Abstract
Vitis vinifera (variety Tempranillo) grapes were pressurized at 400 MPa by high hydrostatic pressure for 10 min and the effect on wild microbial populations, phenol extraction and wine composition was monitored. After treatment, the grapes were inoculated and fermented with Saccharomyces cerevisiae and several non-Saccharomyces yeasts: Schizosaccharomyces pombe, Torulaspora delbrueckii, Metschnikowia pulcherrima and Lachancea thermotolerans. S. pombe was used as sole fermentative yeast, but T. delbrueckii, M. pulcherrima and L. thermotolerans were used in sequential cultures with S. cerevisiae to completely ferment the sugars. The HHP treatment reduces strongly or eliminates wild microorganisms, especially yeasts, facilitating the growth and development of non-Saccharomyces yeasts. Thus, it helps to get either a better expression of enzymatic activities or metabolites production of non-Saccharomyces affecting wine quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High hydrostatic pressure (HHP) is an emerging food preservation technology that is being used extensively in many food industries because of its efficiency into extend shelf life and reduce microbial counts. At the same time, is a gentle technology because of the low temperature (adiabatic compression heat is only 2–3° C per 100 MPa); it is considered inside cold pasteurization technologies. Also, HHP energy is too weak to break covalent bonds so it is unable to degrade molecules with sensory repercussion such as pigments or aromatic compounds (Yen and Lin 1996; Considine et al. 2008).
Conventional industrial scale treatments involve pressures of 400–600 MPa for 3–10 min (Morata 2010; Buzrul 2012). It has been reported that 400-MPa HHP treatment produces the elimination of wild yeast population in grapes (Morata et al. 2015a), although bacterial populations remain but with reduced counts. Also, it has been observed that HHP increase the extraction of pigments and other phenolic compounds (Morata et al. 2015a) and the effect is stronger than with other emerging technologies such as e-beam irradiation (Morata et al. 2015b).
Currently, the use of non-Saccharomyces is a hot topic in wine industry; many biotechnology companies are producing these theses yeasts as dried or liquid cultures (Morata & Suárez-Lepe 2016). Non-Saccharomyces are used to enhance aromatic compounds and increase complexity (Ciani et al. 2010), hydrolyse glycosylated terpenols (Ciani et al. 2010), release cell wall polysaccharides (Palomero et al. 2009; Kulkarni et al. 2015), produce or degrade organic acids affecting pH and stability (Gobbi et al. 2013; Suárez-Lepe et al. 2012) and promote the formation of stable pigments (Benito et al. 2011; Morata et al. 2012).
Schizosaccharomyces pombe is a yeast used in wine biotechnology for biological deacidification because it is able to produce maloalcoholic fermentation (Suárez-Lepe et al. 2012), also favours the formation of stable pigments during fermentation (Morata et al. 2012) and release high concentrations of polysaccharides during ageing on lees (Kulkarni et al. 2015). Torulaspora delbrueckii is used for improving aromatic profile in wines (Loira et al. 2014) but since its fermentative power range 7–9 % v/v must be used in sequential cultures with Saccharomyces cerevisiae (Ciani & Maccarelli 1998). Metschnikowia pulcherrima develops several enzymatic activities that help enhance varietal aromatic compounds, also by forming fermentative esters (Fernández et al. 2000). Lachancea thermotolerans is used to enhance aromatic compounds and to increase acidity by producing lactic acid (Comitini et al. 2011).
Many times non-Saccharomyces yeasts have difficulties to develop and ferment because of the competition with grape wild yeasts that frequently show either higher fermentative power or fast fermentation speed. Therefore, new cold pasteurization technologies improve the possibilities of using non-Saccharomyces yeasts in oenology. Moreover, many non-Saccharomyces yeasts are more sensitive to SO2 than S. cerevisiae and HHP has been described as a way to reduce the concentrations of sulphites in wines, especially in red ones because of its less sensibility to oxidation (Santos et al. 2013).
The aim of the present work was to study how the HHP treatments affect the wild yeast populations on grapes and the effect in the development of non-Saccharomyces yeast inoculated either as sole yeast or in sequential fermentation. Also, the repercussion of those yeasts in both wine composition and quality was assessed.
Materials and Methods
Treatment of Grapes by HHP
Vitis vinifera L. grapes (variety Tempranillo) were manually destemmed and 400 g were vacuum packed in polypropylene bags. Pressure treatments were performed using a pilot-scale model FPG7100:912C HHP device (Stansted Fluid Power Ltd., Harlow, Exxes, UK) designed to provide of up to 900 MPa at temperatures between −20 and 110 °C. The vessel volume was 2 L; water was used as the compressing fluid. Treatments were performed at 400 MPa for 10 min. The rates of compression and expansion were 7 and 60 MPa/s, respectively. During the treatments, the pressure vessel was thermostatically controlled at 20 °C. Two thermocouples were installed to monitor temperature, one near the vessel wall (thermostatically set at 20 °C, controlled by heat exchangers), and the other close to the grapes in the centre of the vessel. After treatment, the samples were stored at 4 °C until fermentation. All experiments were conducted in triplicate.
Fermentations
After pressurization, the vacuum-packed grapes were examined in a laminar flow cabinet; the grapes were gently crushed and placed in sterilized 500 mL Erlenmeyer flasks. Samples of 5 mL were taken to analyse wild microbial population. The remaining must was inoculated with a liquid culture of the corresponding yeast. S. cerevisiae strain 7VA (Dept. of Food Science and Technology, UPM, Madrid, Spain) and S. pombe strain 938 (CSIC, Madrid, Spain) were used as sole fermentative yeasts. T. delbrueckii strain 291 (Lallemand, Danstar Ferment AG, Montreal, Canada), M. pulcherrima FLAVIA MP346 (Lallemand, Danstar Ferment AG, Montreal, Canada) and Lachancea thermotolerans CONCERTO (CHR Hansen, Hoersholm, Denmark) were inoculated in sequential cultures with S. cerevisiae strain 7VA. In sequential fermentations, S. cerevisiae was inoculated on day 7 of fermentation. The population was 108 cfu/mL. After inoculation, musts were fermented isothermally at 22 °C in the absence of SO2. Controls in spontaneous fermentation with wild yeast population were also undertaken both in non-pressurized grapes and those treated at 400 MPa. Samples were taken at day 0, 3, 7, 12 and 18 in order to count yeast populations. Ethanol content was analysed on day 7 by HPLC-RI. At the end of fermentation (day 18), all the wines were analysed to measure anthocyanins, total phenols, organic acids, sugars, ethanol and volatile compounds.
Yeast Populations
Viable yeast cells were determined by plate counting. In the sequential fermentations, total yeasts (non-Saccharomyces plus Saccharomyces) were determined on YPD agar after incubation at 25 °C for 3 days, at different intervals over the fermentation period. Saccharomyces cell numbers were determined by incubating plates at 37 °C for 2 days (at this temperature non-Saccharomyces are unable to grow). Non-Saccharomyces counts were performed in a synthetic lysine agar (Oxoid, Hampshire, UK).
Determination of Anthocyanins
The following anthocyanins and pyranoanthocyanins were determined using an Agilent Technologies (Palo Alto, CA) series 1100 HPLC chromatograph equipped with a diode array detector and a quadrupole mass spectrometer with an electrospray interface: delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, petunidin-3-O-glucoside, peonidin-3-O-glucoside, malvidin-3-O-glucoside, malvidin-3-O-glucoside-acetaldehyde adduct (vitisin B, Vit B), malvidin-3-O-glucoside-pyruvate adduct (vitisin A; Vit A), malvidin-3-O-(6″-acetylglucoside)-pyruvate adduct (acetylvitisin A; Vit A Ac), delphinidin-3-O-(6″-acetylglucoside), petunidin-3-O-(6″-acetylglucoside), peonidin-3-O-(6″-acetylglucoside), malvidin-3-O-(6″-acetylglucoside), peonidin-3-O-(6″-p-coumaroylglucoside), malvidin-3-O-(6″-p-coumaroylglucoside), malvidin-3-O-glucoside-4-vinylphenol and malvidin-3-O-glucoside-4-vinylguaiacol. Gradients of solvent A (water/formic acid, 95:5 v/v) and B (methanol/ formic acid, 95:5 v/v) were used in a reverse-phase Poroshell 120 C18 column (Phenomenex, Torrance, CA, USA) (50 × 4.6 mm; particle size 2.7 μm) as follows: minutes 0–2, 15 % B (working flow 0.8 mL/min); minutes 2–10, 15–50 % B linear; minutes 10–12, 50 % B; minutes 12–13, 50–15 % B linear and minutes 13–15 re-equilibration. Detection was performed by scanning in the 400–600 nm range. Quantification was performed by comparison against an external standard at 525 nm and expressed as milligrams per litre of malvidin-3-glucoside (r 2 = 0.9999). Anthocyanins were identified by their retention times and by comparing their UV-visible and mass spectra with data in the literature. Mass spectrometry was performed in positive scanning mode (m/z 100–1000, fragmenter voltage 150 V from minute 0 to 15). Twenty-microlitre sample of previously filtered (0.45 μm membrane) wines were injected into the HPLC apparatus. The detection limit was 0.1 mg/L.
Analysis of Volatile Compounds by Gas Chromatography with Flame Ionization Detection
Volatile compounds were determined using an Agilent Technologies 6850 gas chromatograph (Network GC System) equipped with an integrated flame ionization detector (GC-FID), as described by Abalos et al. (2011). A DB-624 column (60 m × 250 μm × 1.40 μm) was used. The following compounds were used as external standards for calibration (r 2 > 0.999): acetaldehyde, methanol, 1-propanol, 1-butanol, 2-butanol, isobutanol, 2-methyl-1-butanol, 3-methyl-1-butanol, 2-phenylethyl acetate, 2-phenylethyl alcohol, diacetyl, acetoin, ethyl acetate, isoamyl acetate, isobutyl acetate, ethyl butyrate, ethyl lactate and hexanol. 4-methyl-2-pentanol was used as internal standard. All compounds were purchased from Fluka (Sigma-Aldrich Corp., Buchs SG, Switzerland). The injector temperature was 250 °C, and the detector temperature 300 °C. The column temperature was 40 °C (5 min), rising linearly by 10 °C/min until 250 °C; this temperature was then held for 5 min. Hydrogen was used as carrier gas. The injection split ratio was 1:10, the in-column flow rate 2.2 L/min, and the detection limit 0.1 mg/L. One hundred microlitres of internal standard (500 mg/L) were added to 1 mL test samples and filtered through syringe membrane filters (pore size 0.45 μm) (Teknokroma, Barcelona, Spain). They were then placed in 2 mL glass vials sealed with a PTFE/silicon septum. One microlitre of this filtrate was injected into the GC apparatus. 3-ethoxy propanol was identified by GC-MS under the same chromatographic conditions.
Analysis of Acetic Acid and Residual Sugars
Acetic acid, lactic acid, malic acid and residual sugars were measured enzymatically using an Y15 enzymatic autoanalyzer (Biosystems, Barcelona, Spain).
Ethanol Quantification
Ethanol was analysed by liquid chromatography with refractive index detection (LC-RI) using a Waters e2695 apparatus (Milford, Massachusetts, USA) equipped with a 2414 Refractive Index Detector. Analyses were performed using a reverse phase Phenosphere XDB C18 column (4.6 × 150 mm, 5 μm particle size) (Phenomenex, Torrance, CA, USA). The solvent was Milli-Q water (used in isocratic mode); the flow rate was 0.4 mL/min. The temperature was set at 30 °C both in the column and in the detector. Calibration was performed using an external ethanol standard (Panreac, Barcelona, Spain). Samples were injected after filtration through 0.45 μm cellulose methyl ester membrane filters (Tecknokroma, Barcelona, Spain). The injection volume was 2 μL.
Statistical Analysis
Means and standard deviations were calculated and differences examined using ANOVA and the least significant difference (LSD) test. All calculations were made using PC Statgraphics v.5 software (Graphics Software Systems, Rockville, MD, USA). Significance was set at p < 0.05.
Results and Discussion
Yeast Populations During Fermentation of Both HHP-Treated Grapes and Controls Inoculated with Non-Saccharomyces Yeasts
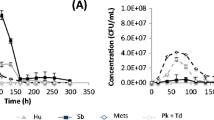
Wild yeast populations in grapes non-treated by HHP were log 2 cfu/mL; however when 400 MPa was applied, the levels were from undetectable to lower than 10 cfu/mL (Fig. 1a). Approximately, 50 % of wild yeast population were non-Saccharomyces able to grow in lysine media. The inoculation of S. cerevisiae at log 6 cfu/mL was successful in both situations, pressurized and unpressurized grapes, due to the low load of wild yeasts that means 4 log orders of difference even in the non-treated grapes (Fig. 1a). The counts of S. cerevisiae reached values higher than log 8 cfu/mL from day 3 until day 12 when populations started to decrease because of nutrient exhaustion.
Changes in the population of both wild and inoculated yeasts during single or sequential fermentations of untreated and HHP-treated grapes. a Single-culture fermentation with S. cerevisiae (7VA). b Single-culture fermentation with S. pombe (938). c–e Torulaspora delbrueckii, Metschnikowia pulcherrima and Lachancea thermotolerans, respectively (circles) during sequential fermentation with S. cerevisiae (triangles) of untreated and HHP-treated grapes. S. cerevisiae (7VA) was inoculated on day 7. Bars represent wild yeast populations, untreated grapes (solid bars); HHP-treated grapes (open bars). Values are means ± standard deviations for three independent fermentations. Dotted lines show the tentative evolution of wild yeast counts in HHP-processed grapes and controls
S. pombe showed a similar trend in both cell counts in pressurized grapes and in controls. It was inoculated at log 6 reaching more than log 8 on day 3 of fermentation. After day 12, populations started to decrease remaining about log 7 at the end of fermentation. Wild yeast counts in grapes processed at 400 MPa remained below log 1 cfu/mL until day 7 and below log 2 until day 18 (Fig. 1b). However, in control grapes, wild yeast population reached log 5 cfu/mL in 3 days producing higher competition with S. pombe especially due to its slow fermentative metabolism. It will affect the final metabolic profile in wine after fermentation. Therefore, HHP is a way to get a better metabolic expression of S. pombe.
T. delbrueckii fermentations had a similar situation with wild yeast population at log 2 and HHP-treated samples at less than log 1 or undetectable. The evolution of wild yeast population was similar remaining in the same values in HHP processed grape but exceeding log 5 in controls. Inoculated population of T. delbrueckii was above log 8 from day 3 of fermentation in both HHP-treated grapes and controls (Fig. 1c). As the fermentative power of Torulaspora is lower than 9 % v/v in ethanol, S. cerevisiae was used in sequential fermentation to completely ferment sugars. S. cerevisiae was inoculated at log 6 and remained at log 7 from day 12 until the end of fermentation.
M. pulcherrima is even weaker in fermentative power (<4 %v/v) than Torulaspora and was also used in sequential culture with S. cerevisiae (Fig. 1d). CFU count evolution was lower than Torulaspora and always was below log 8. Moreover, counts strongly declined from day 12 being below of log 3 at the end of fermentation (Fig. 1d). Wild yeast populations in untreated samples exceeded log 6 cfu/mL on day 7 being in strong competition with M. pulcherrima, and also with the S. cerevisiae that was subsequently inoculated on day 7 at log 6 cfu/mL, as usual. At the end of fermentation (day 18), counts of wild yeasts from grapes were higher than those of M. pulcherrima. In the case of a delicate and weak yeast like Metschnikowia, the use of HHP at 400 MPa is the only way to ensure a good development because wild yeast populations remain below of log 2 until the end of fermentation (Fig. 1d).
L. thermotolerans also has intermediate fermentative power (7–8 % v/v in ethanol) the behaviour was similar to T. delbrueckii, but cell counts decreased faster after day 7 reaching about log 6 at the end of fermentation (Fig. 1e). It also needs to be sequentially inoculated with S. cerevisiae in order to finish fermentation without residual sugars. S. cerevisiae was inoculated at log 6 on day 7 and reached about log 8 on day 12.
Residual Sugars, Ethanol, and Organic Acids After Fermentations by Non-Saccharomyces
All fermentations finished with residual sugar contents below 0.5 g/L except pressurized grapes in spontaneous fermentation that never started to ferment and had 194.5 g/L of sugar in average at day 18 (Table 1). All wines finished with an alcoholic degree higher than 13 % v/v except spontaneous fermentation of pressurized grapes at 400 MPa that never started to ferment properly (Fig. 2) because of the low residual yeast remaining after the HHP cold pasteurization. Sequential fermentations with S. cerevisiae were used to ferment completely sugars; the inoculation of S. cerevisiae was done at day 7 of fermentation (Fig. 2, black square dots). Low fermentative power was observed in M. pulcherrima with an average value of 4 % v/v (Morata & Suárez-Lepe 2016). T. delbrueckii and L. thermotolerans showed a medium-high fermentative power (9 and 11 % v/v, respectively, Fig. 2) but not enough to ferment completely all the sugars. It is also noticeable that HHP treatment in absence of inoculation produces less than 1 % v/v of alcohol after 6 days at 22 °C and 2 % v/v in average after 18 days at 22 °C. So HHP treatment is a very efficient technique to remove wild yeasts in musts but also to keep the must stable during more than 2 weeks at room temperature and without SO2.
Alcoholic degree in fermentations at day 7, before the sequential inoculations (black dots) and at the end of fermentation (grey bars). Values are means ± standard deviations for three independent fermentations. Different letters in the same bars indicate significant differences between means (p < 0.05)
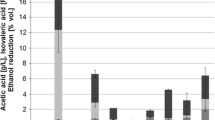
Production of volatile acidity was at a moderate level ranging from 0.35 to 0.72 g/L in most of the fermentations. Only S. pombe fermentations exceeded 1 g/L that is the normal behaviour of this species showing a high acetic acid production. Most of the trials fermented at values below 0.5 g/L that is really low when fermentations are done in small volumes. The highest values of malic acid remained in HHP-treated fermentations without yeast inoculation (Table 1). All the other fermentations, even spontaneous, showed some degree of malic acid degradation due to yeast effect. This metabolization was especially relevant in S. pombe fermentations because this species is able to perform maloalcoholic fermentation (Suárez-Lepe et al., 2012). No residual malic acid was found in S. pombe fermentations (Table 1). L. thermotolerans has been described as strong producer of lactic acid and it has been used to improve total acidity in enology (Gobbi et al., 2013). The problem is its medium fermentative power what makes difficult its development in competition with S. cerevisiae. To get a better prevalence, it is usually necessary to use high inoculation doses. HHP reduce competitive yeasts thus allowing a greater production of lactic acid, almost two times higher (Table 1). So, by using HHP, it is easier to get a better acidification of musts during fermentation with L. thermotolerans.
Volatile Fermentative Compounds
Fermentations of S. pombe showed the highest contents of acetoin (Table 2), and even higher in pressurized grapes probably due to lower development of wild yeasts from grapes that allow a better expression of its metabolism. However, in no case its perception threshold in wine was exceeded (150 mg/L; Selli et al. 2004). The other sequential or single fermentations showed similar values of acetoin without significant differences.
Also, the production of ethyl acetate in S. pombe fermentations was stronger than in S. cerevisiae fermentations and even higher in the case of sequential fermentations involving M. pulcherrima. Otherwise, sequential fermentations of T. delbrueckii and L. thermotolerans produced intermediate levels of ethyl acetate, more similar to concentrations in single fermentations of S. cerevisiae. However, although it can be already detected in tasting (perception threshold 12 mg/L; Peinado et al. 2004) concentrations in which it appears are still considered positive for the quality of wine (<150 mg/L; Rapp 1993).
T. delbrueckii produced the highest concentrations of 3-ethoxy propanol (Loira et al. 2014) described as responsible of blackcurrant aroma in red wines (Tao & Zhang 2010). Concentration of this volatile compound was significantly higher in the HHP-treated sample. Its perception threshold is very low; 0.1 mg/L (Peinado et al. 2004) so that it may be expressed in the sensory profile of the wines produced by contributing to fruity character.
Production of acetate esters was enhanced in sequential fermentations with M. pulcherrima; the concentrations of ethyl acetate, isobutyl acetate and isoamyl acetate were higher than in the other inoculated fermentations. Also, it produced much higher concentrations of 2-phenylethyl alcohol with positive repercussion in aroma (rose petals; Francis & Newton 2005). The expression is significantly higher for this molecule in pressurized grapes probably due to a better development of the yeast in absence of competitive wild yeasts (Table 2).
The formation of ethyl lactate was higher when L. thermotolerans was used in sequential fermentations probably because of the high production of the precursors (lactic acid, Table 1). In average, the value was slightly higher in pressurized grapes but without significant differences. Similarly, sequential fermentations with M. pulcherrima and L. thermotolerans increased the formation of fermentative fruity esters with regard to the other species, but again no significant differences could be observed.
Methanol contents were always significantly lower in HHP-treated grapes fermentations with respect to the untreated samples.
Anthocyanins
Vitisin A was the vitisin pigment that reached higher concentrations in all the fermentations (Fig. 3a), and especially in those performed with S. pombe because of its production of pyruvic acid (Morata et al. 2012; Loira et al. 2015). In average, vitisin A production was higher in HHP-treated grapes with most of the yeast species; however, the differences were not significant. Sequential fermentations with L. thermotolerans and spontaneous fermentations showed the lowest concentrations of vitisins. Formation of vinylphenolic pyranoanthocyanins was very low in all fermentations and non-observed in S. pombe and L. thermotolerans (Fig. 3b).
Conclusions
HHP treatment of grapes at 400 MPa for 10 min reduce wild yeast counts at less than log 1 cfu/mL. It makes possible the development of non-Saccharomyces with low fermentative power or slow fermentation rate allowing the competitive use of these yeasts and ensuring new biotechnologies in wine industry. At the same time, the better development of non-Saccharomyces allows an enhanced expression of non-Saccharomyces metabolome enabling new opportunities in wine industry, in terms of organoleptic properties of the product, shelf life and aroma profile.
References
Abalos, D., Vejarano, R., Morata, A., González, C., & Suárez-Lepe, J. A. (2011). The use of furfural as a metabolic inhibitor for reducing the alcohol content of model wines. European Food Research and Technology, 232, 663–669.
Benito, S., Morata, A., Palomero, F., González, C., & Suárez-Lepe, J. A. (2011). Formation of vinylphenolic pyranoanthocyanins by Saccharomyces cerevisiae and Pichia guillermondii in red wines produced following different fermentation strategies. Food Chemistry, 124, 15–23.
Buzrul, S. (2012). High hydrostatic pressure treatment of beer and wine: a review. Innovative Food Science and Emerging Technologies, 13, 1–12.
Ciani, M., Comitini, F., Mannazzu, I., & Domizio, P. (2010). Controlled mixed culture fermentation: a new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Research, 10, 123–133.
Ciani, M., & Maccarelli, F. (1998). Oenological properties of non-Saccharomyces yeasts associated with wine-making. World Journal of Microbiology and Biotechnology, 14, 199–203.
Comitini, F., Gobbi, M., Domizio, P., Romani, C., Lencioni, L., Mannazzu, I., & Ciani, M. (2011). Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiology, 28, 873–882.
Considine, K. M., Kelly, A. L., Fitzgerald, G. F., Hill, C., & Sleator, R. D. (2008). High-pressure processing-effects on microbial food safety and food quality. FEMS Microbiology Letters, 281, 1–9.
Fernández, M., Úbeda, J. F., & Briones, A. I. (2000). Typing of non-Saccharomyces yeasts with enzymatic activities of interest in wine-making. International Journal of Food Microbiology, 59, 29–36.
Francis, I. L., & Newton, J. L. (2005). Determining wine aroma from compositional data. Australian Journal of Grape and Wine Research, 11, 114–126.
Gobbi, M., Comitini, F., Domizio, P., Romani, C., Lencioni, L., Mannazzu, I., & Ciani, M. (2013). Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: a strategy to enhance acidity and improve the overall quality of wine. Food Microbiology, 33, 271–281.
Kulkarni, P., Loira, I., Morata, A., Tesfaye, W., González, M. C., & Suárez-Lepe, J. A. (2015). Use of non-Saccharomyces yeast strains coupled with ultrasound treatment as a novel technique to accelerate ageing on lees of red wines and its repercussion in sensorial parameters. LWT-Food Science and Technology, 64, 1255–1262.
Loira, I., Morata, A., Comuzzo, P., Callejo, M. J., González, C., Calderón, F., & Suárez-Lepe, J. A. (2015). Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Research International, 76, 325–333.
Loira, I., Vejarano, R., Bañuelos, M. A., Morata, A., Tesfaye, W., Uthurry, C., Villa, A., Cintora, I., & Suárez-Lepe, J. A. (2014). Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. LWT-Food Science and Technology, 59, 915–922.
Morata, A., & Suárez-Lepe, J. A. (2016). Advances in food biotechnology, first edition. In V. Ravishankar Rai (Ed.), New biotechnologies for wine fermentation and ageing (pp. 288–289). West Sussex: John Wiley & Sons, Ltd..
Morata, A. (2010). Nuevas tecnologías de conservación de alimentos (p. 16). Madrid: Antonio Madrid Vicente.
Morata, A., Bañuelos, M. A., Tesfaye, W., Loira, I., Palomero, F., Benito, S., Callejo, M. J., Villa, A., Gonzalez, M. C., & Suarez-Lepe, J. A. (2015b). Electron beam irradiation of wine grapes: effect on microbial populations, phenol extraction and wine quality. Food and Bioprocess Technology, 8, 1845–1853.
Morata, A., Benito, S., Loira, I., Palomero, F., González, M. C., & Suárez-Lepe, J. A. (2012). Formation of pyranoanthocyanins by Schizosaccharomyces pombe during the fermentation of red must. International Journal of Food Microbiology, 159, 47–53.
Morata, A., Loira, I., Vejarano, R., Bañuelos, M. A., Sanz, P. D., Otero, L., & Suárez-Lepe, J. A. (2015a). Grape processing by high hydrostatic pressure: effect on microbial populations, phenol extraction and wine quality. Food and Bioprocess Technology, 8, 277–286.
Palomero, F., Morata, A., Benito, S., Calderón, F., & Suárez-Lepe, J. A. (2009). New genera of yeasts for over-lees aging of red wine. Food Chemistry, 112, 432–441.
Peinado, R. A., Moreno, J., Medina, M., & Mauricio, J. C. (2004). Changes in volatile compounds and aromatic series in sherry wine with high gluconic acid levels subjected to aging by submerged flour yeast cultures. Biotechnology Letters, 26, 757–762.
Rapp, A. (1993). Foreign and undesirable flavours in wine. En: Les acquisitions récentes en chromatographie du vin. Applications à l’analyse sensorielle des vins (pp 151–163). Paris: TEC&DOC-Lavoisier.
Santos, M. C., Nunes, C., Cappelle, J., Gonçalves, F. J., Rodrigues, A., Saraiva, J. A., & Coimbra, M. A. (2013). Effect of high pressure treatments on the physicochemical properties of a sulphur dioxide-free red wine. Food Chemistry, 141, 2558–2566.
Selli, S., Cabaroglu, T., Canbas, A., Erten, H., Nurgel, C., Lepoutre, J. P., & Gunata, Z. (2004). Volatile composition of red wine from cv. Kalecik Karasi grown in central Anatolia. Food Chemistry, 85, 207–213.
Suárez-Lepe, J. A., Palomero, F., Benito, S., Calderón, F., & Morata, A. (2012). Oenological versatility of Schizosaccharomyces spp. European Food Research and Technology, 235, 375–383.
Tao, Y., & Zhang, L. (2010). Intensity prediction of typical aroma characters of Cabernet Sauvignon wine in Changli County (China). LWT-Food Science and Technology, 43, 1550–1556.
Yen, G. C., & Lin, H. T. (1996). Comparison of high pressure treatment and thermal pasteurization effects on the quality and shelf-life of guava puree. International Journal of Food Science and Technology, 31, 205–213.
Acknowledgments
This work was funded by the Ministerio de Economía y Competitividad (AGL2013-40503-R). The authors thank J. A. Sánchez (Dept. Química y Tecnología de Alimentos), A. Villa, P. Santos (Dept. Biotecnología) and R. Domínguez (ICTAN, CSIC) for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bañuelos, M.A., Loira, I., Escott, C. et al. Grape Processing by High Hydrostatic Pressure: Effect on Use of Non-Saccharomyces in Must Fermentation. Food Bioprocess Technol 9, 1769–1778 (2016). https://doi.org/10.1007/s11947-016-1760-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-016-1760-8