecular and physiological analyses of the effects of red and blue LED light irradiation on postharvest senescence of pak choi. Postharvest Biology and Technology, 164.
Toledo, M. E. A., Ueda, Y., Imahori, Y., & Ayaki, M. (2003). l-ascorbic acid metabolism in spinach (Spinacia oleracea L.) during postharvest storage in light and dark. Postharvest Biology and Technology, 28(1), 47–57.
Vamos-Vigyazo, L. (1981). Polyphenol oxidase and peroxidase in fruits and vegetables. Critical Reviews in Food Science and Nutrition, 15(1), 49–127.
Verhoeven, D. T., Verhagen, H., Goldbohm, R. A., van den Brandt, P. A., & van Poppel, G. (1997). Mini review-A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chemico-Biological Interactions, 103, 79–129.
Vollmer, K., Chakraborty, S., Bhalerao, P. P., Carle, R., Frank, J., & Steingass, C. B. (2020). Effect of pulsed light treatment on natural microbiota, enzyme activity, and phytochemical composition of pineapple (Ananas comosus [L.] Merr.) juice. Food and Bioprocess Technology, 13(7), 1095–1109.
Vunnam, R., Hussain, A., Nair, G., Bandla, R., Gariepy, Y., Donnelly, D. J., Kubow, S., & Raghavan, G. S. (2014). Physico-chemical changes in tomato with modified atmosphere storage and UV treatment. Journal of Food Science and Technology, 51(9), 2106–2112.
Waghmare, R. B., & Annapure, U. S. (2015). Integrated effect of sodium hypochlorite and modified atmosphere packaging on quality and shelf life of fresh-cut cilantro. Food Packaging and Shelf Life, 3, 62–69.
Wilawan, N., Ngamwonglumlert, L., Devahastin, S., & Chiewchan, N. (2019). Changes in enzyme activities and amino acids and their relations with phenolic compounds contents in okra treated by LED lights of different colors. Food and Bioprocess Technology, 12(11), 1945–1954.
Wilson, M. D., Stanley, R. A., Eyles, A., & Ross, T. (2019). Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Critical Reviews in Food Science and Nutrition, 59(3), 411–422.
Wu, Q., Gao, H., Zhang, Z., Li, T., Qu, H., Jiang, Y., & Yun, Z. (2020). Deciphering the metabolic pathways of pitaya peel after postharvest red light irradiation. Metabolites, 10(3).
Xu, F., & Liu, S. (2017). Control of postharvest quality in blueberry fruit by combined 1-methylcyclopropene (1-MCP) and UV-C irradiation. Food and Bioprocess Technology, 10(9), 1695–1703.
Xu, F., Shi, L., Chen, W., Cao, S., Su, X., & Yang, Z. (2014). Effect of blue light treatment on fruit quality, antioxidant enzymes and radical-scavenging activity in strawberry fruit. Scientia Horticulturae, 175, 181–186.
Yan, Z., Zuo, J., Zhou, F., Shi, J., Xu, D., Hu, W., Jiang, A., Liu, Y., & Wang, Q. (2020). Integrated analysis of transcriptomic and metabolomic data reveals the mechanism by which LED light irradiation extends the postharvest quality of pak-choi (Brassica campestris L. ssp. chinensis (L.) Makino var. communis Tsen et Lee). Biomolecules, 10(2).
Yang, D., Li, D., Xu, W., Liao, R., Shi, J., Fu, Y., Wang, J., Wang, Y., & He, X. (2018). Design and application of a passive modified atmosphere packaging for maintaining the freshness of Chinese cabbage. LWT, 94, 136–141.
Yang, J., Zhu, Z., Wang, Z., & Zhu, B. (2009). Effects of storage temperature on the contents of carotenoids and glucosinolates in pakchoi (Brassica rapa L. ssp.Chinensis var .communis) Journal of Food Biochemistry, 34(2010), 1186–1204.
Yang, L., Liu, J., Wang, X., Wang, R., Ren, F., Zhang, Q., Shan, Y., & Ding, S. (2019). Characterization of volatile component changes in jujube fruits during cold storage by using headspace-gas chromatography-ion mobility spectrometry. Molecules, 24(21).
Zhan, L., Hu, J., Ai, Z., Pang, L., Li, Y., & Zhu, M. (2013). Light exposure during storage preserving soluble sugar and l-ascorbic acid content of minimally processed romaine lettuce (Lactuca sativa L.var. longifolia). Food Chemistry, 136(1), 273–278.
Zhan, L., Hu, J., Lim, L. T., Pang, L., Li, Y., & Shao, J. (2013). Light exposure inhibiting tissue browning and improving antioxidant capacity of fresh-cut celery (Apium graveolens var. dulce). Food Chemistry, 141(3), 2473–2478.
Zhan, L., Hu, J., Pang, L., Li, Y., & Shao, J. (2014). Effects of light exposure on chlorophyll, sugars and vitamin C content of fresh-cut celery (Apium graveolensvar. dulce) petioles. International Journal of Food Science & Technology, 49(2), 347–353.
Zhan, L., Li, J., Huang, W., Song, C., Li, J., Pang, L., & Li, Y. (2020). Light irradiation affects the total antioxidant capacity, total phenolic compounds, phenolic acids, and related enzyme activities of minimally processed spinach (Spinacia oleracea L.). Journal of Food Processing and Preservation, 44(10).
Zhan, L., Li, Y., Hu, J., Pang, L., & Fan, H. (2012). Browning inhibition and quality preservation of fresh-cut romaine lettuce exposed to high intensity light. Innovative Food Science & Emerging Technologies, 14, 70–76.
Zhang, M., Zhan, Z. G., Wang, S. J., & Tang, J. M. (2008). Extending the shelf-life of asparagus spears with a compressed mix of argon and xenon gases. LWT - Food Science and Technology, 41(4), 686–691.
Zhang, X., Zhang, M., Devahastin, S., & Guo, Z. (2019). Effect of combined ultrasonication and modified atmosphere packaging on storage quality of pakchoi (Brassica chinensis L.). Food and Bioprocess Technology, 12(9), 1573–1583.
Zhou, F., Gu, S., Zuo, J., Gao, L., Wang, Q., & Jiang, A. (2019). LED irradiation delays the postharvest senescence of garland chrysanthemum (Chrysanthemum carinatum Schousb.). Journal of Food Measurement and Characterization, 13(4), 3005–3014.
Funding
We acknowledge the financial supports from National Key R&D Program of China (No. 2018YFD0700303), Jiangsu Province Key Laboratory Project of Advanced Food Manufacturing Equipment and Technology (No. FMZ202003), Jiangsu Province (China) Key Project in Agriculture (Contract No. BE2015310217), and National First-Class Discipline Program of Food Science and Technology (No. JUFSTR20180205). All these above enable us to carry out and promote this research smoothly.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Xj., Zhang, M., Chitrakar, B. et al. Novel Combined Use of Red-White LED Illumination and Modified Atmosphere Packaging for Maintaining Storage Quality of Postharvest Pakchoi. Food Bioprocess Technol 15, 590–605 (2022). https://doi.org/10.1007/s11947-022-02771-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02771-x