Abstract
Arthrospira Spirulina biomass is a source of intracellular compounds with great commercial interest, such as phycocyanin and phenolic compounds. In this work, different cell disruption methods of the microalgae biomass dried in spouted bed and in conventional tray were performed, aiming lead to the better extraction yields of these compounds. The samples of both drying techniques with the most suitable cell disruption were used to biofilms production. FTIR, DSC, and SEM for all samples were performed. The samples dried in spouted bed with cell disruption by milling and by microwave showed the best results for the powder products, with phycocyanin contents of 75.0 and 85.4 mg g−1, and total phenolic compounds of 41.6 and 41.9 mg g−1, respectively. However, the tray drying/milling produced the biofilms with the best characteristics (tensile strength of 3.69 MPa and water vapor permeability of 1.67 × 10−11 g m s−1 m−2 Pa−1) and the highest thermal stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spirulina (Arthrospira) sp. is one of the most popular microalgae, and for the World Health Organization, it is one of the greatest foods on earth (Chacón-Lee and González-Mariño, 2010). Spirulina is a filamentous microalga considered as an antioxidants valuable source, such as phenolic compounds, carotenoids and phycocyanin, and of polyunsaturated fatty acids. Phycocyanin is a natural blue pigment, which is used as a food colorant, and this pigment is the phycobiliprotein most abundant on the Spirulina, constituting up to 20% of its dry weight. Several authors have investigated different techniques to provide better yields in the phycocyanin extraction (Martelli et al., 2014).
The drying operation is performed to obtain the dry biomass for subsequent extraction. However, studies have reported that the phycocyanin is unstable to heat, resulting in precipitation and loss of its natural color, which limits their use in food and pharmaceutical industries. The tray drying is a traditional technique, which is widely used to obtain dried biomass (Oliveira et al., 2010). However, the long drying time at higher temperature can lead to losses of thermosensitive compounds, such as phycocyanin and phenolic compounds (Costa et al., 2015). The spouted bed is a technique used in pastes/suspensions drying, and it presents the possibility to use lower temperatures when compared to the conventional spray dryer (Epstein and Grace, 2011). The cell disruption methods have been performed to increase the yield of several compounds from the microalgae biomass, such as lipids for biodiesel production, proteins, and pigments. The cell disruption leads to the release of intracellular compounds by application of various techniques, such as ultrasonic, abrasive treatments, chemical treatment, enzymatic lysis, freezing, changes in osmotic pressure, microwave and autoclave (Prabakaran and Ravindran, 2011; Show et al., 2015).
Dehydrated products from microalgae can be used such as dietary supplements for human consumption, among other applications. There are some films developed of algae and marine materials, however, the development of films using the crude extract of Spirulina has not yet been explored. Thus, this work aimed to study different cell disruption methods in Spirulina dried in spouted bed dryer and in a tray dryer, evaluating the behavior of the phycocyanin and phenolic compounds extraction. The most suitable cell disruption method for the samples, in both drying techniques, was used for biofilms production by casting technique, and its mechanical and physical properties were evaluated. FTIR, DSC and SEM analysis for all samples (powder and films) were also performed.
Materials and methods
Raw material
Arthrospira Spirulina (strain LEB-18) was produced using water from the Mangueira Lagoon, localized in southern Brazil. Lagoon water was supplemented with 20% (v/v) Zarrouk synthetic medium for the inoculum maintenance and biomass production. Spirulina was grown in raceway-type open bioreactors following the procedure reported by Morais et al. (2009), in which the water was mechanically stirred by 18 rpm. When the concentration reached 0.5 g L−1, the microalgae biomass was separated by filtration and pressed, resulting in a wet paste with about 0.20 g g−1 of solid.
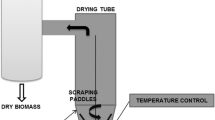
Biomass drying in spouted bed and in tray
The spouted bed dryer used for the Spirulina biomass drying consisted of a drying cell of conical conventional geometry, built in previous work (Larrosa et al., 2015). The solids concentration of the Spirulina suspension was standardized in 0.5 g L−1 with distilled water (because this is the concentration reached in end of the microalgae cultivation). Spirulina suspension was fed in center of the column in a semi-continuous system (syringe), being atomized with compressed air at 200 kPa absolute pressure. The dried powder was collected in a cyclone. The operation conditions in the spouted bed dryer for the Spirulina suspension drying was determined through the preliminary tests, being the suspension flow rate of 0.4 kgbiomass kg −1inert h−1 and the inlet air temperature at 95 ± 1 °C. The minimum spouting air velocity was of 0.33 m s−1 and, to ensure the stability of the spouted bed operation, the air circulation rate was the double of the minimum spouting velocity (Epstein and Grace, 2011). The assays were carried out in triplicate.
The Spirulina biomass samples were dried in a conventional tray dryer using the best conditions found in previous work (Oliveira et al., 2010). The biomass samples were put into a perforated tray, with a sample load of 4 kg m−2. The samples were in cylindrical pellets forms with a diameter of 4 mm (same thickness of tray). The temperature, velocity and absolute humidity of the drying air were of 55 °C, 2.5 m s−1 and 0.015 kg kg−1, respectively. Afterwards, the dried samples were ground in a knife mill (Willey model, Philadelphia, USA) and sieved passing in Tyler 35 mesh (dp < 425 µm). The assays were carried out until the final moisture contents of the samples reach 0.10 kg kg−1 (w.b.) and they were performed in triplicate.
Biomass disruption methods
The dried biomass samples were disrupted by three different methods. By milling, using 150 g of sample in a ball mill (jar volume of 0.0043 m3, with a porcelain balls volume of 20% of total volume) for 2 h at 60 rpm. By microwave oven, using 0.5 g of sample, which was inserted in a microwave oven (Sanyo, EM804TGR, São Paulo, Brazil) for 120 s, with the frequency of 2450 MHz and power of 1400 W. By autoclaving, using 5 g of sample, which were placed in an autoclave (Stermax, vertical model 16052, Pinhais, Brazil) for 30 min, at 121 °C and 200 kPa absolute pressure. The samples obtained by autoclaving method were placed in a vacuum dryer (Quimis, model Q819V2, São Paulo, Brazil) at 40 °C and 14 kPa for 6 h, to remove the water adsorbed in the process. The operation conditions of each disruption method were chosen of previous work (Pohndorf et al., 2016) and the all disrupted assays were carried out in triplicate. Spirulina samples were identified according to the following legend: SB: nondisrupted sample dried in spouted bed; SB-M: sample dried in spouted bed and disrupted by milling; SB-MW: sample dried in spouted bed and disrupted by microwave; SB-AT: sample dried in spouted bed and disrupted by autoclaving; TD: nondisrupted sample dried in tray; TD-M: sample dried in tray and disrupted by milling; TD-MW: sample dried in tray and disrupted by microwave; TD-AT: sample dried in tray and disrupted by autoclaving.
Biomass samples analyses
Phycocyanin content was determined by the method proposed in Moraes et al. (2010). The Spirulina samples were mixed with distilled water on biomass:solvent ratio of 0.16 g mL−1 (dry matter). The phycocyanin concentration (mg mL−1) was determined in optical densities measured at 620 and 652 nm. Phycocyanin content (PC) was expressed in mg g−1 in relation to in natura biomass, for all assays.
Total phenolic compounds were extracted with methanol and the precipitation of non-phenolics with Ba(OH)2 and ZnSO4, according to Assis et al. (2014). The Folin–Ciocalteau reagent was used to determine the total phenolic content, which was measured at 750 nm in a spectrophotometer (SP-220, Bioespectro, Curitiba, Brazil). Gallic acid was used as the standard, and the results were expressed as mgGAE g −1sample .
The aqueous extracts of phycocyanin and the methanolic extracts of total phenols were analyzed by infrared spectrums (FTIR) to identify the main peaks, which represent the functional groups in the Spirulina samples. Distilled water and methanol were used as blank standards. The analysis was based on spectroscopy FT-IR (Shimadzu, Prestige 21, model The-210045, Kyoto, Japan), with horizontal attenuated total reflectance, through a crystal plate with an opening angle of 45°. The analyses were performed at room temperature (20 °C) using a scan frequency in the range of 4000-800 cm−1, with forty-five swept by aliquot. The analyses of differential scanning calorimetry (DSC) (Shimadzu, model DSC60, Kyoto, Japan) were evaluated in the dried samples. The samples (around 4–6 mg) were loaded in pans and sealed and, an empty pan was used as a reference material. The runs were carried out in a temperature range from 15 to 200 °C at a heating rate of 10 °C min−1, under nitrogen flow (50 mL min−1). The structural surfaces of the dried samples were examined by a scanning electron microscope (SEM) (Jeol, model JSM-6610LV, Tokyo, Japan). The samples were coated with gold in a sputter coater (Sputtering Denton Vacuum Desk V., New Jersey, USA) before the SEM analysis to be performed.
Biofilms preparation of dried Spirulina
The Spirulina biofilms were prepared as follows: the dried biomass samples (1.5 g) were dissolved in 50 mL of distilled water under constant stirring (Dremel Stylus, 1100–1101, Rancine, WI, USA) at 25,000 rpm for 10 min. Afterwards, glycerol (10%, w/w) was added as plasticizer on the filmogenic solutions, which were homogenized by magnetic stirring (Marte, MAG-01H, São Paulo, Brazil) at 600 rpm for 15 min. The uniform biofilms thicknesses were maintained by casting onto acrylic plates and drying on air circulation oven, at 30 ± 2 °C for about 24 h for solvent evaporation. The biofilms samples were removed from plates and conditioned in desiccators (relative humidity of 55%) at 25 ± 1 °C for at least 48 h.
The biofilms thicknesses were measured with a digital micrometer (model Insize IP54, Series 3103-25, São Paulo, Brazil) of 0.0010 mm resolution. The mean thicknesses were determined by ten measurements performed at different locations on the samples. Mechanical properties (tensile strength and elongation) were measured using a Texture Analyzer TA-XT-2i (Stable Micro Systems, Surrey, UK) (ASTM, 2000a). The samples were cut in sizes of 25 × 100 mm (width × length). The water vapor permeability was measured using a desiccator under controlled conditions (ASTM, 2000b). The color of the Spirulina films was analyzed using a colorimeter (CR-300, Minolta Corporation, Ramsey, USA). The biofilms samples were placed on a blank standard plate and the color parameters were measured at three different points on the samples surfaces. The values of the standard plate were L′ = 97.3, a′ = –0.14 and b′ = 1.86. The chroma (C), hue angle (Hue) and total color difference (ΔE) were calculated according to the literature (Larrosa et al., 2015). The Spirulina films were characterized by FTIR, DSC and SEM analysis with the same methodologies described previously for the powder samples. The analyses were carried out in two repetitions.
Statistical analysis
The effects of cell disruption in the samples by different drying methods and on Spirulina biofilms were statistically analyzed using Tukey’s test, to determine the significant differences between the obtained values at 95% level (p < 0.05) (Box et al., 2005).
Results and discussion
The samples of in natura Spirulina showed the following chemical composition (wet basis): moisture content of 0.790 ± 0.013 g g−1, ashes content of 0.013 ± 0.004 g g−1 and proteins content of 0.139 ± 0.023 g g−1 by AOAC (1995) methodology. Lipids content of 0.019 ± 0.002 g g−1 was according to Folch et al. (1957), and carbohydrates content of 0.039 ± 0.004 g g−1 was by difference.
The microalgae samples dried in spouted bed presented the final moisture content around 9% (w.b.), and in the conventional tray dryer was of 10% (w.b.) in a total drying time of 240 min.
Effect of cell disruption in microalgae biomass
For phycocyanin contents shown in Table 1, the dried samples in spouted bed and disrupted by microwave presented the best results followed by milling disruption, showing in a large increase in relation to the nondisrupted dried sample. For tray drying, the milling disruption was the most effective to extract the phycocyanin. The Spirulina has a rigid cell wall of several layers, being difficult the extraction of intracellular compounds, however, due to the breaking processes, the phycocyanin is released in higher concentration, because it is present in the thylakoid of the chloroplasts.
In relation to the total phenolic compounds (Table 1), the samples obtained from spouted bed drying and disrupted by milling, microwave and autoclaving not showed significant differences at 95% level (p < 0.05). However, independently of the rupture method, the samples obtained in spouted bed drying showed higher concentrations of total phenolic compounds than in tray drying and they showed an increase of 33% compared to the nondisrupted sample. Similarly, as the phycocyanin, the polyphenols are intracellular compounds, which are found in higher concentrations into the vacuoles.
The bands from the phycocyanin structure were identified by evaluating the FT-IR spectrums of the aqueous phycocyanin extracts for the samples dried in spouted bed and in the conventional tray, without cell disruption (shown in Fig. S1 of the Supplementary Material). In sample dried in the tray without cell disruption, some bands were observed with less intensity than on dried sample in spouted bed without cell disruption in the regions of 3000, 1700 and 1100 cm−1. In addition, in the region 1100 cm−1 had a group of compounds with C–O–H and, the overlap of nearby bands can indicate a preservation of these groups, which may indicate lower losses of phycocyanin in the sample dried in the tray in relation to the samples dried in the spouted bed. The sample dried in spouted bed with microwave disruption presented a higher phycocyanin content than the other samples, showing peaks in the regions of 3000 cm−1 (C–H) and 1700 cm−1 (C=O) with greater intensity than the samples disrupted by milling and by autoclaving. The sample dried in the tray with milling disruption showed the grouping C–H in the region of 3000 cm−1 and overlap of C–O–H derivatives on region 1100 cm−1 with higher intensities than the other samples. In addition, it was observed that all samples obtained by cell disruption showed a decreased of the carbonyl group (C=O) in the region of 1700 cm−1, because the formation of derivatives of this group showed that the cell disruption led to a disturbance in the groups and, consequently, on the phycocyanin content.
In the FT-IR spectrums of the methanolic extracts of total phenolic compounds of the dried samples, in spouted bed and tray, without and with cell disruption, were identified the phenolic characteristic bands (shown in Fig. S2 of the Supplementary Material). The bands in 3520 and 3462 cm−1 correspond to the stretching of N–H; 3151 and 3198 cm−1 correspond to OH stretching; 2925, 2847, 2875 and 2750 cm−1 correspond to the aldehydes grouping; 1645 cm−1 corresponds to C=O vibrational stretching; 1164, 1167 and 1003 cm−1 correspond to the C–O stretching and, 870 cm−1 corresponds to the angular deformation of aromatic rings (Venkatesan et al., 2012). These functional groups are responsible for acid phenolics, which are the most predominant in the Spirulina. In the spectrums analysis of the samples dried in spouted bed without cell disruption can be observed some differences between the peaks. In the 2847–2875 cm−1 bands, the samples with cell disruption showed similar transmittances and the 1405 cm−1 band disappeared.
The DSC curves for the samples dried in spouted bed showed similar behavior for all samples subjected to the cell disruptions process (shown in Fig. S3 of the Supplementary Material). In the same way, for the samples dried in the tray, however, the milling sample showed a different curve of the other samples. Based on peak temperatures (Fig. S3) and in the calculated enthalpy values, the samples with higher thermal stabilities were the dried samples without cell disruption in spouted bed with the values of 98.5 °C and 216.1 J g−1 and, the dried samples in tray with the values of 88.6 °C and 243.2 J g−1, respectively. The samples dried in spouted bed with cell disruption showed thermal degradation peaks in the range from 79.4 to 84.1 °C, corresponding to an enthalpy variation from 130.2 to 152.5 J g−1. While the samples dried in the tray with cell disruption showed peaks from 81.7 to 109.6 °C and the enthalpy variation from 164 to 200.0 J g−1. Thus, the dried samples with cell disruption in tray drying were thermally more stable than the samples in spouted bed drying with disruption.
SEM photographs evaluated the surfaces of the dried Spirulina samples in powder (shown in Fig. S4 of the Supplementary Material). The samples without cell disruption dried in spouted bed (< 100 µm size) and in tray (around 500 µm size) bed showed particles of irregular structures, compacts and rough. The samples disrupted by milling showed decreased particles size, with the formation of agglomerated. For the samples disrupted by microwave was not showed differences in morphological structure, and the samples disrupted by autoclaving presented a roughness reduction of particles due to overheating.
Effect of cell disruption on properties of the biofilms samples
Based on the results shown to cell rupture for the extraction of intracellular compounds, the milling method was chosen as the cell disruption for the preparation of biofilms. Although the microwave method had better results in spouted bed drying, however, the milling process was the suitable cell disruption method for both drying techniques.
In relation to mechanical properties shown in Table 2, the TD-M biofilm showed higher resistance than the other films, with significant difference at 95% level (p < 0.05). The SB biofilm and the SB-M biofilm were not showed significant differences at 95% level (p > 0.05) on the tensile strength, while the lowest resistance was observed in the TD biofilm. In relation to elongation, TD biofilm showed higher extensibility than the other biofilms. This behavior can be explained by particles size of the dried product, because the TD sample used for the film preparation was of coarse particles (around 500 µm size) and, consequently, resulted in a biofilm with higher thickness. The TD-M, SB and SB-M biofilms samples showed less thicknesses due to the fine particles (< 100 µm size), caused by the milling and the atomization in the spouted bed. In addition, it was observed that SB and SB-M films were more brittle and fragile, although the values of tensile strength were higher than the TD biofilm.
In Table 2, for the water vapor permeability (WVP), the TD biofilm showed the highest value, however, this was not expected, because it had higher thickness. The SB and SB-M biofilms not presented significant differences (p > 0.05) in WVP values and, were observed fissures along on its surfaces due to the brittle and fragile characteristics. In general, the protein-based films have high vapor permeability of water when compared to most synthetic polymers, due to its hydrophilic characteristic. According to Sothornvit and Krochta (2001), the combination of intermolecular disulfide bonding, hydrophobic interactions and electrostatic forces between protein chains leads to brittle films. However, even with the addition of plasticizer, which allows a reduction in the intensity of these intermolecular forces, only the SB biofilm presented higher fragility. According to results in Table 2 can be concluded that the TD-M biofilm showed better properties for packing due to the highest tensile strength and the lowest water vapor permeability.
The values of the color analysis were determined for the Spirulina biofilms samples (shown in Table S1 of the Supplementary Material). The lightness of biofilms did not show significant differences at 95% level (p < 0.05), being characterized as dark films by the small lightness (near to zero). The hue angle values of the TD, SB, and SB-M biofilms were in the region around 180°, representing the green-yellowness color; however, the TD-M biofilm showed the lowest value of hue angle (114°), which represent the yellow–greenness color. This difference was, probably, due to the chlorophyll and phycocyanin contents, which are the main pigments present on Spirulina biomass. The chroma (C) values showed that, for drying techniques, were more intense in films without cell disruption. In relation to the total color difference (ΔE) was observed that TD biofilm showed the highest significant difference at 95% level (p < 0.05) between Spirulina films, with a higher color modification in relation to the color of in natura biomass. The other biofilms showed minor ΔE values, due to the lowest particles sizes resultant of the milling process in the ball mill and of the drying in spouted bed, which produced particles with sizes below 100 µm, and thus, the pigments were most exposed.
The functional groups of the Spirulina biofilms were identified by FTIR, and the spectrums are presented in Fig. 1. The 3300 cm−1 band can be assigned by N–H stretching vibration, which represents the presence of secondary amines in proteins and lipids. The 2970, 2935 and 2870 cm−1 bands are representing the aliphatic C–H stretching vibration. The 1745 cm−1 band can be attributed to the C=O stretching vibration referent ester and amino acids. The scissor bending of NH2 group can be observed at 1635 cm−1, the 1560 and 1500 cm−1 bands are the interaction N–H bending with C–N stretching and, the 1415 cm−1 band is relative to the stretching vibration to the C–N of the primary amide. The C–O stretching and O–H bending presence of alcohol were observed at 1370 cm−1, and bands observed in 1120 and 1051 cm−1 were attributed the symmetric C–H stretching present in antioxidant enzymes and the SO3 symmetric stretching vibration present in acids and RSO3, respectively. The bands in the region of 830–970 cm−1 were attributed the aromatic –CH stretching vibrations and –P–O, –S–O (Silverstein et al., 2005). The 3300 cm−1 band was not observed in the biofilms of TD and TD-M samples, however, in the biofilms of SB and SB-M samples this band presented a small intensity, indicating that these biofilms showed that the interactions between primary amines and other compounds present in the biofilms were weaker.
In DSC curves shown in Fig. 2 was observed that, between all samples, the SB and TD biofilms presented higher temperatures peaks (from 97.9 to 90.3 °C), showing that were more thermally stable. The biofilms with cell rupture showed peaks in the range of 80 °C, being that TD-M biofilm showed the highest enthalpy variation (152.2 J g−1), needing to absorb more energy than the SB-M biofilm (114.4 J g−1) for leads to a change in its structure. Therefore, among the biofilms with cell disruption, the TD-M biofilm showed the highest thermal stability.
The biofilms of Spirulina biomass were evaluated by its surfaces (shown in Fig. 3). The biofilm of TD sample showed a rough and striated surface due to coarse particles, and the other biofilms showed continuous phases. However, the biofilm of TD-M sample showed a little rough and irregular surface, while the biofilms of the SB and SB-M samples presented more smooth and homogeneous surfaces.
The cell disruption of the dried Spirulina biomass led to an increase of bioactive compounds. Spouted bed drying and the disruption methods by the mill and microwave oven led to the best results in the powder product, showing the highest values of phycocyanin contents and total phenolic compounds, being thus, the most suitable for use in the food industry and dietary supplements. Tray drying associated to the milling disrupted method produced the biofilms with the best mechanical properties, lower water vapor permeability, yellow-greenness color and higher thermal stability, showing better characteristics for use in food packings. The evaluations of the drying techniques and cell disruption methods for intracellular components extraction, with the purpose of added this biomass in food products, as well as, for the biofilms production, showed to be an important study due to the useful potentials of these compounds.
References
AOAC. Official Method of Analysis of AOAC Intl. 16th ed. Association of Official Analytical Chemists, Arlington, VA, USA (1995)
Assis LM, Machado AR, Motta AS, Costa JAV, Souza-Soares LA. Development and characterization of nanovesicles containing phenolic compounds of microalgae Spirulina strain LEB-18 and Chorella pyrenoidosa. Adv. Mater. Phys. Chem. 4: 6–12 (2014)
ASTM. Standard Test Methods for Tensile Properties on Thin Plastic Sheeting. Method D00882–00. American Society for Testing and Materials, Philadelphia, USA, pp. 160–168 (2000a)
ASTM. Standard Methods of Water Vapor Transmission of Materials. Method E00996–00. American Society for Testing and Materials, Philadelphia, USA, pp. 907–914 (2000b)
Box GEP, Hunter JS, Hunter WG. Statistics for Experiments: Design, Innovation, and Discovery. 2nd ed. John Wiley & Sons, Hoboken, NJ, USA (2005)
Chacón-Lee TL, González-Mariño GE. Microalgae for healthy foods—possibilities and challenges. Comp. Rev. Food Sci. Food Saf. 6: 655-675 (2010)
Costa BR, Rocha SF, Rodrigues MCK, Pohndorf RS, Larrosa APQ, Pinto LAA. Physicochemical characteristics of the Spirulina sp. dried in heat pump and conventional tray dryers. Int. J. Food Sci. Technol. 50: 2614–2620 (2015)
Epstein N, Grace JR. Spouted and Spout-Fluid Beds: Fundamentals and Applications. Cambridge University Press, Cambridge, UK (2011)
Folch J, Lees M, Stanley GHS. A simple method for isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226: 497–509 (1957)
Larrosa APQ, Cadaval Jr TRS, Pinto, LAA. Influence of drying methods on the characteristics of a vegetable paste formulated by linear programming maximizing antioxidant activity. LWT Food Sci. Technol. 60: 178–185 (2015)
Martelli G, Folli C, Visai L, Daglia M, Ferrari D. Thermal stability improvement of blue colorant C-phycocyanin from Spirulina platensis for food industry applications. Process Biochem. 49: 154–159 (2014)
Moraes CC, Burkert JFM, Kalil SJ. C-Phycocyanin extraction process for large-scale use. J. Food Biochem. 34: 133–148 (2010)
Morais MG, Radmann EM, Andrade MR, Teixeira GG, Brusch LRF, Costa JAV. Pilot scale semicontinuous production of Spirulina biomass in southern Brazil. Aquaculture 294: 60–64 (2009)
Oliveira EG, Duarte JH, Moraes K, Crexi VT, Pinto LAA. Optimisation of Spirulina platensis convective drying: evaluation of phycocyanin loss and lipid oxidation. Int. J. Food Sci. Technol. 45: 1572–1578 (2010)
Pohndorf RS, Camara AS, Larrosa APQ, Pinheiro CP, Strieder MM, Pinto LAA Production of lipids from microalgae Spirulina sp.: influence of drying, cell disruption and extraction methods. Biomass Bioenerg. 93: 25–32 (2016)
Prabakaran P, Ravindran AD. A comparative study on effective cell disruption methods for lipid extraction from microalgae. Lett. Appl. Microbiol. 53: 150–154 (2011)
Silverstein RM, Webster FX, Kiemle DJ. Spectrometric Identification of Organic Compounds. 7th ed. John Wiley & Sons, New York, USA (2005)
Show KY, Lee DJ, Tay JH, Lee TM, Chang JS. Microalgal drying and cell disruption—recent advances. Bioresour. Technol. 184: 258–266 (2015)
Sothornvit R, Krochta JM. Plasticizer effect on mechanical properties of B-lactoglobulin films. J. Food Eng. 50: 149–155 (2001)
Venkatesan S, Pugazhendy K, Sangeetha D, Vasantharaja C, Prabakaran S, Meenamba M. Fourier transform infrared (FT-IR) spectroscopic analysis of Spirulina. Int. J. Pharmacol. Biol. Arch. 3: 969–972 (2012)
Acknowledgements
Authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Brazil by financial support, the Biochemical Engineering Laboratory/EQA/FURG/Brazil by biomass Spirulina, and CEME-SUL/FURG/Brazil by SEM analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Larrosa, A.P.Q., Camara, Á.S., Moura, J.M. et al. Spirulina sp. biomass dried/disrupted by different methods and their application in biofilms production. Food Sci Biotechnol 27, 1659–1665 (2018). https://doi.org/10.1007/s10068-018-0397-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-018-0397-y