Abstract
Non-edible seeds are potential candidates for the production of biofuels and other value added products. The specific objective of this work is to measure the dielectric properties (dielectric constant, loss factor, loss tangent and penetration depth) of karanja seed. The data on dielectric properties of karanja seed will be helpful to understand its microwave absorbing capacity and heating behaviour in the microwave pyrolysis process. In this work, the dielectric properties of karanja seed are determined at ambient temperature and in the frequency range 0.1–3.0 GHz. The loss tangent values of karanja seed obtained at the two recognized commercial frequencies of 915 MHz and 2.45 GHz are compared with those of some biomass available in the literature. Finally, the karanja seed is pyrolyzed in a domestic microwave oven at four different power inputs of 500 W, 600 W, 700 W and 800 W. The microwave pyrolysis characteristics of karanja seed is determined in terms of temperature variation inside the reactor and pyrolysis products yields at different power inputs. The chemical composition and fuel properties of the produced bio-oil are measured. The loss tangent of karanja seed at 2.45 GHz is 1.3, which is higher than lignocellulosic biomass like oil palm fibre (0.08). The results of the dielectric properties of the present work shows that karanja seed has better microwave absorption characteristics compared to lignocellulosic biomass. In the actual pyrolysis process in a domestic microwave oven at different power inputs, the heating rate is found to range between 0.661 and 1.157°C/s which is considerably higher than that achieved using electrical heating (0.16–0.33°C/s). The bio-oil yield increases as the power input is increased from 500 to 700 W. The maximum bio-oil yield of 47% is obtained at 700 W. The GC-MS analysis of bio-oil has shown the presence of larger amounts of hydrocarbons, esters, alcohols, very little amount of aromatics and zero sulphur-containing compounds. The fuel properties of bio-oil show that it is a non-acidic and safer fuel. The calorific value of bio-oil is 40% lower than diesel, and its viscosity is 8.5 times higher than diesel. The results reveal that the karanja seed could be pyrolyzed by microwave heating to produce bio-oil which can be used as fuel for diesel engines by suitably upgrading it by blending with diesel fuel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the recent decades, the concern on converting biomass resources into biofuels, bio-energy and other bio-based products has increased due to their renewability, carbon neutrality and availability. Pyrolysis is one of the biomass energy conversion technologies adopted worldwide. In particular, the microwave pyrolysis technique has received significant attention due its number of benefits like (i) efficient heating characteristics (non-contact, volumetric, faster and material selective heating), (ii) operating characteristics (quick start-up and stopping, safety and automation of the equipment) and (iii) improved products’ characteristics [1, 2]. Non-contact heating aids in uniform heat distribution among molecules. Higher heating rate helps to achieve a rapid temperature rise of the feedstock and quick release of volatiles. Selective heating causes zero energy waste. Microwave pyrolysis produces clean, crack-free char with larger specific surface area [3, 4]. It produces liquid product with greater energy value owing to its higher carbon and lower oxygen contents [5,6,7,8]. Microwave pyrolysis also seems to be a favourable method for the production of gas that is rich in H2 and CO [6, 9].
Several researchers have reported the microwave pyrolysis of biomass: larch wood blocks [3], pine wood sawdust [10], corn cob and rice straw [11, 12], cellulose [13], wood sawdust and corn stover [11, 12, 14, 15], algae [16], peanut shell, pine sawdust [17, 18], palm kernel shell [19], Prosopis juliflora [20], oil palm fibre [21], rice husk, sugarcane bagasse, sugarcane peel, coffee grounds, bamboo [11], soapnut seed [22] and tamarind seed [23]. Solar energy is utilized to carry out microwave pyrolysis of rice straw [24] and corn stover [25].
The ability of feedstock to absorb microwave energy and convert it into heat energy depends on its dielectric properties (dielectric constant, loss factor, loss tangent and penetration depth). The microwave frequency and temperature largely affect the dielectric properties [26, 27]. Therefore, it is essential to know the dielectric properties of the biomass feedstock before using it for microwave pyrolysis. The dielectric properties of lignocellulosic biomass including wood and woody biomass, herbaceous biomass and agricultural residues (oil palm residues) are presented in the literature. Most of the lignocellulosic biomass show inferior dielectric properties. In the literature, the change in dielectric properties during actual pyrolysis process is available for the two commercially used microwave frequencies of 915 MHz and 2.45 GHz for switch grass [2], hay [27], corn stover [28], oat and barley straw [29] and oil palm shell [30]. Some authors have reported the variation of dielectric properties of empty fruit bunch [31] and oil palm [32] in the frequency range of 0.1–20 GHz at ambient temperature.
Numerous non-edible seeds are processed via chemical or thermochemical methods for deriving second-generation biofuels like biodiesel, bio-oil and biochar. For instance, karanja seed, mahua seed, neem seed and tamarind seed are subjected to transesterification reaction for the production of biodiesel [33,34,35,36]. These seeds are also considered as suitable feedstock for conventional pyrolysis [37,38,39,40]. In particular, karanja seed is extensively investigated for biodiesel [41] and bio-oil production [42,43,44].
Though the dielectric properties of lignocellulosic biomass are investigated widely, dielectric properties of the seeds are investigated rarely. Generally, the dielectric properties of seeds are examined for the purpose of determining their oil content, viability, moisture content, hydration and metabolic mechanisms [45,46,47,48] in the frequency range of 5 kHz–10 MHz. Fennell et al. [48] investigated the dielectric properties of the tallow tree seeds at 915 MHz and 2.45 GHz to determine its drying characteristics for biodiesel production. So, no published paper is available that reveals dielectric properties and microwave heating characteristics of seeds.
Thus, the specific objective of this work is to measure the dielectric properties (dielectric constant, loss factor, loss tangent and penetration depth) of karanja seed. The data on loss tangent and penetration depth of karanja seed will be helpful to understand its microwave absorbing capacity and hence its heating behaviour in the microwave pyrolysis process [25, 49]. In this work, the dielectric properties of karanja seed are determined at ambient temperature and in the frequency range of 0.1–3.0 GHz. The loss tangent values of karanja seed obtained at the two recognized commercial frequencies of 915 MHz and 2.45 GHz are compared with those of some biomass materials available in the literature. Finally, the karanja seed is pyrolyzed in a domestic microwave oven at four different power inputs of 500 W, 600 W, 700 W and 800 W. The microwave pyrolysis characteristics of karanja seed is determined in terms of temperature variation inside the reactor and pyrolysis products yields at different power inputs. The chemical composition and fuel properties of the produced bio-oil are measured.
2 Materials and methods
2.1 Materials
Well-matured karanja fruits are collected from the karanja trees in Puducherry, India. The seeds (Fig. 1a) are separated from the dried fruits. Feedstock particle size affects the heating rate, maximum temperature and the bio-oil yield [5], and generally smaller particle size is preferred for pyrolysis. Therefore, in this investigation, the karanja seed powder is milled and sieved to particle size of less than or equal to 2 mm (Fig. 1b).
The dielectric characterization experimentations are conducted on pelletized karanja seed powder pellets. The purpose of pelletizing is to decrease the air gap for getting accurate data [28]. The karanja seed is found to have moisture, 15%; volatile matter, 75%; ash, 3%; and fixed carbon, 7% in percentage by weight on as received basis.
2.2 Methods
2.2.1 Dielectric properties
The karanja seed is characterized for its dielectric properties using a dielectric probe kit 85070E connected with a computer-controlled E4991A impedance analyser and vector network analyser. The dielectric properties are frequency dependant and are the basic parameters that determine the microwave-absorbing characteristics of the material. The dielectric property measurements are carried out in the frequency range of 0.1–3.0 GHz and at 25 ± 1°C. The measurements are taken 3 times, and the average value is considered for the analysis. The dielectric constant, loss factor, loss tangent and penetration depth at different frequencies are determined from the measured data.
The parameter dielectric constant represents the ability of the material to store electrical energy whereas loss factor quantifies the ability of the material to absorb or dissipate electrical energy [26, 28, 29, 50]. Loss tangent is the ratio between loss factor and dielectric constant, and it gives the ability of the material to convert microwaves into heat at a specific frequency and temperature. The penetration depth is the depth into the material at which the power flux has fallen to 1/e (=0.368) of its surface value [4, 26].
2.2.2 Microwave pyrolysis: experimental setup
The microwave pyrolysis of karanja seed is carried out in a customized domestic microwave (Make: LG India). The experimental setup (Fig. 2) consists of (1) three-neck quartz round bottom reactor (250 mL capacity) that can withstand temperature of around 1000°C, (2) microwave oven (800W, 2.45 GHz, cavity size 28 × 12 × 10 cm), (3) nitrogen gas (99.99% purity) cylinder, (4) K-type thermocouple with Arduino Uno microcontroller and (5) computer, (6) Allihn condensers and (7) conical flasks. The thermocouple is placed on the surface of the biomass. The Allihn condensers possess series of bulbs that increase the surface area for effective vapour condensation.
2.2.3 Microwave pyrolysis: experimental procedure
The karanja seed powder sample (100 g) is placed in the quartz reactor. Nitrogen gas is allowed into the reactor at a constant flow rate of 100 mL/min to provide an inert atmosphere in the reactor. The seed sample is heated at the desired input power (500 W, 600 W, 700 W and 800 W). Since no temperature change is evidenced after 15 min, the microwave oven is switched off at 20 min. After the reactor attains room temperature, the bio-oil and biochar are collected and weighed. The non-condensable gas is not collected during the experiments, and hence, its amount is calculated by difference. Each experiment is conducted three times and the mean value of the results is considered.
2.2.4 Chemical composition and fuel properties of bio-oil
The chemical composition of the bio-oil is measured using an Agilent make gas chromatography-mass spectroscopy (GCMS) analyser having a HP Agilent capillary column. Helium gas is used as the carrier gas at a flow rate of 0.90 mL/min. The sample of 5 μL is injected in split mode at a split ratio of 1:25. The oven temperature is initially set at 60°C for about 1 min. Then, it is increased at a rate of 10°C/min to 320°C. The injector temperature is maintained at 250°C. For MS, the temperature of the ion source and interface is set at 200°C and 300°C, respectively. The scan range is fixed to 50–1000 m/z with a scan speed of 3333. The chemical compounds present in the bio-oil are identified by comparing their mass spectra with the WILEY data library.
Kinematic viscosity of bio-oil is measured using Redwood viscometer at 40°C (ASTM D2270), flash point using Cleveland open cup apparatus (ASTM D92) and calorific value using a bomb calorimeter (ASTM E711-87). The pH value of bio-oil is measured using pH meter (Labman Digital PH Meter, Model No.: LMPH-10) as per ASTM E70 standard.
3 Results and discussion
3.1 Dielectric properties of karanja seed
Figure 3 shows the variation of dielectric constant and loss factor of karanja seed with respect to frequency at room temperature. It is seen that the dielectric constant decreases with increase in frequency from 0.1 to 3.0 GHz. Figure 3 shows that there is a notable decrease in dielectric constant up to 1.2 GHz and thereafter remains almost constant up to 3.0 GHz. The similar tendency of decrease in dielectric constant with frequency is noted for oil palm fibre and oil palm shell [4]. The decrease in dielectric constant with frequency may be due to the (i) decrease in the dipole moment, (ii) change in orientation of molecules [25] and (iii) change in wavelength. The reduction in dielectric constant is about 63% for karanja seed, when the frequency is increased from 0.1 to 3.0 GHz. Salema et al. [4] observed 16% reduction in dielectric constant for oil palm fibre as the frequency is amplified from 0.2 to 10 GHz. Larger decrease in dielectric constant of karanja seed with increase in frequency may be due to the differences in physical and chemical composition between karanja seed and oil palm fibre. Karanja seed is a lipid-based biomass (28% oil content) whereas palm fibre is a lignocellulosic biomass (43–65% cellulose and 13–25% lignin) [51].
Figure 3 shows that there is a rapid increase in loss factor between 0.1 and 0.875 GHz. The cell walls and cell cavities of karanja seed are partially filled with moisture. The water vapour molecules and cell walls have opposite polarity. An active dipole moment is created on applying the electric field. Now the free electrons and ions displace and polarize on the cell walls [52] enabling increase in loss factor between 0.1 and 0.875 GHz. The loss factor at commercial microwave frequencies of 915 MHz and 2.45 GHz is 2.62 and 2.46, respectively.
Figure 4 shows the loss tangent of karanja seed at different frequencies. It can be observed that the loss tangent increases gradually with frequency and at 2.45 GHz it attains a maximum. Depending on the electric field, various types of polarization takes place (ionic, orientation or interfacial) in the material influencing the dielectric absorptivity of the material [53]. The higher the loss tangent, the higher will be the microwave absorptivity. Thus, higher loss tangent of karanja seed at 2.45 GHz of the microwave oven will be helpful to increase the conversion of microwave energy into heat energy.
Table 1 shows the loss tangent values of karanja seed and some lignocellulosic biomass and other biomass sources. The lignocellulosic biomass like aspen bark, pine wood, pine bark, oil-palm fibre, oil palm shell and empty fruit bunch show low values of loss tangent due to greater cellulose and lignin contents [56]. Compared to these lignocellulosic biomass, karanja seed is found to be a good microwave absorber due to its higher loss tangent value of 1.3. Higher loss tangent may be attributed to the higher lipid content of karanja seed. Zhang et al. [5] have shown that, generally, carbohydrates (tangent loss = 0.035 for cellulose) and proteins (e.g. egg white powder tangent loss = 0.068) are poor microwave absorbers whereas lipids (beef fat; tangent loss = 0.105–0.424) are good microwave absorbers.
The penetration depth at different frequencies is shown in Fig. 5. There is a rapid decrease in penetration depth from 0.1 to 0.2 GHz, and subsequently it remains constant up to 3.0 GHz. As microwave possess short wavelength (1–1 mm), it cannot penetrate deeply into the materials. Penetration may also vary with distinct parameters such as temperature, type of the material and its microstructural property [31, 32, 57]. The wavelength and penetration depth of the biomass influence the kind of polarization that takes place in the feedstock. It is observed that the penetration depth of karanja seed is 1.26 cm at 2.45 GHZ frequency, which is likely to be a good depth for microwaves to penetrate [54].
3.2 Microwave pyrolysis characteristics of karanja seed
3.2.1 Real-time temperature profiles, heating rate and maximum temperature attained
Figure 6 shows the temperature distribution inside the reactor with respect to time during the microwave pyrolysis of karanja seed at four different power inputs. The temperature profiles show two zones: Zone 1 (≈ 0–12 min) indicates a rapid increase in temperature of the feedstock with respect to reaction time. Normally, the microwave heating results in faster heating compared to electrical heating due to its distinctive heating mechanism. Zone 2 (≈12–20 min) shows that the temperature rise is nearly stable. The temperature profiles are observed to be sinusoidal in nature, which is attributed to the cyclic on/off working of the magnetron in the domestic oven [49]. Further, it is seen that as the power input is increased, the average heating rate and the maximum temperature is increased. Liu et al. [58] observed similar temperature profiles in the microwave pyrolysis of tobacco stem.
Table 2 shows the microwave pyrolysis characteristics of karanja seed. It is seen that the heating rate ranges between 0.661 and 1.157°C/s, which is considerably higher than that achieved using electrical heating (0.16–0.33°C/s) [59]. The average heating rates for the lignocellulosic biomass during microwave pyrolysis is reported as 0.85–1.16°C/s, and Table 2 shows that the heating rate of karanja seed is closer to this range. Further, Table 2 shows that the average heating rate and maximum temperature increase with increase in power input. At 500 W, the average heating rate is 0.661°C/s and maximum temperature is 354°C. As the input power is amplified to 800 W, the heating rate and the maximum temperature is increased by 75% and 56%, respectively. This finding matches with the research results of others [58].
The temperature required for the pyrolysis of karanja seed is 200–400°C [37]. Figure 6 shows that karanja seed is heated to ≈200°C within 2–6 min depending on the power input. The seed powder then attains the respective maximum temperature (Table 2) and remains almost at this maximum temperature until the end of the reaction time. These results reveal that karanja seed could be pyrolyzed at all power inputs (500–800 W) and within 20 min.
3.2.2 Product yields at different microwave power inputs
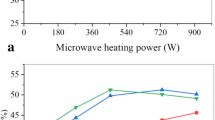
Figure 7 shows the bio-oil, biochar and non-condensable gases yields obtained by the microwave pyrolysis of karanja seed at different power inputs. It is seen that the bio-oil yield increases as the power input is increased from 500 to 700 W. The maximum bio-oil yield of 47% is obtained at 700 W. The increase in power input increases the microwave density in the cavity and hence the microwave absorption capacity of the karanja seed favouring the bio-oil yield. However, the bio-oil yield is reduced to 45% at 800 W. The extreme microwave power causes higher temperature increase (550°C) which promotes secondary pyrolysis reactions that helps in the formation of non-condensable gases and decrease in bio-oil yield [8, 15, 59, 60].
Figure 7 shows that the biochar yield increases with increase in power input. However, an opposite trend is seen for gas yield. Specifically, pyrolysis occurring at low temperature favours biochar yield whereas high temperature favours gas yield [58, 61]. The maximum biochar yield of 38% is obtained at 500 W, and maximum gas yield of 40% is obtained at 800 W.
3.3 Chemical composition and fuel properties of bio-oil
The highest bio-oil yield (47%) is obtained at 700 W, and hence, the bio-oil produced at 700 W is taken for the analysis of chemical composition and properties. Table 3 shows the results of chemical composition of bio-oil measured by GC-MS analysis. The karanja seed bio-oil is a combination of several complex organic compounds. The compounds in the bio-oil may be grouped into aromatics, alkanes, alcohols, olefins, aldehydes, ketones and acids. The GC-MS analysis of bio-oil has shown the presence of larger amounts of hydrocarbons, esters and alcohols; very little amount of aromatics; and zero sulphur-containing compounds.
Table 4 shows the fuel properties of bio-oil produced by the pyrolysis of karanja seed at 700 W. The bio-oil is dark brown colour. It has distinct smoky odour due to the presence of volatile organic compounds. The flash point of bio-oil is higher than diesel, revealing that it is a safe fuel to store and transport. The bio-oil is not acidic (pH>7) [60]. The calorific value of the bio-oil seems to be low compared to diesel fuel due to the presence of oxygen [63], and it is 40% lower than that of diesel. The viscosity of karanja seed bio-oil is 8.5 times higher than diesel. This may be due to the presence of heavier organic compounds present in the bio-oil. The chemical composition (higher hydrocarbon and ester content, lower aromatic and zero sulphur compounds) and fuel properties (non-acidic and higher flash point) reveal that the bio-oil produced by the microwave pyrolysis of karanja seed is a potential fuel for CI engines. It can be upgraded by blending with diesel fuel to reduce its viscosity and increase its energy content.
4 Conclusion
The results of the dielectric properties of karanja seed shows that it has better microwave absorption characteristics compared to lignocellulosic biomass and thus it is a potential feedstock for microwave pyrolysis. In the actual pyrolysis process in a domestic microwave oven at different power inputs, the heating rate is found to range between 0.661 and 1.157°C/s which is considerably higher than that achieved using electrical heating (0.16–0.33°C/s). The microwave pyrolysis of karanja seed produces bio-oil yield of 47% by weight of karanja seed. The chemical composition (higher hydrocarbon and ester content, lower aromatic and zero sulphur compounds) and fuel properties (non-acidic and higher flash point) reveal that the bio-oil produced by the microwave pyrolysis of karanja seed is a potential fuel for diesel engines. It can be upgraded by blending with diesel fuel to reduce its viscosity and increase its energy content
References
Huang YF, Te Chiueh P, Lo SL (2016) A review on microwave pyrolysis of lignocellulosic biomass. Sustain Environ Res 26(3):103–109. https://doi.org/10.1016/j.serj.2016.04.012
Motasemi F, Afzal MT, Salema AA, Mouris J, Hutcheon RM (2014) Microwave dielectric characterization of switchgrass for bioenergy and biofuel. Fuel 124:151–157. https://doi.org/10.1016/j.fuel.2014.01.085
Miura M, Kaga H, Sakurai A, Kakuchi T, Takahashi K (2004) Rapid pyrolysis of wood block by microwave heating. J Anal Appl Pyrolysis 71(1):187–199. https://doi.org/10.1016/S0165-2370(03)00087-1
Salema AA, Yeow YK, Ishaque K, Ani FN, Afzal MT, Hassan A (2013) Dielectric properties and microwave heating of oil palm biomass and biochar. Ind Crop Prod 50:366–374. https://doi.org/10.1016/j.indcrop.2013.08.007
Zhang Y, Chen P, Liu S, Peng P, Min M, Cheng Y, Anderson E, Zhou N, Fan L, Liu C, Chen G, Liu Y, Lei H, Li B, Ruan R (2017) Effects of feedstock characteristics on microwave-assisted pyrolysis – a review. Bioresour Technol 230:143–151. https://doi.org/10.1016/j.biortech.2017.01.046
Domínguez A, Menéndez JA, Inguanzo M, Pís JJ (2006) Production of bio-fuels by high temperature pyrolysis of sewage sludge using conventional and microwave heating. Bioresour Technol 97(10):1185–1193. https://doi.org/10.1016/j.biortech.2005.05.011
Czernik S, Bridgwater AV (2004) Overview of applications of biomass fast pyrolysis oil. Energy Fuel 18(2):590–598. https://doi.org/10.1021/ef034067u
Huang YF, Te Chiueh P, Kuan WH, Lo SL (2015) Effects of lignocellulosic composition and microwave power level on the gaseous product of microwave pyrolysis. Energy 89:974–981. https://doi.org/10.1016/j.energy.2015.06.035
Huang YF, Kuan WH, Lo SL, Lin CF (2010) Hydrogen-rich fuel gas from rice straw via microwave-induced pyrolysis. Bioresour Technol 101(6):1968–1973. https://doi.org/10.1016/j.biortech.2009.09.073
Chen MQ, Wang J, Zhang MX, Chen MG, Zhu XF, Min FF, Tan ZC (2008) Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J Anal Appl Pyrolysis 82(1):145–150. https://doi.org/10.1016/j.jaap.2008.03.001
Lo SL, Huang YF, Te Chiueh P, Kuan WH (2017) Microwave pyrolysis of lignocellulosic biomass. Energy Procedia 105:41–46. https://doi.org/10.1016/j.egypro.2017.03.277
Ravikumar C, Senthil Kumar P, Subhashni SK, Tejaswini PV, Varshini V (2017) Microwave assisted fast pyrolysis of corn cob, corn stover, saw dust and rice straw: experimental investigation on bio-oil yield and high heating values. Sustain Mater Technol 11:19–27. https://doi.org/10.1016/j.susmat.2016.12.003
Al Shra’Ah A, Helleur R (2014) Microwave pyrolysis of cellulose at low temperature. J Anal Appl Pyrolysis 105:91–99. https://doi.org/10.1016/j.jaap.2013.10.007
Borges FC, du Z, Xie Q, Trierweiler JO, Cheng Y, Wan Y, Liu Y, Zhu R, Lin X, Chen P, Ruan R (2014) Fast microwave assisted pyrolysis of biomass using microwave absorbent. Bioresour Technol 156:267–274. https://doi.org/10.1016/j.biortech.2014.01.038
Mahmoud Fodah AE, Ghosal MK, Behera D (2020) “Bio-oil and biochar from microwave-assisted catalytic pyrolysis of corn stover using sodium carbonate catalyst,” J Energy Inst, no. xxxx, https://doi.org/10.1016/j.joei.2020.09.008.
Li L, Ma X, Xu Q, Hu Z (2013) Influence of microwave power, metal oxides and metal salts on the pyrolysis of algae. Bioresour Technol 142:469–474. https://doi.org/10.1016/j.biortech.2013.05.080
Mamaeva A, Tahmasebi A, Tian L, Yu J (2016) Microwave-assisted catalytic pyrolysis of lignocellulosic biomass for production of phenolic-rich bio-oil. Bioresour Technol 211:382–389. https://doi.org/10.1016/j.biortech.2016.03.120
Mohamed BA, Kim CS, Ellis N, Bi X (2016) Microwave-assisted catalytic pyrolysis of switchgrass for improving bio-oil and biochar properties. Bioresour Technol 201:121–132. https://doi.org/10.1016/j.biortech.2015.10.096
Omoriyekomwan JE, Tahmasebi A, Yu J (2016) Production of phenol-rich bio-oil during catalytic fixed-bed and microwave pyrolysis of palm kernel shell. Bioresour Technol 207:188–196. https://doi.org/10.1016/j.biortech.2016.02.002
Suriapparao DV, Pradeep N, Vinu R (2015) Bio-oil production from Prosopis juliflora via microwave pyrolysis. Energy Fuel 29(4):2571–2581. https://doi.org/10.1021/acs.energyfuels.5b00357
Hossain MA, Jewaratnam J, Ganesan P, Sahu JN, Ramesh S, Poh SC (2016) Microwave pyrolysis of oil palm fiber (OPF) for hydrogen production: Parametric investigation. Energy Convers Manag 115:232–243. https://doi.org/10.1016/j.enconman.2016.02.058
Mathiarasu A, Pugazhvadivu M (2019) Production of bio-oil from soapnut seed by microwave pyrolysis. IOP Conf Ser Earth Environ Sci 312:012022. https://doi.org/10.1088/1755-1315/312/1/012022
Mathiarasu A, Pugazhvadivu M (2020) “Studies on microwave pyrolysis of tamarind seed,” AIP Conf Proc, vol. 2225, no. March, https://doi.org/10.1063/5.0005644.
Fodah AEM, Ghosal MK, Behera D (2020) Studies on microwave-assisted pyrolysis of rice straw using solar photovoltaic power. Bioenergy Res:25–30. https://doi.org/10.1007/s12155-020-10172-1
Torgovnikov GI (2015) Dielectric Properties of Wood and Wood-Based Materials, vol. 1
Meredith R (1998) “Engineers’ handbook of industrial microwave heating,” Engineers’ Handbook of Industrial Microwave Heating, https://doi.org/10.1049/pbpo025e.
Motasemi F, Afzal MT, Salema AA (2014) Microwave dielectric characterization of hay during pyrolysis. Ind Crop Prod 61:492–498. https://doi.org/10.1016/j.indcrop.2014.07.046
Motasemi F, Salema AA, Afzal MT (2015) Dielectric characterization of corn stover for microwave processing technology. Fuel Process Technol 131:370–375. https://doi.org/10.1016/j.fuproc.2014.12.006
Tripathi M, Sahu JN, Ganesan P, Dey TK (2015) “Effect of temperature on dielectric properties and penetration depth of oil palm shell (OPS) and OPS char synthesized by microwave pyrolysis of OPS,” Fuel, vol. 153, no. March, pp. 257–266, https://doi.org/10.1016/j.fuel.2015.02.118.
Motasemi F, Salema AA, Afzal MT (2015) Microwave dielectric properties of agricultural biomass at high temperature in an inert environment. Trans ASABE 58(3):869–877. https://doi.org/10.13031/trans.58.11006
Omar R, Idris A, Yunus R, Khalid K, Aida Isma MI (2011) Characterization of empty fruit bunch for microwave-assisted pyrolysis. Fuel 90(4):1536–1544. https://doi.org/10.1016/j.fuel.2011.01.023
Salema AA, Ani FN (2012) Microwave-assisted pyrolysis of oil palm shell biomass using an overhead stirrer. J Anal Appl Pyrolysis 96:162–172. https://doi.org/10.1016/j.jaap.2012.03.018
Ghadge SV, Raheman H (2005) Biodiesel production from mahua (Madhuca indica) oil having high free fatty acids. Biomass Bioenergy 28(6):601–605. https://doi.org/10.1016/j.biombioe.2004.11.009
Sharma YC, Singh B (2008) Development of biodiesel from karanja, a tree found in rural India. Fuel 87(8–9):1740–1742. https://doi.org/10.1016/j.fuel.2007.08.001
Sivalakshmi S, Balusamy T (2013) “Effect of biodiesel and its blends with diethyl ether on the combustion , performance and emissions from a diesel engine,” vol. 106, pp. 106–110, https://doi.org/10.1016/j.fuel.2012.12.033.
Alagu RM, Ganapathy Sundaram E (2018) Preparation and characterization of pyrolytic oil through pyrolysis of neem seed and study of performance, combustion and emission characteristics in CI engine. J Energy Inst 91(1):100–109. https://doi.org/10.1016/j.joei.2016.10.003
Nayan NK, Kumar S, Singh RK (2012) Characterization of the liquid product obtained by pyrolysis of karanja seed. Bioresour Technol 124:186–189. https://doi.org/10.1016/j.biortech.2012.08.004
Shadangi KP, Mohanty K (2014) “Comparison of yield and fuel properties of thermal and catalytic Mahua seed pyrolytic oil,” Fuel, vol. 117, no. PART A, pp. 372–380, https://doi.org/10.1016/j.fuel.2013.09.001.
Kader MA, Islam MR, Parveen M, Haniu H, Takai K (2013) Pyrolysis decomposition of tamarind seed for alternative fuel. Bioresour Technol 149:1–7. https://doi.org/10.1016/j.biortech.2013.09.032
Prasad Shadangi K, Mohanty K (2013) “Characterization of nonconventional oil containing seeds towards the production of bio-fuel,” J Renew Sustain Energy vol. 5, no. 3, https://doi.org/10.1063/1.4808029.
Nabi MN, Hoque SMN, Akhter MS (2009) Karanja (Pongamia pinnata) biodiesel production in Bangladesh, characterization of karanja biodiesel and its effect on diesel emissions. Fuel Process Technol 90(9):1080–1086. https://doi.org/10.1016/j.fuproc.2009.04.014
Shadangi KP, Mohanty K (2014) Kinetic study and thermal analysis of the pyrolysis of non-edible oilseed powders by thermogravimetric and differential scanning calorimetric analysis. Renew Energy 63:337–344. https://doi.org/10.1016/j.renene.2013.09.039
Shadangi KP, Mohanty K (2014) “Thermal and catalytic pyrolysis of Karanja seed to produce liquid fuel,” Fuel, vol. 115, no. July, pp. 434–442, https://doi.org/10.1016/j.fuel.2013.07.053.
Nelson SO, Trabelsi S (2010) “Measurement of grain and seed microwave permittivity for moisture and density determination,” Conf. Proc. - IEEE SOUTHEASTCON, pp. 463–466, https://doi.org/10.1109/SECON.2010.5453809.
Trabelsi S, Nelson SO (2012) Microwave dielectric properties of cereal grains. Trans ASABE 55(5):1989–1996. https://doi.org/10.13031/2013.19599
Kardjilova K, Bekov E, Hlavacova Z, Kertezs A (2012) Measurement of electrical properties of rapeseed seeds with LCR meter good will 8211. Int J Appl Sci Technol 2(8):35–44 [Online]. Available: www.ijastnet.com
Sacilik K, Colak A (2010) Determination of dielectric properties of corn seeds from 1 to 100 MHz. Powder Technol 203(2):365–370. https://doi.org/10.1016/j.powtec.2010.05.031
Fennell LP, Boldor D (2013) Dielectric characterization of the seeds of invasive Chinese tallow tree. J Microw Power Electromagn Energy 47(4):237–250. https://doi.org/10.1080/08327823.2013.11689861
Salema AA, Ani FN, Mouris J, Hutcheon R (2017) Microwave dielectric properties of Malaysian palm oil and agricultural industrial biomass and biochar during pyrolysis process. Fuel Process Technol 166:164–173. https://doi.org/10.1016/j.fuproc.2017.06.006
Antunes E, Jacob MV, Brodie G, Schneider PA (2018) Microwave pyrolysis of sewage biosolids: dielectric properties, microwave susceptor role and its impact on biochar properties. J Anal Appl Pyrolysis 129:93–100. https://doi.org/10.1016/j.jaap.2017.11.023
Shinoj S, Visvanathan R, Panigrahi S, Kochubabu M (2011) Oil palm fiber (OPF) and its composites: a review. Ind Crop Prod 33(1):7–22. https://doi.org/10.1016/j.indcrop.2010.09.009
Zimmermann MH, Springer Series in Wood Science
Liu S, Zhang Y, Tuo K, Wang L, Chen G (2018) “Structure, electrical conductivity, and dielectric properties of semi-coke derived from microwave-pyrolyzed low-rank coal,” Fuel Process Technol, vol. 178, no. February, pp. 139–147, https://doi.org/10.1016/j.fuproc.2018.05.028.
Mushtaq F, Mat R, Ani FN (2014) A review on microwave assisted pyrolysis of coal and biomass for fuel production. Renew Sust Energ Rev 39:555–574. https://doi.org/10.1016/j.rser.2014.07.073
Vos B, Mosman J, Zhang Y, Poels E, Bliek A (2003) Impregnated carbon as a susceptor material for low loss oxides in dielectric heating. J Mater Sci 38(1):173–182. https://doi.org/10.1023/A:1021138505264
Salema AA, Yeow YK, Ishaque K, Ani FN, Afzal MT, Hassan A (2013) “Dielectric properties and microwave heating of oil palm biomass and biochar,” Ind Crops Prod, vol. 50, no. August, pp. 366–374, https://doi.org/10.1016/j.indcrop.2013.08.007.
Venu H, Appavu P (2019) Combustion and emission characteristics of tamarind seed biodiesel-diesel blends in a compression ignition engine. Int J Ambient Energy 0(0):1–22. https://doi.org/10.1080/01430750.2019.1611652
Liu H, Jiaqiang E, Deng Y, Xie C, Zhu H (2016) Experimental study on pyrolysis characteristics of the tobacco stem based on microwave heating method. Appl Therm Eng 106:473–479. https://doi.org/10.1016/j.applthermaleng.2016.06.042
Ellison C, McKeown MS, Trabelsi S, Boldor D (2017) Dielectric properties of biomass/biochar mixtures at microwave frequencies. Energies 10(4):1–11. https://doi.org/10.3390/en10040502
Wu Q, Wang Y, Jiang L, Yang Q, Ke L, Peng Y, Yang S, Dai L, Liu Y, Ruan R (2020) Microwave-assisted catalytic upgrading of co-pyrolysis vapor using HZSM-5 and MCM-41 for bio-oil production: Co-feeding of soapstock and straw in a downdraft reactor. Bioresour Technol 299:122611. https://doi.org/10.1016/j.biortech.2019.122611
Salema AA, Afzal MT, Bennamoun L (2017) “Pyrolysis of corn stalk biomass briquettes in a scaled-up microwave technology,” Bioresour Technol, vol. 233, no. February, pp. 353–362, https://doi.org/10.1016/j.biortech.2017.02.113.
Nayan NK, Kumar S, Singh RK (2013) Production of the liquid fuel by thermal pyrolysis of neem seed. Fuel 103:437–443. https://doi.org/10.1016/j.fuel.2012.08.058
Chukwuneke JL, Ewulonu MC, Chukwujike IC, Okolie PC (2019) Physico-chemical analysis of pyrolyzed bio-oil from Swietenia macrophylla (mahogany) wood. Heliyon 5(6):e01790. https://doi.org/10.1016/j.heliyon.2019.e01790
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mathiarasu, A., Pugazhvadivu, M. Studies on dielectric properties and microwave pyrolysis of karanja seed. Biomass Conv. Bioref. 13, 2895–2905 (2023). https://doi.org/10.1007/s13399-021-01349-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01349-5