Abstract
Since cucumbers suffer from a short postharvest life, applying different technologies is increasingly used as effective ways to increase their shelf life and quality. In this study a combination of chitosan-limonene coating and MAP storage has been used as a postharvest treatment to maintain cucumber quality. Samples were stored in three different packages: A (21% O2, macro-perforated package to be in equilibrium with air); package B (active MAP, starting concentrations 10% O2 + 5% CO2); package C (passive MAP starting concentrations 21% O2 + 0.1% CO2); they were stored at three temperatures (20, 10, and 4 °C). Quality parameters of cucumber such as weight loss, firmness, color, pH, fungal growth, Tg, organoleptic properties were determined. Interactive effects of coating, package, temperature, and storage time showed that coating and MAP in general had positive effects on several quality aspects. Coating combined with active MAP had the most positive effect on most postharvest attributes. However, using active MAP at higher temperature led to quality problems and is only useful if storage time is short. The combined usage of active MAP and chitosan-based coating on cucumber represents an innovative and interesting method for commercial application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cucumber (Cucumis sativus L.), belonging to the family Cucurbitaceae, is an economically important vegetable crop worldwide, particularly for Iran. Iran is the third largest producer of cucumber in the world, after China and Turkey, producing 1,532,860 tonnes in 2011 (FAO, http://faostat3.fao.org/compare/E). Cucumber is a non-climacteric vegetable crop and is rich in vitamin K, Mo, K, Mn, and Mg [1]. Cucumber has a short shelf life which is mostly due to fungal decay. Several preservation technologies including cold storage, minimal processing, modified atmosphere packaging and edible coatings have been used to prolong the shelf-life of cucumber [2].
Applying edible coatings is increasingly used as an effective way to increase the shelf life of fruit and vegetables and maintain their quality [3, 4]. One of the most efficient materials used as a coating on fruits and vegetables is chitosan. Chitosan has been used as an edible coating on pear [5], sweet cherry fruit [6], cucumber [7], and mango [8], and kiwifruit [9]. To obtain an antibacterial effect, chitosan can also be enriched with essential oils (EOs) which are bioactive compounds and have been used as food preservatives [10]. Doses of essential oils should be reduced because using them alone is cost effective and causes intense aroma. In this case, an interesting method might be to incorporate these compounds into the formulation of edible coatings. Some studies have shown inhibitory effect of EOs in combination with edible coatings on tomatoes [11], strawberries [12], bell pepper [13], and cucumber [14]. Hemalatha et al. [15] reported the efficacy of chitosan films with basil essential oil for food packaging. Limonene is an example of plant-derived essential oils which has the GRAS status of the US FDA (US EPA, 1994) and exhibits antifungal activity against fungal pathogens of fruit [16]. Perdones et al. [17] have reached significant results using chitosan coating enriched with limonene on strawberries.
Modified atmosphere packaging is another way to retard decay and enhance shelf life of food products. For this purpose, Muriel-Galet et al. [18] have studied changing quality of fruits and vegetables under modified atmosphere packaging (MAP). The beneficial effect of MAP on shelf life could be due to reduction in respiration rate and biochemical activity, control of microbial growth, and to the restriction of water loss [18]. Kader et al. [19] reported that optimum atmosphere for storing cucumber is 3–5% O2 + 3–10% CO2. Saltveit [1] recommended storage period of 2–3 weeks under 3–5% O2 + 3–5% O2. A combination of MAP with a coating treatment may have a greater effect on prolonging shelf life of fresh produce [20]. For instance, a combination of pectin-based edible coating and MA packaging on persimmon fruit focusing on microbial growth have shown higher antimicrobial effects in comparison with samples individually treated under coating or MA packaging [21]. In addition, the use of both coating treatment and MA packaging on minimally processed kiwifruit showed greater preservation effects on the product by decreasing sensory decays and mold growth comparing to using MA packaging alone [22]. However, few reports represent studying the combined usage of MAP and chitosan-based coating on fresh produce shelf life.
The objective of the present work was to evaluate the effect of chitosan based coating (0.5%) combined with passive MAP (21% O2 + 0.1% CO2) or active MAP (10% O2 + 5% CO2) on quality-related properties of immature cucumber stored at different temperatures (4, 10, 20 °C).
Materials and methods
Fruit preparation
Cucumbers (Cucumis sativus L. cv. Royal) were obtained at commercial harvest maturity (about 15 cm) from an organic farm, free from physical damage and fungal infection, were transported at low temperature to the laboratory. Prior to the study, the fruits were washed with Tap water, surface sterilized in 0.5% ClO2 solution for 5 min, washed twice with tap water and surface dried at ambient condition in a slow air draft. Samples were coated on the same day.
Preparation of coating
Briefly, chitosan-limonene (CS-LEO) solution was prepared by dissolving 5 g low molecular weight CS (Sigma-Aldrich, CAS: 9012-76-4) in 50 ml of aqueous acetic acid solution (1% v/v) at ambient temperature overnight. 800 ml distilled water added to the solution. 2 ml tween 80 (CAS: 9005-65-6) as a surfactant and 5 ml glycerol (99%, CAS: 56-81-5) as a plasticizer were then added to the solution and stirred at 45 °C for 2 h to obtain a homogeneous mixture. 3 ml limonene (Sigma-Aldrich, CAS: 5989-27-5) was gradually dropped into the stirring mixture of CS solution, and agitation was carried out for 30 min to obtain an oil-in-water emulsion. Finally, the volume was adjusted to 1000 ml using distilled water to obtain a solution of 0.5% chitosan and 0.3% limonene.
Coating application and packaging
Cucumbers were divided into two groups, coated and uncoated. Those for coating application were dipped in the coating solution for 1 min, the excess solution was drained off and the coated fruits were air-dried. Both coated and uncoated cucumbers were divided into three groups and packed under three atmosphere conditions in polyethylene (PE) bags (thickness: 80 µm; \({{\text{P}}_{{{\text{O}}_{\text{2}}}}}\): 3617 ml µm m−2 h−1 atm−1; \({{\text{P}}_{{\text{C}}{{\text{O}}_{\text{2}}}}}\): 9341 ml µm m−2 h−1 atm−1) and heat-sealed. A MAP Henkelman machine (Gustav Muller and Co., Bad Homburg, Germany) was used to perform MAP packaging and the initial internal gas composition was set for the groups. Package A: macro-perforated (12 holes of 6-mm diameter), ambient air composition, (21% O2 and < 0.1% CO2); package B: 10% O2 and 5% CO2; package C: ambient air composition, non-perforated (21% O2 and about 0.1% CO2). Samples were stored under 3 temperatures (4, 10, 20 °C), RH (85–90%) and at intervals of 5 days over a period of 15 days observations were carried out. At each sample time, three packages were removed from storage and used for measurement of gaseous condition and for fruit quality measurements.
Gas analysis
The headspace gas composition (% O2 and % CO2) of packages was determined every day using a gas analyzer (WITT, GmbH & Co KG D-38,454, Germany).
Weight loss
During storage, the weight loss was measured by weighing the individual fruit on each day of the experiment by a digital precision balance (A&D CO, LTD, Japan). Weight loss was determined using the Eq. (1):
where WA is the weight on the first day and WB the weight in the test day.
Firmness
A compression test was performed on cucumbers using a Lloyd Universal Testing Machine (Model LRX-2500N, Lloyd Instruments Ltd., Fareham, Hans, UK). The samples were compressed 5 mm in depth, using a 5 mm diameter round tipped puncture probe, at a speed of 50 mm min−1 [23]. From each package, three representative fruit were selected for firmness measurement; measurement was done on three parts of the fruits.
Color analysis
Color of the cucumbers was measured using a Computer vision system (CVS) containing a Lighting system, a Color Digital Camera (model Power Shot A70 (Canon, USA)) and ImageJ software (version 1.49). ImageJ software is able to process the color image to LAB components percentages. In food research, color is frequently represented using the L* a* b* color space. L* is the luminance or lightness component that goes from 0 (black) to 100 (white), and parameters a* (from green to red) and b* (from blue to yellow) are the two chromatic components, varying from − 120 to + 120. Images of samples were captured then color measurements within the L* a* b* color system was computed [24].
pH
To determine pH of the samples were prepared according to Maftoonzad and Ramaswamy [25] using a pH meter (Metrohm Ltd. Herisau, Switzerland).
Fungal contamination
The most important microbial factor that results in spoilage in cucumber is yeasts and molds. The stomacher bags with 25 g cucumber pieces (stored refrigerated at 5 °C) were filled up with 225 g of peptone water to create a 10−1 solution and put into the stomacher (Seward Stomacher 400 Circulator) on the highest stomach-intensity for 1 min. Several decimal dilutions were prepared from a 10−1 dilution and each was spread-plated (0.1 ml) using sabouraud dextrose agar (SDA) with chloramphenicol incubated at 25 °C for 5 days.
Glass transition temperature
Three samples were chosen for Tg determination (untreated sample day 1, uncoated sample of package A at day 5, and coated sample in package B at day 5) Glass transition temperature (Tg) of the cucumber samples were determined using a differential scanning calorimeter (DSC; model DSC823e, Mettler-Toledo, Schwerzenbach, Switzerland). The instrument was calibrated using indium standard. 10–15 mg of cucumber was placed into a Mettler-Toledo DSC pan (ME-00026763) that was then sealed hermetically. An empty pan was used as reference. The sample was first cooled to − 120 °C, at − 10 °C min−1, and then scanned from − 120 to 80 °C at a rate of 10 °C min−1 to determine its thermal behaviour. Tg was recorded as the middle temperature on the curves of the heat flow vs. temperature, where a step change in the heat capacity is evidenced [26].
Sensory analysis
Sensory analysis was carried out using 20 panelists within the age range of 20–30 years who were chosen on the basis that they regularly consume and purchase cucumbers. For this regard, two cucumbers of each package were used, one of them was cut into round pieces and the other one was put in full for appearance checking. It is noteworthy that each time of evaluation an untreated sample served as the control. The following characteristics were evaluated: hardness, color, taste, appearance, and general acceptance (GA). The sensory evaluation was rated on a five-point Hedonic: 1, very poor; 2, poor; 3, not bad or not good; 4, good; 5, excellent [27]. It was assumed that the sensory attribute rejection would occur when the score declined below 3.5.
Statistical analysis
The experiment had factorial structure with two coatings (0 and 0.5%), three packaging, four storage time (0, 5, 10, and 15 days), and 3 temperatures (4, 10, 20 °C). The experiment had a complete random design for each factor combination with three replications. The effects of the factors on each qualitative characteristic were determined by analysis of variance using SPSS 12.0 (Version, 2008). Also, Duncan’s multiple range tests (DMRT) at 5% probability were performed to compare the means of different treatments.
Results and discussion
Head space composition
Due to respiration, fresh products consume O2 and produce CO2 inside a package leading to a change in the atmosphere of the package [20]. Since in the macro-perforated package (Package A) conditions were equal to ambient atmospheric conditions, data are not shown. Figure 1 shows the change in head space composition of the packages B and C during 15 days. During the initial 80 h O2 dropped rapidly with corresponding sharp increase in CO2 which might be because of high respiration rate of cucumbers in the transient state of equilibration. Chitosan coating acts as a gas barrier and reduces the respiration rate of samples in both packages in all temperatures.
In most of the cases the drop in oxygen concentrations and the increase in CO2 were more pronounced in uncoated than in coated samples. Several studies have revealed the positive effects of polysaccharide and protein-based edible coatings on reducing the respiration rate of fresh products attributed to their efficient oxygen barrier [28]. Ghidelli et al. [29] observed a lower CO2 production rate in coated eggplant fruit. Lower CO2 production has also been reported in fresh-cut apple and melon dipped in alginate-based edible coating compared to uncoated samples [30, 31]. Generally, the main reason for advantageous results of MA packaging on fresh fruits emanates from the lowered O2 and increased CO2 to reduce respiration rate [25]. This effect is obvious in samples of package B (active MAP) compared to package C (passive MAP). Dawange et al. [32] also observed rapid increase in CO2 concentration in passive MAP reached 56 (± 2.02) % during 16 days. Storage of samples at elevated temperature resulted in higher respiration rates, which was previously reported [25, 33]. At 20 °C, CO2 content in package C (both coated and uncoated samples) followed a sharp increase and did not reach the equilibrium level. This could be due to using up O2 in the packages turning the condition to anaerobic which caused fermentation and more production of CO2. Packages stored in 4 °C surprisingly contained lower O2 and higher CO2 levels compared to the ones in 10 °C which could be associated with chilling injury resulting in higher respiration rate [33].
Weight loss
Anova analysis (data not shown) showed that the effect of all four factors: chitosan coating, packaging, temperature, and storage time on weight loss were statistically significant (P < 0.05). Weight loss significantly increased as the storage time increased. Weight loss was highest at 20 °C (up to 12% at day 15) followed by 4 °C (up to 7–8% at day 15); Weight loss was lowest at 10 °C. Effects of the three different packages on weight loss were statistically significantly different. The highest weight loss was observed in samples stored in package A; the lowest weight loss in samples in package B, but differences were small. On average weight loss was about 25% less in coated samples compared to non-coated samples.
Figure 2 shows the interactive effect of the four factors on weight loss. Both coated and uncoated samples in package A at all times of storage showed the more weight loss than package B. However, there is no significant difference between weight loss of uncoated cucumbers stored under B and C atmosphere, but coated cucumbers stored under B atmosphere showed significantly less weight loss than uncoated fruit. Studying the storage time illustrated that at day 10, weight loss of cucumbers in the different packages were not significantly different, but in longer storage this difference gets more significant, so that package C has the highest weight loss at day 15, 20 °C. It is believed that migration of moisture from fruit to the environment is the major cause of post-harvest weight loss of fruit during storage causing loss of firmness and freshness [7, 34]. It has been reported that loss of weight can be due to fermentation and respiration degrading sugars to acids and gasses [19]. Weight loss affects the quality (crispness, freshness) and marketability of the product. Both coating and MAP act as semi-permeable barriers against oxygen, carbon dioxide and moisture reducing respiration and water loss which result in less weight loss [35]. These results are accordance with those of many other studies which demonstrate the effect of coating and MAP, on reducing the weight loss of food products, such as cucumber [36], baby carrots [20], and pear wedges [37].
Firmness
Based on analysis of variance, the effect of all four factors: chitosan coating, packaging, temperature, and time of storage on firmness were significant (P < 0.05). Fruit firmness is a major attribute of the postharvest life and quality of fruit. Cucumber is a fruit which is exposed to a rapid loss of firmness during storage due to water loss and susceptibility to fungal contamination.
Firmness of cucumbers with different treatments is shown in Fig. 3. In almost all of the treatments, firmness decreased over time. The pattern of firmness loss was similar at 4 and 10 °C. At both these temperatures, firmness was best preserved in package B and coated fruit showed slightly less firmness loss than uncoated fruit. At 20 °C, rapid loss of firmness occurred in the MAP packages B and C. This may be related to extensive fermentation leading to tissue decay and softening. Coating and MAP were reported influential in preserving textural water resulting in reduced firmness loss [7]. Coatings were also reported to slow down softening, probably due to the effects of chitosan coating on fruit, which acts as a barrier for O2 uptake thereby slowing down the metabolic activity, and consequently the ripening process [38].
However, samples in ventilated package A at 20 °C and day 15 had significant high firmness due to dehydration because of water evaporation. Samples of package B and C at 20 °C showed the least firmness after 15 days of storage which might be due to fermentation having decay and softening effect on texture. Degradation of middle lamella of cell wall of cortical parenchyma cells, cell wall strength, and cell to cell contact and cellular swell may influence the fruit firmness [39]. Our findings are similar to those reported by Arnon et al. [40], and Xiao et al. [37].
Color analysis
Besides firmness, color is one of the most important quality parameters of consumer acceptance. L* represents (Fig. 4) the lightness. At 4 and 10 °C lightness generally went down over the storage time. This indicates that the fruit got lighter green during the storage. Only in package B at 10 °C lightness was maintained at the initial level. There were no differences between coated and uncoated fruit at both these temperatures. At 20 °C L* went down in package A, but went up in both MAP packages.
Both coated and uncoated samples in package B and C stored at ambient temperature had higher L* on day 10 and 15, which might be due to effect of more light and temperature enhancing respiration causing discoloration of cucumbers and more water accumulation on surface [41].
a* is related to greenness and redness of samples (− 120 to + 120). At 4 and 10 °C the change in a* was relatively small and no differences were apparent between coated and uncoated fruit and between the packages. Storage time was significantly influential in a* while increasing time resulted in lower a*. At 20 °C the change in a* was much greater, especially in the MAP treatments, presumably due to tissue degeneration under conditions of high CO2. Cucumbers in package B and C stored at room temperature for 10 and 15 days had the most a* showing change of color to brown [42]. This is due to conversion of chlorophyll to pheophytin in higher level of CO2 and acidic conditions established by fermentation in these packages [25]. The coated fruit in package B at 10 °C showed the least change in a*, indication complete preservation of green color.
b* presenting blueness and yellowness of samples (− 120 to + 120) is significantly higher in most uncoated cucumbers. Effect of temperature is significant on b*. The highest b* was observed in 4 °C followed by 10 and 20 °C. However, there is no significant difference between 10 and 20 °C. Increase in time of storage resulted in higher b*. There was a significant increase in yellowness (b values) of uncoated and coated air packed samples stored at room temperature while cucumbers in package B and C stored at 20 °C suffered the lowest b* (darker surface) which correspond with results of a*. The change was greater in uncoated than in coated fruit. All in all, colorimetric parameters in coated cucumbers of package B stored at 10 °C were much similar to the control which is due to better adjustment atmosphere, low temperature and minimum effect of light inside cold chamber preserving quality of these samples. Results reported previously confirm our findings [25, 43, 44].
pH
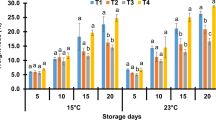
pH is one of the most important factors in determining cucumber flavour. The change in pH is associated with number of reasons; it might be due to the effect of any treatments (such as thermal and enzymatic) on the biochemical condition of the fruit and slower rate of respiration and metabolic activity [45]. Figure 5 illuminates that pH significantly decreased over time (P < 0.05) because sugars in fruits changed to acids in texture. Increase in temperature accelerates this trend because of higher respiration rate. Lack of refrigeration at any time during a MAP product’s life could allow the growth of organisms which had been inhibited during storage at low temperature [46]. MAP samples stored at ambient temperature, after 5 days, suffered the least pH because the atmosphere inside the package tends to anoxic conditions. These conditions favor the growth of facultative anaerobes and/or obligate anaerobes. Therefore, living tissue ferments and develops more acids lowering the pH. pH of coated samples of package B during the first 5 days did not change significantly in comparison to fresh cucumbers. Previous reports verify our findings [3, 20, 36].
Fungal decay
Fresh fruit, due to the high amounts of moisture and sugar, is a favourable environment for microbial growth on its surface. Variance analysis showed the significant effect of four factors (coating, gas concentration, temperature, and time) on mold and yeast population (P < 0.05). At all temperatures, mold infection was much less in coated samples, irrespective the type of package (Fig. 6). Highest numbers were found at 4 °C, followed by 10 and 20 °C.
Fewest fungi were detected in coated cucumbers stored at room temperature in package A. This might be due to the lower humidity in this package. Coating and MAP change the atmosphere and reduce the respiration rate having inhibitory effect on fungal growth [19]. Coated samples stored in package B particularly at 4 and 10 °C showed the least fungal growth. This might be due to the changed atmosphere from aerobic to anaerobic which is not suitable for molds and yeasts. As molds have an absolute requirement for O2, packaging in low O2 and high CO2 condition can be extremely successful in controlling the fungal growth [47].
Antifungal effect of chitosan has been reported previously [35]. Inhibitory effect of chitosan coating on fungal growth in cucumbers during the whole storage time could be because of its effect as an antimicrobial agent [48]. This effect might follow two mechanisms: (1) chitosan may induce the synthesis of chitinase in plant tissues, which degrades microbial cell walls; (2) polycationic nature of chitosan may scavenge the major anionic species on the surface of fruits altering the membrane cell permeability [49]. Moreover, limonene essential oil acts as an antimicrobial factor which can exert potent spectrum antimicrobial activity. Use of essential oils in food preservation is often limited because of their application costs, their intense aroma and potential toxicity. The incorporation of limonene into the chitosan-based coating reduces the doses of essential oils while maintaining their effectiveness as a natural preservative which have synergistic antimicrobial effect with chitosan [50]. These results are similar to what previously reported about apple [51], pear [5], and tomato [11].
Glass transition
Glass transition (Tg) is the name given to phenomena observed when a glass (rigid solid) is changed into a rubber (supercooled melt) during heating, or to the reverse transformations during cooling [52].
As the glass transition occurs within a temperature range, changes in the physical properties of foods related to this state transition may occur or start within that range. In this sense, the onset, mid-point or end-point of the glass transition may be taken as Tg. As the most important changes in the food must have occurred towards the end of the glass transition, the mid-point and the final temperatures were considered in this study (Fig. 7). Midpoint of Tg were − 85.87, − 95.46, and − 104.106 respectively in uncoated samples in Package A at day 5, untreated sample at day 1, and coated sample of package B at day 5. Water molecules act as plasticizer resulting in lower Tg. This effect is obviously seen in package B coated which is due to coating and MAP storage have had positive effect on preserving moisture content. Moreover, water accumulation in package due to respiration causes increase in moisture content in samples. On the other hand, Tg significantly increased in uncoated sample in package A which lost moisture content in ambient condition. Similar observation was reported by other researchers, who studied glass transitions and state diagrams for various fruits and vegetables [53, 54]. Roos and Karel [55] found dependence of Tg on water content, as water has a plasticising effect on the Tg of pectin, which leads to increased free volume and a weakening of the inter-chain interactions during storage. This plasticising activity of water may be based on the weakening of hydrogen bonds and dipole–dipole intra and inter-macromolecular interactions due to the shielding of these mainly attractive forces by water molecules [53]. In food, plasticization (Tg decrease) is mainly due to water, but other small solutes may also act as plasticizers such as glycerol used in our chitosan coating [52].
Sensory analysis
Figure 8 shows sensory attributes of cucumbers and dotted line (3.5) is the acceptance limit. All attributes of the samples at day 5 were acceptable except for appearance of package C at 20 °C.
20 °C at day 10 also led to rejection of all attributes of coated and uncoated cucumbers in package C as well as hardness and flavor of package B samples. This might be due to lower pH affecting the texture and flavor. At day 15 and 4 °C, just coated sample in package B was generally accepted although color of all samples was rejected. All attributes of package C were discarded at day 15, 10 °C, but all other samples were generally accepted. However, package B was the only acceptable package regarding the color. At 20 °C of last day, no sample was acceptable but hardness of air-packed samples (package A) which might be due to dehydration. As it is observed, samples at 10 °C got higher scores. In spite reduction of scores over time, coated and uncoated samples in package B and coated samples in package were more acceptable. Positive effect of coating and MAP on sensory attributes of mandarin [56] and carrots [32] has been reported previously. These results show that the panelists believed that 20 °C for a long storage time particularly under passive MAP (package C) were not effective ways to preserve quality attributes of cucumbers.
Conclusion
This research indicates that chitosan-limonene coating in combination with MAP could prolong postharvest life of cucumber by maintaining fruit quality properties such as weight loss, firmness, color, pH, Tg, and organoleptic properties, and reduce decay by suppression of fungal growth. Both coating and MAP act as semi-permeable barriers to reduce respiration and water loss which causes the lower weight loss. Colorimetric parameters in coated cucumbers of package B stored at 10 °C were much similar to the control which is due to better adjustment atmosphere, low temperature and minimum effect of light inside cold chamber preserving quality of these samples. Coated samples stored in package B particularly at 4 and 10 °C showed the least fungal growth. It is concluded that coating and MAP treatment and storage at 10 °C up to 15 days may be useful as a biochemical way of maintaining cucumber quality and extending its postharvest life. However, this treatment is not desirable in ambient condition (20 °C) which might be due to production of more CO2. Despite considerable findings of this article, further studies are needed to be done on combination of other treatments to preserve quality of cucumbers.
References
M.A. Saltveit, A summary of CA requirements and recommendations for vegetables. Postharvest. Acta Hortic. 600, 723–727 (2003)
M. Moalemiyan, H.S. Ramaswamy, Quality retention and shelf-life extension in Mediterranean cucumbers coated with a pectin-based film. J. Food Res. 1, 159–168 (2012)
S. Benítez, I. Achaerandio, M. Pujol, F. Sepulcre, Aloe vera as an alternative to traditional edible coatings used in fresh cut fruits: a case of study with kiwifruit slice. LWT-Food Sci. Technol. 61, 184–193 (2013)
N. Mahfoudhi, S. Hamdi, Use of almond gum and gum Arabic as novel edible coating to delay postharvest ripening and to maintain sweet cherry (Prunus avium) quality during storage. J. Food Process. Preserv. 39, 1499–1508 (2015)
C.E. Ochoa-Velasco, J.A. Guerrero-Beltrán, Postharvest quality of peeled prickly pear fruit treated with acetic acid and chitosan. Postharvest. Biol. Technol. 92, 139–145 (2014)
M.S. Pasquariello, D. Di Patre, F. Mastrobuoni, L. Zampella, M. Scortichini, M. Petriccione, Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest. Biol. Technol. 109, 45–56 (2015)
A. Mohammadi, M. Hashemi, S.M. Hosseini, Chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil enhance the shelf life of cucumber during cold storage. Postharvest. Biol. Technol. 110, 203–213 (2015)
M. Cissé, J. Polidori, D. Montet, G. Loiseau, M.N. Ducamp-Collin, Preservation of mango quality by using functional chitosan-lactoperoxidase systems coatings. Postharvest. Biol. Technol. 101, 10–14 (2015)
Z. Huang, J. Li, J. Zhang, Y. Gao, G. Hui, Physicochemical properties enhancement of Chinese kiwi fruit (Actinidia chinensis Planch) via chitosan coating enriched with salicylic acid treatment. J. Food Meas. Charact. 11, 184–191 (2017)
W.S. Jo, H.Y. Song, N.B. Song, J.H. Lee, S.C. Min, K.B. Song, Quality and microbial safety of ‘Fuji’ apples coated with carnauba-shellac wax containing lemongrass oil. LWT-Food Sci. Technol. 55, 490–497 (2014)
M. Ramos-García, E. Bosquez-Molina, J. Hernández-Romano, G. Zavala-Padilla, E. Terrés-Rojas, I. Alia-Tejacal, L. Barrera-Necha, M. Hernández-López, S. Bautista-Baños, Use of chitosan-based edible coatings in combination with other natural compounds, to control Rhizopus stolonifer and Escherichia coli DH5α in fresh tomatoes. Crop. Prot. 38, 1–6 (2012)
A.C. Guerreiro, C.M.L. Gago, M.L. Faleiro, M.G.C. Miguel, M.D.C. Antunes, The use of polysaccharide-based edible coatings enriched with essential oils to improve shelf-life of strawberries. Postharvest. Biol. Technol. 110, 51–60 (2015)
A. Ali, N. Mohd Noh, M.A. Mustafa, Antimicrobial activity of chitosan enriched with lemongrass oil against anthracnose of bell pepper. Food Packag. Shelf Life 3, 56–61 (2015)
A. Mohammadi, M. Hashemi, S.M. Hosseini, Integration between chitosan and Zataria multiflora or Cinnamomum zeylanicum essential oil for controlling Phytophthora drechsleri, the causal agent of cucumber fruit rot. LWT-Food Sci. Technol 65, 349–356 (2016)
T. Hemalatha, T. UmaMaheswari, R. Senthil, G. Krithiga, K. Anbukkarasi, Efficacy of chitosan films with basil essential oil: perspectives in food packaging. J. Food Meas. Charact. 11, 2160–2170 (2017)
Á Perdones, L. Sanchez-Gonzalez, A. Chiralt, M. Vargas, Effect of chitosan-lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest. Biol. Technol. 70, 32–41 (2012)
Á Perdones, I. Escriche, A. Chiralt, M. Vargas, Effect of chitosan-lemon essential oil coatings on volatile profile of strawberries during storage. Food Chem. 197, 979–986 (2016)
V. Muriel-Galet, J.P. Cerisuelo, G. López-Carballo, M. Lara, R. Gavara, P. Hernández-Mu˜noz, Development of antimicrobial films for microbiological control of packaged salad. Int. J. Food Microbiol. 157, 195–201 (2012)
A.A. Kader, D. Zagory, E.L. Kerbel, C.Y. Wang, Modified atmosphere packaging of fruits and vegetables. Crit. Rev. Food Sci. 28, 1–30 (1989)
I. Leceta, S. Molinaro, P. Guerrero, J.P. Kerry, K. de la Caba, Quality attributes of map packaged ready-to-eat baby carrots by using chitosan-based coatings. Postharvest. Biol. Technol. 100, 142–150 (2015)
E. Sanchís, C. Ghidelli, C.C. Sheth, M. Mateos, L. Palou, M.B. Pérez-Gago, Integration of antimicrobial pectin-based edible coating and active modified atmosphere packaging to preserve the quality and microbial safety of fresh-cut persimmon (Diospyros kaki Thunb. cv. Rojo Brillante). J. Sci. Food Agric. 97, 252–260 (2017)
M. Mastromatteo, A. Conte, M.A. Del Nobile, Combined effect of active coating and MAP to prolong the shelf life of minimally processed kiwifruit (Actinidia deliciosa cv. Hayward). Food Res. Int. 44, 1224–1230 (2011)
P. Hernández-Munoz, E. Almenar, V.D. Valle, D. Velez, R. Gavara, Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria × ananassa) quality during refrigerated. Food Chem. 110, 428–435 (2008)
D. Wu, D.W. Sun, in Instrumental Assessment of Food Sensory Quality, ed. by D. Kilcast (Woodhead Publishing, Cambridge, 2013), pp. 165–189
N. Maftoonazad, H.S. Ramaswamy, Effect of pectin-based coating on the kinetics of quality change associated with stored avocados. J. Food Process. Preserv. 32, 621–643 (2008)
N. Djendoubi Mrad, C. Bonazzi, N. Boudhrioua, N. Kechaou, F. Courtois, Influence of sugar composition on water sorption isotherms and on glass transition in apricots. J. Food Eng. 111, 403–411 (2012)
V. Chamanara, B. Shabanpour, S. Gorgin, M. Khomeiri, An investigation on characteristics of rainbow trout coated using chitosan assisted with thyme essential oil. Int. J. Biol. Macromol. 50, 540–544 (2012)
G.I. Olivas, G.V. Barbosa-Cánovas, Edible coatings for fresh-cut fruits. Crit. Rev. Food Sci. Nutr. 45, 657–670 (2005)
C. Ghidelli, M. Mateos, C. Rojas-Argudo, M.B. Pérez-Gago, Extending the shelf life of fresh-cut eggplant with a soyprotein–cysteine based edible coating and modifiedatmosphere packaging. Postharvest. Biol. Technol. 95, 81–87 (2014)
R.M. Raybaudi-Massilia, J. Mosqueda-Melgar, O. Martín-Belloso, Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int. J. Food Microbiol. 121, 313–327 (2008)
M.A. Rojas-Graü, R.M. Raybaudi-Massilia, R. Soliva-Fortuny, R.J. Avena-Bustillos, T.H. Mc Hugh, O. Martín-Belloso, Apple puree-alginate coating as carrierof antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest. Biol. Technol. 45, 254–264 (2007)
S.P. Dawange, S.K. Dash, L.M. Bal, M.K. Panda, Quality of minimally processed carrots in perforation-mediated modified-atmosphere packaging (PM-MAP). J. Food Meas. Charact. 10, 746–754 (2016)
P.V. Mahajan, F.A.R. Oliveira, M.J. Sousa, S.C. Fonseca, L.M. Cunha, in Handbook of Food Science, Technology, and Engineering, ed. by Y.H. Hui (CRC Press, Taylor & Francis Group, Boca Raton, 2006)
J. Duan, R. Wu, B.C. Strik, Y. Zhao, Effects of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest. Biol. Technol. 59, 71–79 (2011)
M. Maqbool, A. Ali, S. Ramachandran, D.R. Smith, P.G. Alderson, Control of postharvest anthracnose of banana using a new edible composite coating. Crop. Prot. 29, 1136–1141 (2010)
Y. Zhang, M. Zhang, H. Yang, Postharvest chitosan-g-salicylic acid application alleviates chilling injury and preserves cucumber fruit quality during cold storage. Food Chem. 174, 558–563 (2015)
C.L. Xiao, L.W. Zhu, W. Luo, X.Y. Song, Y. Deng, Combined action of pure oxygen pretreatment and chitosan coating incorporated with rosemary extracts on the quality of fresh-cut pears. Food Chem. 121, 1003–1009 (2010)
M.V. Bhaskara Reddy, K. Belkacemi, R. Corcuff, F. Castaigne, J. Arul, Effect of pre-harvest chitosan sprays on post-harvest infection by Botrytis cinerea quality of strawberry fruit. Postharvest. Biol. Technol. 20, 39–51 (2000)
O.B. Sogvar, M. Koushesh Saba, A. Emamifar, Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest. Biol. Technol. 114, 29–35 (2016)
H. Arnon, Y. Zaitsev, R. Porat, E. Poverenov, Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest. Biol. Technol. 87, 21–26 (2014)
M. Manjunatha, R.K. Anurag, Effect of modified atmosphere packaging and storage conditions on quality characteristics of cucumber. J. Food Sci. Technol. 51, 3470–3475 (2014)
K.N. Waliszewski, H.D. Cortes, V.T. Pardio, M.A. Garcia, Color parameter changes in banana slices during osmotic dehydration. Dry Technol. 17, 955–960 (1999)
J. Jeong, D.J. Huber, S.A. Sargent, Delay of avocado (Persea americana) fruit by 1-methylcyclopropene and wax treatments. Postharvest. Biol. Technol. 28, 247–257 (2003)
N. Maftoonazad, H.S. Ramaswamy, Postharvest shelf-life extension of avocados using methyl cellulose-based coating. LWT-Food Sci. Technol. 38, 617–624 (2005)
P. Jitareerat, S. Paumchai, S. Kanlayanarat, S. Sangchote, Effect of chitosan on ripening, enzymatic activity, and disease development in mango (Mangifera indica) fruit. New Zeal. J. Crop. Hortic. 35, 211–218 (2007)
S.K. Wolf, Use of CO and CO2-enriched atmospheres for meat, fish, and produce. Food Technol. 34, 55–58 (1980)
G.A. Gonzalez-Aguilar, E. Valenzuela-Soto, J. Lizardi-Mendoza, F. Goycoolea, M.A. Martinez-Tellez, M.A. Villegas-Ochoa, I.N. Monroy-Garcia, J.F. Ayala-Zavala, Effect of chitosan coating in preventing deterioration and preserving the quality of fresh-cut papaya ‘Maradol’. J. Sci. Food Agric. 89, 15–23 (2009)
P.J. Chien, F. Sheu, F.H. Yang, Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J. Food Eng. 78, 225–229 (2007)
D. Lin, Y. Zhao, Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 6, 60–75 (2007)
M. Bill, D. Sivakumar, L. Korsten, A.K. Thompson, The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Prot. 64, 159–167 (2014)
H.Y. Song, W.S. Jo, N.B. Song, S.C. Min, K.B. Song, Quality change of apple slices coated with aloe vera gel during storage. J. Food Sci. 78, C817–C822 (2013)
M. Le Meste, D. Champion, G. Roudaut, G. Blond, D. Simatos, Glass transition and food technology: a critical appraisal. J. Food Sci. 67, 2444–2458 (2002)
V.R.N. Telis, P.J.A. Sobral, Glass transitions for freeze-dried and air-dried tomato. Food Res. Int. 35, 435–443 (2002)
H. Wang, S. Zhang, G. Chen, Glass transition and state diagram for fresh and freeze-dried Chinese gooseberry. J. Food Eng. 84, 307–312 (2008)
Y.H. Roos, M. Karel, Differential scanning calorimetry study of phase transitions affecting the quality of dehydrated materials. Biotechnol. Prog. 6, 159–163 (1990)
F. Khorram, A. Ramezanian, S.M.H. Hosseini, Effect of different edible coatings on postharvest quality of ‘Kinnow’ mandarin. J. Food Meas. Charact. 11, 1827–1833 (2017)
Acknowledgements
This work was financially supported by Ferdowsi University of Mashhad. We are thankful to Ferdowsi University of Mashhad and Wageningen University for providing facilities for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Rights and permissions
About this article
Cite this article
Maleki, G., Sedaghat, N., Woltering, E.J. et al. Chitosan-limonene coating in combination with modified atmosphere packaging preserve postharvest quality of cucumber during storage. Food Measure 12, 1610–1621 (2018). https://doi.org/10.1007/s11694-018-9776-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-018-9776-6