Abstract
The role of glycogen synthase kinase-3 (GSK-3) in nitric oxide (NO)-enhanced chilling tolerance in postharvest peach fruit was investigated. The fruits were immersed in sodium nitroprusside (SNP; exogenous NO donor) and bikinin (GSK-3 inhibitor). Results showed that the chilling injury (CI) index declined following the exposure of the peach fruit to exogenous SNP. SNP treatment also induced GSK-3 expression. Furthermore, SNP treatment reduced malondialdehyde (MDA) content and electrolyte leakage in the peach fruit. In addition, SNP treatment induced the increase in alternative oxidase (AOX) activity and the upregulation of the gene expression of 18.1-kDa class I heat shock protein (HSP), WRKY2, and C-repeat binding factor (CBF). The effects of SNP treatment were partly weakened by the addition of bikinin. These findings indicate that GSK-3 mediated the reduction of MDA content and electrolyte leakage and the activation of AOX, 18.1-kDa class I HSP, WRKY2, and CBF by NO, thereby inducing chilling tolerance in peach fruit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cold storage is a useful method to delay decay and to maintain the quality of postharvest fruit. However, peach (Prunus persica) is susceptible to chilling injury (CI). Classic visual symptoms of CI in peach fruit are internal browning and flesh mealiness. These symptoms limit postharvest life and reduce consumer acceptability (Lurie and Crisosto 2005). Consequently, understanding of the mechanisms of mitigation of CI by potential technologies in fruit is imperative.
Many technologies have been employed to reduce CI in fruit. Heat treatment has been shown to modulate soluble sugar metabolism and induce chilling tolerance in loquat fruit (Shao et al. 2013). Brassinolide (Aghdam and Mohammadkhani 2014), methyl jasmonate (MeJA) (Jin et al. 2014), and gibberellic acid (GA3) (Zhu et al. 2016) applications have been found to effectively enhance chilling tolerance in tomato fruit. A previous study by our group verified that application of an exogenous nitric oxide (NO) donor, sodium nitroprusside (SNP), reduced the levels of lipoxygenase and phospholipase D and activated antioxidant enzymes and small ubiquitin-like modifier (SUMO) in peach fruit, thereby reducing the CI index (Jiao et al. 2019). The mitigation of CI in fruit requires the involvement of endogenous signal compounds (Jiao et al. 2019). It is of great importance to explore the possible downstream signal molecules that mediate NO-mitigated CI in peach fruit. Glycogen synthase kinase-3 (GSK-3), a serine/threonine kinase, has been shown to be involved in many physiological responses in plants under exogenous stimuli, such as wound (Jonak et al. 2000) and salt stress (Chen et al. 2003). Especially, another previous work from our group has reported that GSK-3 mediated NO-induced isoflavone synthesis in soybean sprouts after UV-B treatment (Jiao et al. 2017). However, there are no reports concerning the mediation of NO-alleviated CI by GSK-3 in peach fruit.
Membrane damage in fruit caused by low temperatures leads to elevated malondialdehyde (MDA) contents and electrolyte leakage, which are qualitative indexes of CI (Dong et al. 2012). It has been reported that NO can reduce MDA content and electrolyte leakage and thereby alleviate membrane damage in loquat fruit (Xua et al. 2012) and mango fruit (Ruan et al. 2015). Furthermore, GSK-3 activation and declines of MDA content and electrolyte leakage are the results of defense responses. However, no reports concern the GSK-3-mediated decreases in MDA content and electrolyte leakage under NO treatment in peach fruit.
Alternative oxidase (AOX), a non-energy-conserving terminal oxidase in the plant mitochondrial electron transport chain, plays important roles in metabolic and signaling homeostasis under stressful conditions. It has been shown to be involved in the alleviation of CI induced by methyl jasmonate (MeJA) and methyl salicylate (MeSA) treatments in tomato and sweet pepper (Wang et al. 2005). Additionally, AOX activity was enhanced by NO in tobacco plants (Luisa et al. 2006). No reports are available about the enhancement of AOX by NO in peach fruit. Furthermore, the mechanism by which GSK-3 mediates AOX activation under NO treatment remains to be revealed.
Heat shock proteins (HSPs), a group of conserved proteins, play central roles in maintaining cellular homeostasis (Carranco et al. 1997). Salicylic acid (Wang et al. 2006) and MeJA and MeSA (Ding et al. 2001) applications induce HSPs, thereby mitigating CI in peach and tomato fruit, respectively. No reports are about the induction of HSP by NO in peach fruit. Furthermore, the mediation of HSP activation by GSK-3 under NO treatment in peach fruit remains to be explored.
Plant-specific WRKYs, comprising a family of transcription factors, have been shown to play important roles in various stress responses. WRKY has been shown to be involved in the enhancement of chilling tolerance due to abscisic acid (ABA) treatment in banana fruit (Luo et al. 2017) and to be upregulated by SNP treatment in Larix olgensis Henry (Hu et al. 2015). However, no reports have been conducted on the induction of WRKY by NO and its role in CI alleviation in peach fruit. Serine/threonine kinases, including GSK-3 and mitogen-activated protein kinase (MAPK), have been confirmed to be involved in some biological processes. A WRKY transcription factor was reported to be phosphorylated by MAPK in Arabidopsis (Mao et al. 2011). No reports are available regarding the stimulation of WRKY by GSK-3 in response to NO treatment in peach fruit. Additionally, C-repeat binding factors (CBFs), as another transcription factor, have been verified to play important roles in plant response to cold stress (Oakenfull et al. 2013). Gibberellic acid treatment has been shown to induce gene expression of CBF, a vital regulator in the cold response in tomato fruit (Zhu et al. 2016), and endogenous NO production under cold stress was found to modulate CBF in tomato via nitrosylation (Sehrawat et al. 2013). However, no reports are about CBF induction by GSK-3 in response to NO treatment in peach fruit.
Based on the above observations, we hypothesized that GSK-3 can mediate NO-alleviated CI in peach fruit. The aims of the present study were to investigate the modulation of the CI index, the reductions of MDA content and electrolyte leakage, and the activation of AOX, HSP, WRKY, and CBF by GSK-3 in peach fruit under NO treatment and to reveal the underlying mechanisms.
Materials and Methods
Fruit Material and Postharvest Treatments
Peach fruit (Prunus persica Batsch ‘Jinqiuhongmi’) were harvested at commercial maturity at a local orchard in Beijing, China. Peaches of uniform size and maturity with absence of mechanical damage were chosen and randomly divided into three groups:
(1) Control group (CK): The fruits were immersed in sterile deionized water.
(2) SNP (NO donor): The fruits were immersed in 15 μmol L−1 SNP.
(3) SNP+bikinin (GSK-3 inhibitor): The fruits were immersed in 15 μmol L−1 SNP plus 10 μmol L−1 bikinin.
The fruits in each group were treated for approximately 10 min and then air-dried for 40 min at room temperature. Subsequently, the fruits in all three groups were stored for 28 days at 4 °C and 80% relative humidity. During the 28 days of storage, twenty fruits from each group were collected at 7-day intervals for assays. Each assay was repeated three times. The samples were stored at − 80 °C for determination.
CI Index Detection
The CI index was calculated according to internal browning symptoms. The severity of browning was assessed in a four-stage scale: 0 = no damage, 1 = superficial damage (< 5% damage), 2 = moderate damage (6–25% damage), 3 = severe damage (26–50% damage), and 4 = very severe damage (> 50% damage). The CI index was obtained using the following formula: CI index =Σ [(CI scale) × (number of fruit at that CI)] / [5 × (total number of fruit in each sample)] × 100%.
MDA Content Determination
A total of 1.0 g of peach fruit was ground with 5 mL of 10% trichloroacetic acid and then centrifuged at 12,000×g for 20 min at 4 °C. MDA content was determined using the thiobarbituric acid (TBA) method according to Ding et al. (2016).
Electrolyte Leakage Detection
Thirty disks (5 mm in diameter) were obtained using a cork borer from the skin tissue of 10 fruits. The disks were immersed in distilled water for 10 min and then dried on filter paper. Then, the disks were immersed in 0.3 mol L−1 mannitol for 3 h in a 50-mL plastic centrifuge tube. Electrolyte leakage was determined according to Ding et al. (2015).
AOX Activity Determination
A total of 0.5 g of peach fruit was ground in 10 mL of 10 mmol L−1 phosphate buffer (pH 7.2) at 4 °C. The peach samples were centrifuged at 10,000g at 4 °C for 15 min. Then, AOX activity was assayed using an assay kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).
Gene Expression
Total RNA from each peach sample was collected using an E.Z.N.A.™ Plant RNA Kit (Omega, Norcross, GA, USA; R6827-01) according to the manufacturer’s protocol. Subsequently, synthesis of first-strand cDNA was carried out as described by Zhu et al. (2016). The primers used for quantitative real-time PCR (QRT-PCR) determination are listed in Table 1. The QRT-PCR cycling conditions were as follows: 1 cycle of 95 °C for 1 min, followed by 40 cycles of 95 °C for 15 s, and then 63 °C for 25 s. Each analysis was performed with 3 independent biological replicate experiments. For each experiment, 3 technical replicates were carried out.
Statistical Analyses
All the data were expressed as the mean ± standard deviation (SD). SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) was used to test for significant differences using the ANOVA procedure and Duncan’s test.
Results
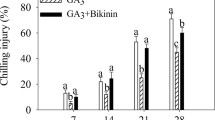
Effects of Exogenous SNP and Bikinin on the CI Index in Peach Fruit
The peach fruit developed CI symptoms after 7 days of cold storage. The CI index gradually increased over the storage period. The development of CI symptoms was delayed in peach fruit treated with exogenous SNP. After 28 days of cold storage, the CI index in peach fruit subjected to SNP treatment was 43%, which represented a 38% decrease relative to the value in the control fruit. More interestingly, the reduction of the CI index by SNP treatment in peach fruit was weakened by bikinin (GSK-3 inhibitor) treatment. After 28 days of storage, the CI index in peach fruit under SNP-plus-bikinin treatment was 55%, representing a 29% increase and 20% decrease relative to the value observed under SNP treatment and control treatment, respectively (Fig. 1).
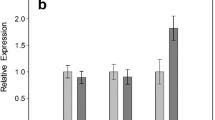
Effects of Exogenous SNP and Bikinin on Gene Expression of GSK-3 in Peach Fruit
During cold storage, the gene expression of GSK-3 first increased and then decreased under control and exogenous SNP treatment. Exogenous SNP induced the expression of GSK-3. GSK-3 expression under SNP treatment reached a maximum (4.2) at 14 days, with a level 1.8 times the control level. After 28 days of cold storage, GSK-3 expression after SNP treatment was 3.1, which was 1.4 times the control level. Interestingly, the induction of GSK-3 expression by SNP treatment was blocked by bikinin. After 28 days of storage, there was no significant difference in GSK-3 expression between the SNP treatment and the SNP-plus-bikinin treatments (Fig. 2).
GSK-3-Mediated Prevention of the Increase in MDA Content and Electrolyte Leakage Under SNP Treatment
Over the course of cold storage, MDA content and electrolyte leakage in peach fruit increased continuously under the control and exogenous SNP treatments. The increase in MDA content and electrolyte leakage was prevented by exogenous SNP. After 28 days of cold storage, MDA content and electrolyte leakage were 3.40 mmol/kg FW and 42% under SNP treatment, representing decreases of 35% and 33%, respectively, relative to the control values. Interestingly, bikinin weakened the declines of MDA content and electrolyte leakage induced by SNP treatment. After 28 days of storage, MDA content and electrolyte leakage under SNP-plus-bikinin treatment were 4.21 mmol/kg FW and 52%, representing increases of 23% and 24%, respectively, relative to that under SNP treatment and decreases of 19% and 17%, respectively, relative to the control values (Fig. 3).
GSK-3 Mediation of AOX Activity Under SNP Treatment
Over the course of cold storage, AOX activity first increased and then decreased under the control and exogenous SNP treatments. Exogenous SNP enhanced AOX activity. AOX activity under SNP treatment reached a maximum (60 U/mg FW) at 14 days, with a value 1.7 times the control value. After 28 days of cold storage, AOX activity under SNP treatment was 43 U/mg FW, 1.5 times the control value. Interestingly, the activation of AOX by SNP treatment was blocked by bikinin. After 28 days of storage, AOX activity under the SNP-plus-bikinin treatment was 32 U/mg FW, representing a 26% decrease relative to the activity under SNP treatment, and there was no significant difference in AOX activity between the control and SNP-plus-bikinin treatments (Fig. 4).
GSK-3-Mediated Induction of Gene Expression of 18.1-kDa class I HSP, WRKY2, and CBF Under SNP Treatment
Over the course of cold storage, the gene expression of 18.1-kDa class I HSP, WRKY2, and CBF in peach fruit first increased and then decreased under the control and exogenous SNP treatments. Exogenous SNP treatment enhanced the gene expression of 18.1-kDa class I HSP, WRKY2, and CBF. The gene expression of 18.1-kDa class I HSP, WRKY2, and CBF under SNP treatment reached maximum values (5, 6.9, and 4.9) at 14 days, 14 days, and 21 days, respectively, with values 1.4, 1.4, and 2.9 times the control values, respectively. After 28 days of storage, there was no significant difference in 18.1-kDa class I HSP expression between the control and SNP treatments, and WRKY2 and CBF expression under SNP treatment was 5.9 and 4.4, respectively, with values 2.0 and 3.7 times the corresponding control levels. More importantly, during storage, bikinin inhibited the gene expression of 18.1-kDa class I HSP, WRKY2, and CBF under SNP treatment. After 28 days of storage, there were no significant differences in 18.1-kDa class I HSP and CBF expression between the SNP and SNP-plus-bikinin treatments, and WRKY2 expression under SNP-plus-bikinin treatment was 4.6, representing a 22% decrease relative to the level under SNP treatment (Fig. 5).
Discussion
Our work showed that during cold storage, SNP treatment significantly reduced the CI index (Fig. 1) in peach fruit, demonstrating that SNP application has potential as a method to mitigate CI in peach fruit.
The elevations of MDA content and electrolyte leakage due to peroxidation and breakdown of unsaturated fatty acids reflect the severity of membrane damage (Ruan et al. 2015). In the present study, SNP treatment effectively prevents both the increase in MDA content and electrolyte leakage in cold-stored peach fruit (Fig. 3). This, in turn, suggests that NO may reduce chilling-induced membrane damages. More interestingly, the mitigation of membrane damage by NO was mediated by GSK-3 (Fig. 3). SNP treatment, as an external signal, did not directly induce defense responses but activated endogenous signaling molecules such as GSK-3 (Fig. 2). Subsequently, the stimulation of the signal compounds modulated the downstream physiological responses (Jian et al. 2005).
Furthermore, AOX activity was increased by SNP treatment (Fig. 4). AOX has been shown to regulate some vital mitochondrial signaling compounds, including superoxide, nitric oxide, and important redox couples (Vanlerberghe 2013). Excess ROS-caused oxidative damage is one of the early responses to CI in fruit (Yao et al. 2018). Apart from the antioxidant enzyme we previously reported (Jiao et al. 2019), AOX also acts as the defense line to scavenge ROS. It has been reported that AOX downregulated superoxide levels, thereby reducing reactive oxygen species (ROS) generation in tobacco leaf (Cvetkovska and Vanlerberghe 2012). Therefore, AOX might enhance chilling tolerance by scavenging ROS. Furthermore, GSK-3 mediated the activation of AOX due to SNP treatment in peach fruit (Fig. 4). Accordingly, AtCBL1 and AtCP1, members of the Ca2+ sensor family called the AtCBL family, can be activated upon the overexpression of AtGSK1 in transgenic Arabidopsis. AtCBL1 is considered to be a Ca2+ sensor involved in stress signal transmission (Kudla et al. 1999), and AtCP1 was identified as a small Ca2+-binding protein with EF hands (Jang et al. 1998). Both of these proteins interact with CBL-interacting protein kinases (CIPKs) (Shi et al. 1999). Hence, GSK-3 acts as an effective mediator of Ca2+ mobilization in plant. Moreover, it has been shown that EGTA (a calcium chelator) weakens the alternative respiratory pathway in chilling-stressed Arabidopsis callus (Leo 2012), indicating that Ca2+ is essential to the induction of the alternative respiratory pathway during cold storage. Thus, Ca2+ might mediate GSK-3-induced AOX activation in peach fruit.
HSP has been identified as another biochemical marker of CI degree in fruit. SNP treatment induced gene expression of 18.1-kDa class I HSP in peach fruit (Fig. 5A). HSP is a member of a group of proteins involved in the responses to stressful conditions that act to protect the plant against damage. Increased HSP expression has been found to be correlated with the enhancement of chilling tolerance (Ding et al. 2001). Moreover, our work suggested that GSK-3 mediated the activation of 18.1-kDa class I HSP stimulated by SNP treatment in peach fruit (Fig. 5A). Accordingly, GSK-3 has been shown to phosphorylate heat shock factor 1 at Ser(303), thereby modulating heat shock factor 1 and HSP expression in mice (Zahra et al. 2010). Therefore, we speculate that NO application induces HSP expression by regulating the phosphorylation of heat shock proteins at some amino acid sites.
Some transcription factors are activated as part of the defense responses to stressful conditions in plants (Cheng et al. 2017). The gene expression of WRKY2 was enhanced by SNP treatment in peach fruit (Fig. 5B). Interestingly, GSK-3 mediated the activation of WRKY2 triggered by SNP treatment in peach fruit (Fig. 5B). It has been reported that the increases in Ca2+ influxes due to injury induce the phosphorylation of WRKY in Arabidopsis thaliana (Yan et al. 2018). Thus, the chilling tolerance enhancement by WRKY possibly involves the activation of Ca2+. Additionally, WRKYs have been shown to regulate a plant immune NADPH oxidase in Nicotiana benthamiana (Adachi et al. 2015). Furthermore, the activities of superoxide dismutase (SOD) and peroxidase (POD), the contents of proline and MDA, and electrolyte leakage have also been shown to be regulated by WRKYs (Yang et al. 2017). Hence, WRKY may alleviate CI through modulating the antioxidant system and alleviating membrane damage. The gene expression of another transcription factor, CBF, was also increased by SNP treatment in peach fruit (Fig. 5C). Accordingly, previous work found that tomato plants overexpressing Arabidopsis CBF1 exhibited greater chilling tolerance than wild-type plants (Hsieh et al. 2004), suggesting that CBF is a key regulator of the cold response. Our work showed that NO could effectively upregulate CBF expression during long-term cold storage in peach, a cold-sensitive fruit. More importantly, our results demonstrated that GSK-3 mediated the enhancement of CBF expression due to SNP treatment in peach fruit (Fig. 5C).
Conclusions
Exogenous SNP treatment protected peach fruit against CI and activated GSK-3. The elevated chilling tolerance in SNP-treated peaches may be due to the decreases in MDA content and electrolyte leakage, resulting in the alleviation of membrane damage. Additionally, the activity of AOX and the gene expression of 18.1-kDa class I HSP, WRKY2, and CBF increased in response to SNP treatment; such increases might activate a positive feedback mechanism and induce chilling tolerance in SNP-treated peaches. However, bikinin, a GSK-3 inhibitor, weakened the SNP-induced effects described above. Overall, GSK-3 mediated the induction of chilling tolerance through reductions in MDA content and electrolyte leakage, activation of AOX, and increases in 18.1-kDa class I HSP, WRKY2, and CBF expression under SNP treatment in postharvest peach fruit. The findings of the current study provide a foundation for in-depth studies of the functional roles of the NO signaling network in fruit during cold storage.
Abbreviations
- GSK-3:
-
Glycogen synthase kinase-3
- NO:
-
Nitric oxide
- SNP:
-
Sodium nitroprusside
- CI:
-
Chilling injury
- MDA:
-
Malondialdehyde
- AOX:
-
Alternative oxidase
- HSP:
-
Heat shock protein
- CBF:
-
C-Repeat binding factor
References
Adachi, H., Nakano, T., Miyagawa, N., Ishihama, N., Yoshioka, M., Katou, Y., Yaeno, T., Shirasu, K., & Yoshioka, H. (2015). WRKY transcription factors phosphorylated by MAPK regulate a plant immune NADPH oxidase in Nicotiana benthamiana. Plant Cell, 27(9), 2645–2663.
Aghdam, M. S., & Mohammadkhani, N. (2014). Enhancement of chilling stress tolerance of tomato fruit by postharvest brassinolide treatment. Food and Bioprocess Technology, 7(3), 909–914.
Carranco, R., Almoguera, C., & Jordano, J. (1997). A plant small heat shock protein gene expressed during zygotic embryogenesis but noninducible by heat stress. Journal of Biological Chemistry, 272(43), 27470–27475.
Chen, G. P., Ma, W. S., Huang, Z. J., Xu, T., Xue, Y. B., & Shen, Y. Z. (2003). Isolation and characterization of TaGSK1 involved in wheat salt tolerance. Plant Science, 165(6), 1369–1375.
Cheng, M., Huang, Z., Hua, Q., Shan, W., Kuang, J., Lu, W., et al. (2017). The WRKY transcription factor HpWRKY44 regulates CytP450-like1 expression in red pitaya fruit (Hylocereus polyrhizus). Horticulture Research, 4, 17039.
Cvetkovska, M., & Vanlerberghe, G. C. (2012). Alternative oxidase modulates leaf mitochondrial concentrations of superoxide and nitric oxide. New Phytologist, 195(1), 32–39.
Ding, C., Wang, C., Gross, K., & Smith, D. (2001). Reduction of chilling injury and transcript accumulation of heat shock proteins in tomato fruit by methyl jasmonate and methyl salicylate. Plant Science, 161(6), 1153–1159.
Ding, Y., Sheng, J., Li, S., Nie, Y., Zhao, J., Zhu, Z., et al. (2015). The role of gibberellins in the mitigation of chilling injury in cherry tomato (Solanum lycopersicum L.) fruit. Postharvest Biology and Technology, 101(101), 88–95.
Ding, Y., Zhu, Z., Zhao, J., Nie, Y., Yu, Z., Sheng, J., et al. (2016). Effects of postharvest brassinolide treatment on the metabolism of white button mushroom (Agaricus bisporus) in relation to development of browning during storage. Food and Bioprocess Technology, 9(8), 1327–1334.
Dong, J., Qin, Y., Li, L., & Xua, M. (2012). Effect of yeast saccharide treatment on nitric oxide accumulation and chilling injury in cucumber fruit during cold storage. Postharvest Biology and Technology, 68(2), 1–7.
Hsieh, T., Lee, J., Yang, P., Chiu, L., Charng, Y., Wang, Y., et al. (2004). Heterology expression of the Arabidopsis C-repeat/dehydration response element binding factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiology, 135(2), 1145–1145.
Hu, X., Yang, J., & Li, C. (2015). Transcriptomic response to nitric oxide treatment in Larix olgensis Henry. International Journal of Molecular Sciences, 16(12), 28582–28597.
Jang, H. J., Pih, K. T., Kang, S. G., Lim, J. H., Jin, J. B., Hai, L. P., et al. (1998). Molecular cloning of a novel Ca2+-binding protein that is induced by NaCl stress. Plant Molecular Biology, 37(5), 839–847.
Jian, Z., Davis, L. C., & Verpoorte, R. (2005). Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnology Advances, 23(4), 283–333.
Jiao, C., Zhu, L., & Gu, Z. (2017). GSK-3 mediates NO-cGMP-induced isoflavone production in soybean sprouts. Food Research International, 101, 203–208.
Jiao, C., Chai, Y., & Duan, Y. (2019). Inositol 1, 4, 5-trisphosphate mediates nitric-oxide-induced chilling tolerance and defense response in postharvest peach fruit. Journal of Agricultural and Food Chemistry, 67(17), 4764–4773.
Jin, P., Duan, Y., Wang, L., Wang, J., & Zheng, Y. (2014). Reducing chilling injury of loquat fruit by combined treatment with hot air and methyl jasmonate. Food and Bioprocess Technology, 7, 2259–2266.
Jonak, C., Beisteiner, D., Beyerly, J., & Hirt, H. (2000). Wound-induced expression and activation of WIG, a novel glycogen synthase kinase 3. Plant Cell, 12(8), 1467–1476.
Kudla, J., Xu, Q., Harter, K., Gruissem, W., & Luan, S. (1999). Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proceedings of the National Academy of Sciences of the United States of America, 96(8), 4718–4723.
Leo, A. D. (2012). Involvement of hydrogen peroxide, calcium, and ethylene in the induction of the alternative pathway in chilling-stressed Arabidopsis callus. Planta, 235(1), 53–67.
Luisa, E., Roberta, M., Andrea, B., Claus, W., Otto, M., Lara, R., et al. (2006). Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants. Plant Physiology, 142(2), 595–608.
Luo, D. L., Ba, L. J., Shan, W., Kuang, J. F., Lu, W. J., & Chen, J. Y. (2017). Involvement of WRKY transcription factors in ABA-induced cold tolerance of banana fruit. Journal of Agricultural and Food Chemistry, 65(18), 3627–3635.
Lurie, S., & Crisosto, C. H. (2005). Chilling injury in peach and nectarine. Postharvest Biology and Technology, 37(3), 195–208.
Mao, G., Meng, X., Liu, Y., Zheng, Z., Chen, Z., & Zhang, S. (2011). Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell, 23(4), 1639–1653.
Oakenfull, R. J., Robert, B., & Knight, M. R. (2013). A C-repeat binding factor transcriptional activator (CBF/DREB1) from European bilberry (Vaccinium myrtillus) induces freezing tolerance when expressed in Arabidopsis thaliana. PLoS One, 8(1), e54119.
Ruan, J., Li, M., Jin, H., Sun, L., Zhu, Y., Xu, M., & Dong, J. (2015). UV-B irradiation alleviates the deterioration of cold-stored mangoes by enhancing endogenous nitric oxide levels. Food Chemistry, 169, 417–423.
Sehrawat, A., Gupta, R., & Deswal, R. (2013). Nitric oxide-cold stress signalling cross-talk, evolution of a novel regulatory mechanism. Proteomics, 13(12-13), 1816–1835.
Shao, X., Zhu, Y., Cao, S., Wang, H., & Song, Y. (2013). Soluble sugar content and metabolism as related to the heat-induced chilling tolerance of loquat fruit during cold storage. Food and Bioprocess Technology, 6(12), 3490–3498.
Shi, J., Kim, K. N., Ritz, O., Albrecht, V., Gupta, R., Harter, K., Luan, S., & Kudla, J. (1999). Novel protein kinases associated with calcineurin B-like calcium sensors in Arabidopsis. The Plant Cell, 11(12), 2393–2405.
Vanlerberghe, G. C. (2013). Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. International Journal of Molecular Sciences, 14(4), 6805–6847.
Wang, C. Y., Fung, R. W. M., & Ding, C. K. (2005). Reducing chilling injury and enhancing transcript levels of heat shock proteins, PR-proteins and alternative oxidase by methyl jasmonate and methyl salicylate in tomatoes and peppers. V International Postharvest Symposium, 6821, 481–486.
Wang, L., Chen, S., Kong, W., Li, S., & Archbold, D. (2006). Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharvest Biology and Technology, 41(3), 244–251.
Xua, M., Zhang, M., Xu, X., & Sun, L. (2012). Cold-induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage. Postharvest Biology and Technology, 65(3), 5–12.
Yan, C., Fan, M., Yang, M., Zhao, J., Zhang, W., Su, Y., Xiao, L., Deng, H., & Xie, D. (2018). Injury activates Ca2+/calmodulin-dependent phosphorylation of JAV1-JAZ8-WRKY51 complex for jasmonate biosynthesis. Molecular Cell, 70(1), 136–149.
Yang, G., Zhang, W., Liu, Z., Yi-Maer, A. Y., Zhai, M., & Xu, Z. (2017). Both JrWRKY2 and JrWRKY7 of Juglans regia mediate responses to abiotic stresses and abscisic acid through formation of homodimers and interaction. Plant Biology, 19(2), 268–278.
Yao, W., Xu, T., Farooq, S. U., Peng, J., & Zheng, Y. (2018). Glycine betaine treatment alleviates chilling injury in zucchini fruit (Cucurbita pepo L.) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biology and Technology, 144, 20–28.
Zahra, K., Hana, C., Sarah, H., Cathrine, M. K., & Zachara, N. E. (2010). O-linked β-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3β-dependent manner. Journal of Biological Chemistry, 285(50), 39096–39107.
Zhu, Z., Ding, Y., Zhao, J., Nie, Y., Yu, Z., Sheng, J., et al. (2016). Effects of postharvest gibberellic acid treatment on chilling tolerance in cold-stored tomato (Solanum lycopersicum L.) fruit. Food and Bioprocess Technology, 9(7), 1202–1209.
Funding
This project was supported by the National Natural Science Foundation of China (31871862).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• GSK-3 mediated NO-alleviated CI in peach fruit.
• GSK-3 mediated NO-reduced MDA content and electrolyte leakage.
• GSK-3 mediated NO-activated AOX.
• GSK-3 mediated NO-enhanced gene expression of 18.1 kDa classIHSP.
• GSK-3 mediated NO-enhanced gene expression of WRKY2 and CBF.
Rights and permissions
About this article
Cite this article
Jiao, C., Duan, Y. The Mediation of NO-Enhanced Chilling Tolerance by GSK-3 in Postharvest Peach Fruit. Food Bioprocess Technol 12, 2028–2035 (2019). https://doi.org/10.1007/s11947-019-02367-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-019-02367-y