Abstract
This study focused on the development of novel active packaging methods based on natural compounds. Its main objective was the quality enhancement of refrigerated packaged fish. For it, the effect of the inclusion of a protein concentrate (PC) extracted from microalga Spirulina platensis into a crosslinked gelatine-based biofilm was investigated during the preservation of hake (Merluccius merluccius) muscle. Thus, microbiological and chemical analyses related to quality loss were monitored throughout a 7-day storage at 4 °C. Statistically significant (p < 0.05) lower counts (log CFU g−1 muscle) were obtained for aerobe mesophiles, psychrotrophs, proteolytics, lipolytics, and Enterobacteriaceae in refrigerated fish muscle as a result of the microalga PC presence in the packaging medium when compared with fish corresponding to the control batch. With respect to the lipid fraction, an inhibitory effect on lipid hydrolysis development (free fatty acids formation) (p < 0.05) was concluded and, furthermore, a higher (p < 0.05) polyunsaturated fatty acid retention (polyene index value) was achieved in the PC-treated fish at the end of the storage time. Fish quality enhancement derived from the use of the spirulina PC in the packaging medium was concluded, this result being linked to the presence of preservative water-soluble molecules in the PC extract. Further research related to the optimisation of processing conditions ought to be addressed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine foods rapidly deteriorate post-mortem as a consequence of a variety of biochemical and microbial breakdown mechanisms (Bondoc and Şindilar 2002; Özoğul 2010). According to the increasing demand for high-quality fresh fish products, different strategies have been tested to delay fish damage as long as possible (Campos et al. 2012). One such strategy has been the use of packaging films including antimicrobial and antioxidant compounds so that a marine product with an increased shelf-life time is attained (Yerlikaya et al. 2020; Cai et al. 2014). Among preservative compounds, adverse health problems (i.e. cancer development, toxicity) resulting from persistent consumption of synthetic antioxidants (butylhydroxytoluene, butylhydroxyanisole, propyl gallate, etc.) have recommended the use of natural antioxidants as an alternative to synthetic ones (Tavakoli et al. 2017). Therefore, the identification and isolation of novel natural preservatives from aquatic and terrestrial sources are gaining increasing interest.
Spirulina platensis is a prokaryotic microalga, order Cyanophyceae, division Cyanophyta (Cyanobacteria). This blue-green microalga is one of the most cultivated in the world, and its use in aquaculture as a food in fish diets has gained increasing importance in the last decade (Rosas et al. 2019). Its biomass nutritional value represents potential extraction of high added-value biocompounds such as carbohydrates, vitamins, pigments, polyphenols, flavonoids, and preservative protein-derived compounds (Budiyono et al. 2014; Ovando et al. 2018). Interestingly, this filamentous microalga is recognised for presenting GRAS (generally recognised as safe) certification granted by the FDA (Food and Drug Administration) (Belay and Houston 2002). S. platensis biomass has proven to stimulate important biological processes related to remarkable antiallergenic, antibacterial, antifungal, anti-inflammatory, antioxidant, and immunomodulating activities (Khan et al. 2005). Furthermore, S. platensis has been extensively used in biomedicine for its beneficial properties such as cholesterol reduction, cancer protection, cardiovascular disease prevention, anti-aging, and immune system enhancer agent (Chu et al. 2010; Wang and Zhang 2015; Ngo-Matip et al. 2015).
Concerning its application to food, S. platensis extracts have attracted great attention because of their preservative properties. Thus, in vitro studies have shown relevant antimicrobial (Mallikarjun Gouda et al. 2015; Sun et al. 2016) and antioxidant (Yu et al. 2016; Andrade et al. 2018) properties. In this sense, application to different kinds of foods such as cookies (Onacik-Gur et al. 2018), yogurt (Barkallah et al. 2017), and snacks (Lucas et al. 2018) has been described. Additionally, bioactive compounds obtained from S. platensis including proteins (phycobiliproteins), pigments, and phenolic compounds have been tested successfully to prepare preservative and novel biofilms (Larrosa et al. 2018; Goettems Kuntzler et al. 2018). Concerning marine product preservation, the employment of S. platensis extracts can be considered scarce, previous research being limited to the potential antimicrobial effect against fish bacterial pathogens (Nanthini Devi et al. 2016) and their antioxidant properties during rainbow trout (Oncorhynchus mykiss) refrigeration (Taghavi Takyar et al. 2019) and during storage of dried-salted fish (Bertolin et al. 2011). Furthermore, both antimicrobial (Najdenski et al. 2013) and antioxidant (Romay et al. 2001) activities have previously been reported for proteins from S. platensis. Indeed, a protein extract from S. platensis, with protein content higher than 60%, showed to increase the antimicrobial and antioxidant activity of gelatine films in in vitro studies (Stejskal et al. 2018, 2019). However, to the best of our knowledge, no previous research has been conducted related to the use of a biopackaging film incorporated with bioactive substances from S. platensis or any other microalga for fish conservation.
The current study was focused on the development of novel active packaging methods based on natural compounds. Its main objective was the quality enhancement of refrigerated packaged fish. For it, the effect of the inclusion of a protein concentrate (PC) extracted from microalga Spirulina platensis into a crosslinked gelatine-based film was investigated during the preservation of hake (Merluccius merluccius) muscle kept under refrigerated (4 °C) conditions. A gelatine-based film was chosen because of its abundance, acceptability, low manufacturing cost, and great biofilm-forming properties (Feng et al. 2017; Dehghani et al. 2018). In the current study, microbiological and chemical analyses related to quality loss were monitored throughout a 7-day storage.
Material and Methods
Materials
Commercially available Spirulina platensis (lyophilised powder, Martin Bauer GmbH & Co, Vestenbergsgreuth, Germany) was used as raw material without further treatment. Bovine gelatine type B, isoionic point, pI 5.3, Bloom 150, was kindly provided by Rousselot Argentina (Villa Tesei, Argentina). Glycerol analytical grade (Gly, 98%) was purchased from Anedra (Buenos Aires, Argentina) and used as a plasticiser. Sodium alginate with moisture content ≤ 15.0% and pH = 6.5–8.6 was purchased from Sigma-Aldrich (St. Louis, USA). Potassium periodate (NaIO4, > 99.0%, Merck, Germany) was used to synthesise oxidised alginate that was used as crosslinking agent of gelatine. Phosphate buffer (pH 10), sodium hydroxide, and hydrochloric acid were obtained from Anedra (Buenos Aires, Argentina).
All other solvents and chemical reagents used in the current study were of reagent grade (Merck, Darmstadt, Germany).
Preparation of Biofilm Systems
S. platensis was submitted to an extraction protocol, based on repeated aqueous, alkaline, and acidic extraction steps followed by several rounds of centrifugation and recovery using precipitation and ultracentrifugation (Chronakis 2001; Benelhadj et al. 2016a). For it, 5 g of commercially lyophilised powder of S. platensis was dissolved in 100 mL of phosphate buffer pH 10 (0.005 mol L−1) under agitation (500 rpm, 60 min). Subsequently, the suspension was centrifuged (10,000×g, 10 min, 3 times) using an ultracentrifuge (Sartorius 4-15). The supernatant was collected (batch A) and the pellet was redissolved and centrifuged again under the same conditions as above described, to obtain the batch B. Both supernatants (batches A and B) were pooled together and the pH was dropped from 10.0 to 3.0 by addition of 0.1 mol L−1 aqueous HCl solution to precipitate the protein fraction. The suspension was centrifuged (10,000×g, 30 min), and the precipitated proteins were freeze-dried (VirTis Bench Top SLC lyophiliser, Warminster, PA, USA), this leading to the spirulina PC.
Biofilms were produced by casting from their film-forming solutions (FFS) (Neira et al. 2019), using glycerol as plasticiser and oxidised sodium alginate (OA) as efficient crosslinking agent through side-chain amino groups in gelatine to stabilise gelatine films against moisture, thus reducing swelling and solubility (Bonani et al. 2010). Oxidised sodium alginate (OA) was obtained according to the method reported by Balakrishnan et al. (2005) with an aldehyde yield of 4.2 ± 0.2 mmol aldehyde g−1 oxidised sodium alginate. Active biofilms were produced by dissolving 8 g of gelatine and 2 g of the freeze-dried PC in 100 mL of phosphate buffer solution pH 10 under stirring at 40 °C. Glycerol (30% wt. on dry protein basis) and OA (5% wt. on dry protein basis) were incorporated to the FFS, the suspension being stirred at 40 °C for 120 min. Then, the FFS were cast onto teflon-coated Petri dishes (diameter 10 cm) and dried at 40 °C in a convection oven for 48 h at controlled relative humidity, until constant weight. Biofilms were conditioned for 48 h in a chamber at 4 ± 1 °C prior to analysis. The resulting biofilm was named as GE-SP.
Two different control-packaging systems were taken into account in the current study. First, a control batch was prepared as mentioned above, but consisting of the gelatine film without spirulina PC (CT-GE packaging condition; gelatine control). Second, a low-density polyethylene film was also employed as control (CT-PE packaging condition; polyethylene control). For both control systems, 10-cm packaging films were prepared as for the GE-SP condition. The low-density polyethylene used to elaborate the packaging films (CT-PE batch) had a water vapour transmission rate of 3.62 g m−2 day−1 when measured at 38 °C and 90% relative humidity, its thickness being 140 μm (Rodríguez et al. 2012).
The content of spirulina PC employed in the present study was based on several preliminary tests carried out in our laboratory. Thus, higher concentrations than 20 g PC L−1 buffer led to modifications in fish sensory descriptors such as muscle colour and odour and to non-suitable mechanical properties of the biofilms (data not shown).
Physico-chemical Characteristics and Preserving Properties of Protein Concentrates and Biofilm Systems
Previous research accounts for characteristics and properties of PC and PC-derived biofilms obtained from S. platensis (Stejskal et al. 2018, 2019). Thus, after PC lyophilisation, the extraction efficiency as determined gravimetrically proved to be 22 ± 3%. The total protein content in PC determined by the Biuret method as the sum of the amount of C-phycocyanin and allophycocyanin was 61.55%. The total phenolic compound estimated from the Folin-Ciocalteu analysis was 35.58 ± 0.15 mg gallic acid g−1 sample, and the in vitro antioxidant activities of the PC, expressed as the radical scavenging activity and reduction power against ferric ion, were 76.35 ± 3.02% and 12.6 ± 0.23 mg ascorbic acid g−1 sample, respectively.
Interestingly, the incorporation of 2% of PC (dry-protein basis) provided antimicrobial activity against E. coli and S. aureus (inhibition halo for both pathogens 10 ± 1 mm) and antioxidant activity (radical scavenging activity, RSA 121.5 ± 5.7 vs. 21.2 ± 6.8 μg ascorbic acid g−1 for gelatine film) to the gelatine films (Stejskal et al. 2018). In addition, the UV-visible barrier properties were enhanced by the presence of PC in the films. The resulting GE-SP film was thicker (373 ± 42 vs. 320 ± 40 μm), 35% more stretchable (195 ± 8 vs. 145 ± 27%), 78% more mechanically resistant (2.5 ± 0.1 vs. 1.4 ± 0.2 MPa), and less permeable to water vapour (WVPGE-SP = 7.2 ± 0.3 10−15 kg m h−1 Pa−1 and WVPCT-GE = 8.8 ± 0.3 10−15 kg m h−1 Pa−1) than control counterpart (CT-GE). Such improvements were associated with the presence of interactions between matrix components and PC (Stejskal et al. 2019). RSA of GE-SP films could not be determined because active and control film dramatically reduced their solubility in water and methanol up to 90% due to crosslinking induced by OA. Conversely, the antimicrobial activity of GE-SP films and the UV-visible barrier properties showed to remain unaltered after crosslinking (Stejskal et al. 2019).
Fish Material, Processing, and Sampling
Fresh hake (Merluccius merluccius) (63 specimens) were caught near the Galician Atlantic coast (north-western Spain) and transported to the laboratory. Throughout this process (10 h), the fish were maintained in ice. The length and weight of the fish specimens ranged from 39 to 44 cm and from 465 to 575 g, respectively.
Upon arrival to the laboratory, 9 individual fish specimens were separated and analysed as initial material (day 0). These fish specimens were divided into three different groups (three specimens per group), the white muscle being analysed independently in each group (n = 3). The remaining fish specimens were distributed into three batches. Then, fish were filleted, cut into pieces (round 35 g), and sealed-packaged individually in any of the three above-mentioned packaging systems (CT-PE, CT-GE, and GE-SP; 36 fish pieces for each packaging condition), respectively. All batches were placed inside a refrigerated room (4 °C). Fish samples from all batches were stored for a 7-day period, being sampled and analysed on days 4 and 7. At each sampling time, 18 fish samples were taken from each batch for analysis and divided into three groups (six fish samples in each group) that were studied independently (n = 3).
Analysis of Microbial Development
Portions of 10 g of fish muscle were dissected aseptically from refrigerated fish, mixed with 90 mL of 0.1% peptone water (Merck, Darmstadt, Germany), and homogenised in sterilised stomacher bags (AES, Combourg, France) as previously described (Ben-Gigirey et al. 1998; Ben-Gigirey et al. 1999). Serial dilutions from the microbial extracts were prepared in 0.1% peptone water in all the cases.
Total aerobes were determined on plate count agar (PCA) (Oxoid Ltd., London, UK) after incubation at 30 °C for 48 h. Psychrotrophs were determined in PCA, after incubation at 7–8 °C for 7 days. Enterobacteriaceae members of the coliforms group were investigated in Violet Red Bile Agar (VRBA) (Merck, Darmstadt, Germany) after incubation at 37 ± 0.5 °C for 24 h. Microorganisms exhibiting proteolytic or lipolytic phenotypes were investigated in casein-agar or tributyrine-agar, respectively, after incubation at 30 °C for 48 h, as previously reported (Ben-Gigirey et al. 2000).
In all the cases, bacterial counts were transformed into log CFU g−1 muscle before undergoing statistical analysis. All analyses were conducted in triplicate.
Analysis of Lipid Damage Development
Lipids were extracted from the hake white muscle by the Bligh and Dyer (1959) method, which employs a single-phase solubilisation of the lipids using a chloroform-methanol (1:1) mixture. The results were calculated as g lipid kg−1 muscle.
Free fatty acid (FFA) content was determined using the lipid extract of the fish muscle by the Lowry and Tinsley (1976) method, which is based on complex formation with cupric acetate-pyridine followed by spectrophotometric (715 nm) assessment. The results were expressed as g FFA kg−1 lipids.
Lipid extracts were converted into fatty acid methyl esters (FAME) by using acetyl chloride and then analysed by gas-liquid chromatography (Perkin-Elmer 8700 chromatograph; Madrid, Spain), as described elsewhere (Vázquez et al. 2018). Peaks corresponding to FAME were identified by comparing their retention times with those of standard mixtures (Qualmix Fish, Larodan, Malmo, Sweden; FAME mix, Supelco, Inc., Bellefonte, PA, USA). Peak areas were automatically integrated. C19:0 fatty acid was used as the internal standard for quantitative purposes. The polyene index (PI) was calculated as the following fatty acid content ratio: (C20:5ω3 + C22:6ω3)/C16:0.
Statistical Analysis
Data obtained from all microbiological and chemical analyses were subjected to the ANOVA method to explore differences resulting from the effect of the packaging system. The comparison of means was performed using the least-squares difference (LSD) method. In all the cases, analyses were carried out using the PASW Statistics 18 software for Windows (SPSS Inc., Chicago, IL, USA); differences among packaging batches were considered significant for a confidence interval at the 95% level (p < 0.05) in all the cases.
Results and Discussion
Analysis of Microbial Development
The initial microbiological quality of the fish specimens was considered good, according to the low microbial counts determined on the initial day for aerobes, psychrotrophs, and Enterobacteriaceae: 2.40 log CFU g−1, 2.51 log CFU g−1, and 1.00 log CFU g−1, respectively.
The GE-SP fish batch exposed to the film including spirulina PC exhibited better microbiological features than the other two batches, namely CT-PE and CT-GE, during refrigerated storage. In the case of the evolution of aerobes, the S. platensis batch evidenced partial inhibition of the growth of this microbial group as compared with the two control batches (Table 1). Thus, such inhibition was slight at moderate storage times (4 days) but rose up to microbial count differences (p < 0.05) of 1.67 log CFU g−1 and 1.38 log CFU g−1 units with respect to the CT-PE and CT-GE batches, respectively, at advanced storage time (7 days). Remarkably, the level of aerobe mesophiles in the batch containing spirulina PC did not reach 6 log units, the value commonly used as limit of acceptance (Bondoc 2014; Barros-Velázquez 2016).
Table 1 also displays the comparative evolution of psychrotrophic bacteria in all the three batches. In this sense, and as in the case of aerobic mesophiles, notable differences were observed in the GE-SP batch as compared with the two control batches, although such differences only resulted to be statistically significant (p < 0.05) at advanced storage time. Thus, after 7 days of chilled storage, the GE-SP batch exhibited psychrotrophs counts that resulted to be 1.24 log CFU g−1 and 0.85 log CFU g−1 lower than the CT-PE and CT-GE control batches, respectively (Table 1). As stated above, this result was in agreement with that observed for aerobes, and confirmed that the presence of spirulina PC in the film used for the refrigerated storage of the fish material exerted a protective effect on the microbiological quality of fish muscle, this being determined by a significant (p < 0.05) slower growth of aerobes and psychrotrophs.
With respect to the development and growth of Enterobacteriaceae in fish muscle, a protective effect derived from the presence of the spirulina PC in the film was also inferred. Thus, as in the cases of aerobes and psychrotrophs, the evolution of Enterobacteriaceae in the packaging batch including spirulina PC was remarkably lower as compared with CT-PE and CT-GE control batches (Table 2). Such differences were statistically significant (p < 0.05) and reached maximum levels at advanced storage time (7 days), rising up to 1.85 log CFU g−1 and 1.29 log CFU g−1 when comparing the GE-SP batch with the CT-PE and CT-GE control batches, respectively.
Bacteria exhibiting a proteolytic phenotype may cause the breakdown of fish muscle structure, also generating undesirable metabolites and off-odours (Rodríguez et al. 2003). In this study, the investigation of the development of this specific spoilage microbial group indicated that the presence of spirulina PC in the packaging film exerted a very intense protective effect. Remarkably, statistically significant (p < 0.05) differences between the GE-SP batch and the two control batches were observed at all sampling times (Table 2). Such differences were more intense on day 7: 2.13 log CFU g−1 and 1.79 log CFU g−1 with respect to the CT-PE and CT-GE batches, respectively.
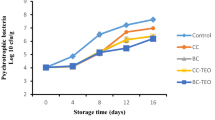
Bacteria exhibiting a lipolytic phenotype may induce lipid hydrolysis in fish muscle, this generating the so-called lipolytic rancidity (Sikorski and Kolakoski 2000). In this study the development and growth of lipolytic bacteria was followed and the results revealed a remarkably better control of this specific spoilage microbial group in the batch including spirulina PC in the packaging medium (Fig. 1). Such effect was found to be statistically significant (p < 0.05) on day 4 but not (p > 0.05) on day 7.
Lipolytic counts determination (log CFU g-1 muscle)* in refrigerated hake muscle stored under different packaging conditions**. *Average values of three replicates (n = 3); standard deviations are indicated by bars. For each refrigeration time, values accompanied by different letters (a, b) indicate significant differences (p < 0.05). **Packaging conditions: CT-PE (control polyethylene), CT-GE (control gelatine), and GE-SP (gelatine-spirulina)
Current results are in agreement with previous research showing antimicrobial effects of S. platensis protein extracts against fish bacterial pathogens under in vitro conditions (Nanthini Devi et al. 2016). In such study, bioactive compounds extracted by a mixture of methanol/ethanol/acetone (1:1:1) inhibited the growth of Proteus mirabilis, Bacillus pumilus, and Staphylococcus sciuri in infected fish. Furthermore, an antibacterial peptide (18 amino acid residues with a molecular mass of 1878.97 Da) isolated from an alkaline protease and papain hydrolysate of S. platensis, exhibited in vitro antimicrobial activity against Escherichia coli and Staphylococcus aureus (Sun et al. 2016). Also based on an in vitro experiment, Mallikarjun Gouda et al. (2015) reported the antimicrobial effect of a butanol extract from S. platensis against several microorganisms (E. coli, S. aureus, Bacillus cereus, and Yersinia enterocolitica); interestingly, phytoconstituents such as phenolic and flavonoid compounds were found to be responsible for this inhibitory effect. Hydrolysis by trypsin and chymotrypsin enzymes of S. platensis proteins revealed that approximately 20–22-kDa proteins and their derivative peptides were able to decrease in vitro bacterial growth (E. coli and S. aureus) (Sadeghi et al. 2018). Finally, S. platensis protein isolates were also included as components of an edible packaging system on the basis of their antimicrobial activity resulting from the presence of molecules such as polyhydroxybutyrate and phenolic compounds (Benelhadj et al. 2016b; Goettems Kuntzler et al. 2018).
The inclusion of macroalgae extracts in biofilms has also provided antimicrobial activity. This is the case of the red macroalga Gelidium corneum, whose presence in an edible film also including persimmon peel and grape fruit seed extracts improved the physical properties and provided antimicrobial activity (Jo et al. 2014). Furthermore, lyophilised Fucus spiralis was included in a polylactic-based film, this leading to a reduced microbial development in megrim (Lepidorhombus whiffiagonis) fillets kept under refrigerated (4 °C) conditions for 11 days (García-Soto et al. 2015). Previous studies have also shown the positive effect of red macroalgae on the physicochemical properties of biodegradable films. This accounts for polyvinyl alcohol-agar films from Hydropuntia cornea (Madera-Santana et al. 2011) and Gloiopeltis tenax and glue plant films from Gloiopeltis furcata (Rhim et al. 2002).
Analysis of Lipid Damage Development
A marked hydrolytic activity could be observed in the muscle of this lean fish species (4.7–6.1 g lipids kg−1 muscle) throughout refrigerated storage (Fig. 2). Thus, FFA formation increased with time in all the batches. However, the presence of a gelatine-film (CT-GE and GE-SP batches) led to lower (p < 0.05) FFA contents, so that a marked inhibitory effect of gelatine on lipid hydrolysis development was concluded. Interestingly, lower average FFA values were determined in fish samples corresponding to the GE-SP batch when compared with their counterparts belonging to gelatine films, although significant differences were not observed (p > 0.05). Chemical results obtained on FFA content agree with the inhibitory trend implied from the above-mentioned results related to the lipolytic counts evolution throughout the refrigerated storage (Fig. 1).
Free fatty acid (FFA) assessment (g FFA kg−1 lipids)* in refrigerated hake muscle stored under different packaging conditions**. *Average values of three replicates (n = 3); standard deviations are indicated by bars. For each refrigeration time, values accompanied by different letters (a, b) indicate significant differences (p < 0.05). **Packaging conditions as expressed in Fig. 1
Previous studies on the effects of S. platensis protein extracts or extracts in general on lipid hydrolysis in a food matrix are scarce. Thus, Taghavi Takyar et al. (2019) reported the inhibitory effect of ethanol extracts on lipid hydrolysis (FFA formation); such extract was added to rainbow trout fillets that were packaged in polyethylene bags and kept at 4 °C up to 16 days. On the other hand, the inhibitory effect of gelatine on lipid hydrolysis during fish products storage was also reported. Thus, a chitosan-gelatine coating slowed down FFA formation in rainbow trout (O. mykiss) fillets (Nowzari et al. 2013) and Belanger’s croaker (Johnius belangerii) fillets (Saki et al. 2018) during refrigerated storage (4 ± 1 °C).
FFA formation has been reported to exert a direct effect on lipid oxidation development, this effect being based on the catalytic effect of the carboxyl group on the formation of free radicals by breakdown of hydroperoxides (Aubourg 2001). Furthermore, a lower oxidative stability in FFA than in their corresponding methyl esters and triglycerides has been reported as a result of a lower steric hindrance to oxidative reactions (Miyashita and Takagi 1986).
Concerning lipid oxidation development in the current study, the polyene content (i.e. PI) was measured and the results displayed in Fig. 3. Thus, higher average values were observed in fish samples corresponding to the GE-SP batch, differences being significant (p < 0.05) at day 7. In this sense, an inhibitory effect of spirulina PC in the packaging film on lipid oxidation of the muscle was concluded.
Polyene index determination* in refrigerated hake muscle stored under different packaging conditions**. *Average values of three replicates (n = 3); standard deviations are indicated by bars. For each refrigeration time, values accompanied by different letters (a, b) indicate significant differences (p < 0.05). **Packaging conditions as expressed in Fig. 1
Previous research has pointed out the antioxidant effect of S. platensis extracts on the basis of the presence of a wide range of antioxidant molecules (i.e. phycocyanins, polyphenols, flavonoids) (Romay et al. 2001; Yu et al. 2016; Andrade et al. 2018). Thus, microalgae ethanolic extracts were added to rainbow trout fillets that were then packaged in polyethylene bags and kept at 4 °C up to 16 days (Taghavi Takyar et al. 2019); as a result, lower peroxide and thiobarbituric acid indices were detected in treated fish fillets. Furthermore, phycocyanin obtained from S. platensis showed antioxidant properties in dried-salted fish (Pacu, Piaractus mesopotamicus) during a 60-day storage time at 25 °C (Bertolin et al. 2011). The antioxidant properties of S. platensis extracts have also been proved in different kinds of non-marine foods such as yogurt (Barkallah et al. 2017), sausage (Luo et al. 2017), and snacks (Lucas et al. 2018). Moreover, Carissimi et al. (2018) reported the antioxidant properties of a starch-based film including an ethanolic extract of microalgae Heterochlorella luteoviridis and Dunaliella tertiolecta; this effect (thiobarbituric acid index decrease) was observed in salmon fillets stored at 6 ± 2 °C for 6 days.
Previous research accounted for an antioxidant effect as a result of the inclusion of macroalgae extracts in biofilms. Thus, alginate-based films prepared from red macroalga (Sargassum fulvellum) provided antioxidant properties (ABTS and DPPH assays) to a biofilm also including black chokeberry (Kim et al. 2018). Furthermore, the presence of lyophilised F. spiralis in a polylactic acid packaging film also showed a marked antioxidant effect during the refrigerated storage (11 days at 4 °C) of megrim (Lepidorhombus whiffiagonis) fillets (García-Soto et al. 2015).
Conclusions
Lower (p < 0.05) counts (log CFU g−1 muscle) were obtained for aerobe mesophiles, psychrotrophs, proteolytics, lipolytics, and Enterobacteriaceae in refrigerated hake muscle as a result of the incorporation of spirulina PC in the packaging medium. Concerning the fish lipid fraction, a protective effect on lipid hydrolysis (i.e. lower (p < 0.05) free fatty acid formation) was concluded. In agreement to the PI assessment, a higher (p < 0.05) polyunsaturated fatty acid retention was achieved in the PC-treated fish at the end of the experiment.
A preservative effect derived from the use of a spirulina PC in the packaging medium of a refrigerated muscle of a fish species is concluded, to our knowledge for the first time. This result is linked to the presence of preserving water-soluble compounds in the microalga PC. In agreement with the availability of S. platensis, the present study opens the way to the development of a novel biopreservation packaging strategy for the refrigerated storage of commercial fish products such as fish fillets or fish muscle portions in general. Further research related to the identification of active components present in S. platensis PC as well as the optimisation of processing conditions (i.e. concentration of PC, as well as biofilm matrix and marine species concerned) ought to be carried out to attain the best quality degree of the fish product.
References
Andrade, I., Andrade, C., Dias, M., Nascimento, C., & Mendes, M. (2018). Chlorella and Spirulina microalgae as sources of functional foods, nutraceuticals and food supplements; an overview. Med Crave Open Journal Food Processing and Technology, 6, 45–58.
Aubourg, S. P. (2001). Fluorescence study of the prooxidant activity of free fatty acids on marine lipids. Journal of the Science of Food and Agriculture, 81(4), 385–390.
Balakrishnan, B., Lesieur, S., Labarre, D., & Jayakrishnan, A. (2005). Periodate oxidation of sodium alginate in water and in ethanol–water mixture: A comparative study. Carbohydrate Polymers, 340(7), 1425–1429.
Barkallah, M., Dammak, M., Louati, I., Hentati, F., Hadrich, B., Mechichi, T., Ali Ayadi, M., Fendri, I., Attia, H., & Abdelkafi, S. (2017). Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. Lebensmittel Wissenschaft und Technologie-Food Science and Technology, 84, 323–330.
Barros-Velázquez, J. (2016). Antimicrobial food packaging (pp. 676). San Diego: Elsevier Science Publishing.
Belay, A., & Houston, M. (2002). The potential application of Spirulina (Arthrospira) as a nutritional and therapeutic supplement in health management. The Journal of the American Nutraceutical Association, 5, 27–48.
Benelhadj, S., Gharsallaoui, A., Degraeve, P., Attia, H., & Ghorbel, D. (2016a). Effect of pH on the functional properties of Arthrospira (Spirulina platensis) protein isolate. Food Chemistry, 194, 1056–1063.
Benelhadj, S., Fejji, N., Degraeve, P., Attia, H., Ghorbel, D., & Gharsallaoui, A. (2016b). Properties of lysozyme/Arthrospira platensis (Spirulina) protein complexes for antimicrobial edible food packaging. Algal Research, 15, 43–49.
Ben-Gigirey, B., Vieites Baptista de Sousa, J., Villa, T., & Barros-Velázquez, J. (1998). Changes in biogenic amines and microbiological analysis in albacore (Thunnus alalunga) muscle during frozen storage. Journal of Food Protection, 61(5), 608–615.
Ben-Gigirey, B., Vieites Baptista de Sousa, J., Villa, T., & Barros-Velázquez, J. (1999). Histamine and cadaverine production by bacteria isolated from fresh and frozen albacore (Thunnus alalunga). Journal of Food Protection, 62(8), 933–939.
Ben-Gigirey, B., Vieites Baptista de Sousa, J., Villa, T., & Barros-Velázquez, J. (2000). Characterization of biogenic amine-producing Stenotrophomonas maltophilia strains isolated from white muscle of fresh and frozen albacore tuna. International Journal of Food Microbiology, 57(1-2), 19–31.
Bertolin, T., Guarientio, C., Farias, D., Souza, F., Gutkoski, L., & Colla, L. (2011). Antioxidant effect of phycocianin on dried-salted fish. Ciência e Agrotecnologia Lavras, 35(4), 751–757.
Bligh, E., & Dyer, W. (1959). A rapid method of total extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8), 911–917.
Bonani, E., Rubini, K., Panzavolta, S., & Bigi, A. (2010). Chemico-physical characterization of gelatin films modified with oxidized alginate. Acta Biomaterialia, 6(2), 383–388.
Bondoc, I. (2014). The veterinary sanitary control of fish and fisheries products. In Control of products and food of animal origin (pp. 264-346), vol. I. Iaşi: Ion Ionescu de la Brad Iaşi Publishing.
Bondoc, I., & Şindilar, E. V. (2002). Veterinary sanitary control of food quality and hygiene (Vol. I, pp. 151–166). Iaşi: Ion Ionescu de la Brad Iaşi Publishing.
Budiyono, Syaichurrozi, I., Sumardiono, S., & Budi Sasongko, S. (2014). Production of Spirulina platensis biomass using digested vinasse as cultivation medium. Trends in Applied Sciences Research, 9, 93–102.
Cai, L., Li, X., Wu, X., Lv, Y., Liu, X., & Li, J. (2014). Effect of chitosan coating enriched with ergothioneine on quality changes of Japanese sea bass (Lateolabrax japonicas). Food and Bioprocess Technology, 7(8), 2281–2290.
Campos, C., Gliemmo, M., Aubourg, S. P., & Barros-Velázquez, J. (2012). Novel technologies for the preservation of chilled aquatic food products. In A. McElhatton & P. A. Sobral (Eds.), Novel technologies in food science (pp. 299–323), chapter 13). New York: Springer.
Carissimi, M., Flôres, S., & Rech, R. (2018). Effect of microalgae addition on active biodegradable starch film. Algal Research, 32, 201–209.
Chronakis, I. S. (2001). Gelation of edible blue-green algae protein isolate (Spirulina platensis strain pacifica): thermal transitions, rheological, properties, and molecular forces involved. Journal of Agricultural and Food Chemistry, 49(2), 888–898.
Chu, W., Lim, Y., Kutty Radhakrishnan, A., & Lim, P. (2010). Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals. BioMed Central Complementary and Alternative Medicine, 10(1), 53 (8 pp.).
Dehghani, S., Hosseini, S. V., & Regenstein, J. M. (2018). Edible films and coatings in seafood preservation. A review. Food Chemistry, 240, 505–513.
Feng, X., Ng, V. K., Mikš-Krajnik, M., & Yang, H. (2017). Effects of fish gelatin and tea polyphenol coating on the spoilage and degradation of myofibril in fish fillet during cold storage. Food and Bioprocess Technology, 10(1), 89–102.
García-Soto, B., Miranda, J., Rodríguez-Bernaldo de Quirós, A., Sendón, R., Rodríguez-Martínez, A., Barros-Velázquez, J., & Aubourg, S. P. (2015). Effect of biodegradable film (lyophilised alga Fucus spiralis and sorbic acid) on quality properties of refrigerated megrim (Lepidorhombus whiffiagonis). International Journal of Food Science and Technology, 50(8), 1891–1900.
Goettems Kuntzler, S., Araujo de Almeida, A., Vieira Costa, J., & Greque de Morais, M. (2018). Polyhydroxybutyrate and phenolic compounds microalgae electrospun nanofibers: a novel nanomaterial with antibacterial activity. International Journal of Biological Macromolecules, 113, 1008–1014.
Jo, W., Song, N., Lee, J., & Song, K. (2014). Physical properties and antimicrobial activities of a persimmon peel/red algae composite film containing grapefruit seed extract. Food Science and Biotechnology, 23(4), 1169–1172.
Khan, Z., Bhadouria, P., & Bisen, P. S. (2005). Nutritional and therapeutic potential of Spirulina. Current Pharmaceutical Biotechnology, 6(5), 373–379.
Kim, S., Back, S., & Song, K. (2018). Physical and antioxidant properties of alginate films prepared from Sargassum fulvellum with black chokeberry extract. Food Packaging and Shelf Life, 18, 157–163.
Larrosa, A., Camara, A., Mora, J., & Pinto, L. (2018). Spirulina sp. biomass dried/disrupted by different methods and their application in biofilms production. Food Science and Biotechnology, 27(6), 1659–1665.
Lowry, R., & Tinsley, I. (1976). Rapid colorimetric determination of free fatty acids. Journal of the American Oil Chemists' Society, 53(7), 470–472.
Lucas, B., Morais, M., Santos, T., & Vieira Costa, J. (2018). Spirulina for snack enrichment: nutritional, physical and sensory evaluations. Lebensmittel Wissenschaft und Technologie-Food Science and Technology, 90, 270–276.
Luo, A., Feng, J., Hu, B., Lv, J., Oliver Chen, C., & Xie, S. (2017). Polysaccharides in Spirulina platensis improve antioxidant capacity of Chinese-style sausage. Journal of Food Science, 82(11), 2591–2597.
Madera-Santana, T., Robledo, D., & Freile-Pelegrin, Y. (2011). Physicochemical properties of biodegradable polyvinyl alcohol-agar films from the red alga Hydropuntia cornea. Marine Biotechnology, 13(4), 793–800.
Mallikarjun Gouda, K., Kavitha, M., & Sarada, R. (2015). Antihyperglycemic, antioxidant and antimicrobial activities of the butanol extract from Spirulina platensis. Journal of Food Biochemistry, 39(5), 594–602.
Miyashita, K., & Takagi, T. (1986). Study on the oxidative rate and prooxidant activity of free fatty acids. Journal of the American Oil Chemists' Society, 63(10), 1380–1384.
Najdenski, H., Gigova, L., Iliev, I., Pilarski, P., Lukavsk, J., Tsvetkova, I., Ninova, M., & Kussovski, V. (2013). Antibacterial and antifungal activities of selected microalgae and cyanobacteria. International Journal of Food Science and Technology, 48(7), 1533–1540.
Nanthini Devi, K., Balachandran Dhayanithi, N., Ajith Kumar, T., Balasundaram, C., & Hari Krishnan, R. (2016). In vitro and in vivo efficacy of partially purified herbal extracts against bacterial fish pathogens. Aquaculture, 458, 121–133.
Neira, L. M., Agustinelli, S. P., Ruseckaite, R. A., & Martucci, J. F. (2019). Shelf life extension of refrigerated breaded hake medallions packed into active edible fish gelatin films. Packaging Technology and Science, 32(9), 471–480.
Ngo-Matip, M., Pieme, C., Azabji-Kenfack, M., Moukette Moukette, B., Korosky, M., Stefanini, P., Yonkeu Ngogang, J., & Moses Mbofung, C. (2015). Impact of a daily supplementation of Spirulina platensis on the immune system of naïve HIV-1 patients in Cameroon: a 12 months single blind, randomized, multicenter trial. Nutrition Journal, 14(1), 70 (7 pp.).
Nowzari, F., Shabanpour, B., & Mahdi Ojagh, S. (2013). Comparison of chitosan-gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chemistry, 141(3), 1667–1672.
Onacik-Gur, S., Zbikowska, A., & Majewska, B. (2018). Effect of spirulina (Spirulina platensis) addition on textural and quality properties of cookies. Italian Journal of Food Science, 30, 1–12.
Ovando, C., de Carvalho, J., de Melo Pereira, G., Jacques, P., Soccol, V., & Soccol, C. (2018). Functional properties and health benefits of bioactive peptides derived from Spirulina: a review. Food Reviews International, 34(1), 34–51.
Özoğul, Y. (2010). Methods for freshness quality and deterioration. In L. Nollet & F. Toldrá (Eds.), Handbook of seafood and seafood products analysis (pp. 189–214), chapter 13). Boca Raton: CRC Press.
Rhim, J., Park, S., & Kim, M. (2002). Preparation of biodegradable films using glue plants. Food Science and Biotechnology, 11, 280–284.
Rodríguez, O., Barros-Velázquez, J., Ojea, A., Piñeiro, C., & Aubourg, S. P. (2003). Evaluation of sensory and microbiological changes and identification of proteolytic bacteria during the iced storage of farmed turbot (Psetta maxima). Journal of Food Science, 68(9), 2764–2771.
Rodríguez, A., Cruz, J. M., Paseiro-Losada, P., & Aubourg, S. P. (2012). Effect of a polyphenol-vacuum packaging on lipid deterioration during an 18-month frozen storage of Coho salmon (Oncorhynchus kisutch). Food and Bioprocess Technology, 5(6), 2602–2611.
Romay, C., Remírez, D., & González, R. (2001). Actividad antioxidante de la ficocianina frente a radicales peroxílicos y la peroxidación lipídica microsomal. Revista Cubana de Investigaciones Biomedicas, 20, 38–41.
Rosas, V. T., Poersch, L. H., Romano, L. A., & Tesser, M. B. (2019). Feasibility of the use of Spirulina in aquaculture diets. Reviews in Aquaculture, 11(4), 1367–1348.
Sadeghi, S., Jalili, H., Siadat, S., & Sedighi, M. (2018). Anticancer and antibacterial properties in peptide fractions from hydrolyzed spirulina protein. Journal of Agricultural Science and Technology, 20, 673–683.
Saki, J., Khodanazary, A., & Mehdi Hosseini, S. (2018). Effect of chitosan-gelatin composite and bi-layer coating combined with pomegranate peel extract on quality properties of Belanger’s croaker (Johnius belangerii) stored in refrigerator. Journal of Aquatic Food Product Technology, 27(5), 557–567.
Sikorski, Z., & Kolakoski, E. (2000). Endogenous enzyme activity and seafood quality: Influence of chilling, freezing, and other environmental factors. In N. Haard & B. Simpson (Eds.), Seafood enzymes (pp. 451–487). New York: Marcel Dekker.
Stejskal, N., Ruseckaite, R. A., & Martucci, J. F. (2018). Spirulina platensis as potential alternative bio-feedstock of sustainable active films. Conference Compass ECO-BIO 2018, Dublin, Ireland. 4-7 March 2018.
Stejskal, N., Ruseckaite, R. A., & Martucci, J. F. (2019). Development and characterisation of active films based on gelatine and algal protein. XXXII Chemical Argentinian Congress, Buenos Aires, Argentina. 12-15 March 2019.
Sun, Y., Chang, R., Li, Q., & Li, B. (2016). Isolation and characterization of an antibacterial peptide from protein hydrolysates of Spirulina platensis. European Food Research and Technology, 242(5), 685–692.
Taghavi Takyar, M. B., Haghighat Khajavi, S., & Safari, R. (2019). Evaluation of antioxidant properties of Chlorella vulgaris and Spirulina platensis and their application in order to extend the shelf life of rainbow trout (Oncorhynchus mykiss) fillets during refrigerated storage. Lebensmittel Wissenschaft und Technologie-Food Science and Technology, 100, 244–249.
Tavakoli, J., Grewer, M., Zaraei-Jelyani, A., & Estakhr, P. (2017). Oxidative stability of olive oil during the thermal process: effect of Pistacia khinjuk fruit oil. International Journal of Food Properties, 20(12), 3256–3265.
Vázquez, M., Fidalgo, L., Saraiva, J. A., & Aubourg, S. P. (2018). Preservative effect of a previous high-pressure treatment on the chemical changes related to quality loss in frozen hake (Merluccius merluccius). Food and Bioprocess Technology, 11(2), 293–304.
Wang, Z., & Zhang, X. (2015). Preparation of bioactive peptides derived from Spirulina platensis and their anti-tumour activities. Modern Food Science and Technology, 11, 25–32.
Yerlikaya, P., Aydan Yatmaz, H., & Kadir Topuz, O. (2020). Applications of edible films and coatings in aquatic foods. In Y. Özoğul (Ed.), Innovative technologies in seafood processing (pp. 71–91), chapter 4). Boca Raton: CRC Press.
Yu, J., Hu, Y., Xue, M., Dun, Y., Li, S., Peng, N., Liang, Y., & Zhao, S. (2016). Purification and identification of antioxidant peptides from enzymatic hydrolysate of Spirulina platensis. Journal of Microbiology and Biotechnology, 26(7), 1216–1223.
Funding
The work was supported by the Project 2013-70E-001 (CSIC, Spain) and PICT 2016-1672 (ANPCyT, Argentina).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stejskal, N., Miranda, J.M., Martucci, J.F. et al. Quality Enhancement of Refrigerated Hake Muscle by Active Packaging with a Protein Concentrate from Spirulina platensis. Food Bioprocess Technol 13, 1110–1118 (2020). https://doi.org/10.1007/s11947-020-02468-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02468-z