Abstract
Vitis vinifera L. (variety Tempranillo) grapes were subjected to electron beam irradiation at nominal doses of 0.5, 1 and 10 kGy, and the effect on microbial populations, phenol extraction and wine quality was examined. No external modifications of fruit shape or colour were observed with any of the doses tested. The 1 kGy dose reduced initial must bacterial and yeast counts by 1 log cycle, while the 10 kGy dose left only a residual population of <10 colony-forming units (cfu)/mL. Irradiation was associated with a dose-dependent increase in phenolic compounds in the must. However, the wines produced from grapes irradiated at different doses showed no significant differences in their total phenolic compound contents. All the wines had a good sensory profile; those irradiated at 10 kGy had an increased fruity odour.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electron beam (e-beam) irradiation has been extensively used to control microbial populations in fruits and vegetables since it preserves food flavour, colour, nutrient content, taste, texture and other quality attributes (Alothman et al. 2009; Farkas et al. 2014; Kong et al. 2014). E-beam irradiation is a type of cold pasteurisation since it reduces bacterial and yeast counts without increasing the temperature of the food (Korkmaz and Polat 2005). It also reduces the presence of residues of chemical additives (Korkmaz and Polat 2005). The safety of irradiated foods for consumption has been extensively studied by international consortia formed by the FAO, the IAEA and the WHO (Farkas and Mohácsi-Farkas 2011).
E-beam irradiation could provide a means of using smaller amounts of SO2 in winemaking. This would be of interest, given the allergenic properties of this compound and the current downward trend in European legislation regarding its acceptable concentration in foodstuffs (Guerrero and Cantos-Villar 2014). SO2 is commonly added to musts at concentrations of around 50 mg/L to encourage the selective growth of fermentative yeasts and to help control oxidation reactions (Fugelsang 1989). Thermal technologies designed to achieve the same ends affect the sensory quality of wines by degrading their aromatic compounds and promoting the oxidation of phenolic compounds.
Grape irradiation could also be of interest in new fermentation technologies which involve the use of non-Saccharomyces yeasts (Suárez-Lepe and Morata 2012). The successful use of these yeasts, which are less competitive than Saccharomyces cerevisiae, depends on the initial population of wild yeasts on the grapes. Techniques that reduce wild yeast and bacterial populations without affecting the sensory quality of the final wine could be put to good use by oenologists.
The aim of the present work was to study the effect of e-beam irradiation on (1) wild yeast and bacterial populations in must and wine, (2) the extraction of phenolic compounds from grape skin and (3) the fermentative and sensory quality of wine.
Materials and Methods
Treatment of Grapes by E-Beam Irradiation
Vitis vinifera L. (variety Tempranillo) grapes were manually destemmed, and 700-g samples were vacuum packed in polypropylene bags. E-beam irradiation was performed at the installations of the Ionisos Company (Tarancon, Spain) using a 10-MeV, 50-kW Rhodotron accelerator (Louvain-la-Neuve, Belgium) at a scan frequency of 100 Hz. Grapes were irradiated in their bags (manually adjusted to present a target under 2 cm thick) at nominal doses of 0 (control), 0.5, 1 and 10 kGy (performed at room temperature and in quadruplicate); the radiation doses actually received were verified by dosimetry (Table 1). After irradiation, the samples were stored at 4 °C until fermentation.
Fermentations
After irradiation, the bags of grapes were opened in a laminar flow cabinet. All grapes from each bag were placed in sterilised 1-L Erlenmeyer flasks. They were then gently crushed using a sterile glass rod, and the must was inoculated with 20 mL of a YPD inoculum of S. cerevisiae 7VA (ETSIA, UPM) (108 colony-forming units (cfu)/mL). Fermentation was then allowed to proceed isothermally at 25 °C for 15 days in the absence of SO2. Samples were taken at the beginning and end of fermentation to examine their microbial populations, colour and anthocyanin and total phenolic compound contents. Aromatic compounds, ethanol, acetic acid and residual sugars were determined at the end of fermentation.
Microbial Populations
Following grape irradiation, microbiological analyses were performed on the must and on the final wines. Ten millilitres of the must from the crushed grapes was taken from each Erlenmeyer flask (see above), and 10-fold dilutions were prepared in saline peptone. One-millilitre samples were then pour plated on selective media for the enumeration of total aerobes and lactic acid bacteria (LAB). A further 100 μL was spread plated for yeast enumeration on glucose chloramphenicol agar (incubated aerobically for 4 days at 25 °C). A medium with lysine (which cannot be used by S. cerevisiae) as the sole nitrogen source was also used to estimate the population of non-Saccharomyces yeasts (Loira et al. 2014). The media used for growing aerobic bacteria were plate count agar (PCA) supplemented with 50 mg/L nystatin (incubations were aerobic and for 3 days at 30 °C) and Man–Rogosa–Sharpe (MRS) agar supplemented with 50 mg/L nystatin (incubations were anaerobic and for 4 days at 30 °C). All the media, but lysine, were from Pronadisa (Barcelona, Spain). The same plating methodologies and media were used to determine the bacterial and yeast counts of the final wines.
Determination of Anthocyanins
Grape anthocyanins and pyranoanthocyanins were determined by HPLC-DAD-ESI/MS (Agilent Technologies 1100LC-DAD and VL1956MSD, Santa Clara, CA, USA) according to Morata et al. (2012). Briefly, solvent A (water/formic acid, 95:5, v/v) and B (methanol/formic acid, 95:5) gradients were used in an RP C18 column (100 × 4.6 mm; 2.6 μm) as follows: 0–27 min, 20–50 % B linear (0.8 mL/min); 27–28 min, 50 % B; 28–29 min, 50–20 % B linear; and 29–30 min, re-equilibration. Detection was performed by scanning in the 500–600 nm range. Quantification was performed by comparison against an external standard at 525 nm and expressed in milligrams per litre of malvidin-3-glucoside (r 2 = 0.9999). Anthocyanins were identified by their retention times and by comparing their UV–visible and mass spectra with data in the literature. Mass spectrometry was performed in positive scanning mode (m/z 100–1000, fragmenter voltage 150 V from 0 to 23 min). One hundred-microlitre samples of previously filtered (0.45-μm membrane) wines were injected into the HPLC apparatus. The detection limit was 0.1 mg/L.
Analysis of Volatile Compounds by Gas Chromatography with Flame Ionisation Detection
Volatile compounds were determined using an Agilent Technologies 6850 gas chromatograph (Network GC System; Santa Clara, CA, USA) equipped with an integrated flame ionisation detector (GC-FID), as described by Abalos et al. (2011). A DB-624 column (60 m × 250 μm × 1.40 μm) was used. The following compounds were employed as external standards for calibration (r 2 > 0.999): acetaldehyde, methanol, 1-propanol, 1-butanol, 2-butanol, isobutanol, 2-methyl-1-butanol, 3-methyl-1-butanol, 2-phenylethyl acetate, 2-phenylethyl alcohol, diacetyl, acetoin, ethyl acetate, isoamyl acetate, isobutyl acetate, ethyl butyrate, ethyl lactate and hexanol. 4-Methyl-2-pentanol was used as an internal standard. All compounds were purchased from Fluka (Sigma-Aldrich Corp., Buchs, SG, Switzerland). The injector temperature was 250 °C, and the detector temperature was 300 °C. The column temperature was 40 °C (5 min), rising linearly by 10 °C/min until 250 °C; this temperature was then held for 5 min. Hydrogen was used as the carrier gas. The injection split ratio was 1:10, the in-column flow rate was 2.2 L/min, and the detection limit was 0.1 mg/L. One hundred microlitres of internal standard (500 mg/L) was added to 1-mL test samples and filtered through syringe membrane filters (pore size 0.45 μm) (Teknokroma, Barcelona, Spain). They were then placed in 2-mL glass vials sealed with a PTFE/silicon septum. One microlitre of this filtrate was injected into the GC apparatus.
Acetic Acid and Residual Sugars
Acetic acid and residual sugars were determined enzymatically using a Y15 Enzymatic Autoanalyzer (Biosystems, Barcelona, Spain).
Ethanol Quantification
Ethanol was analysed by liquid chromatography with refractive index detection (LC-RI) using a Waters e2695 apparatus (Milford, MA, USA) equipped with a 2414 Refractive Index Detector. Analyses were performed using a reversed phase PhenoSphere XDB C18 column (4.6 × 150 mm, particle size 5 μm) (Phenomenex, Torrance, CA, USA). The solvent was Milli-Q water (used in isocratic mode); the flow rate was 0.4 mL/min. The temperature was set at 30 °C in the column and detector. Calibration was performed using an external ethanol/glucose standard (Panreac, Barcelona, Spain). Samples were injected after filtration through 0.45-μm cellulose methyl ester membrane filters (Teknokroma, Barcelona, Spain). The injection volume was 2 μL.
Colour and Total Polyphenol Index
Wine absorbance at 420, 520 and 620 nm was determined using a V-530 spectrophotometer (JASCO, Tokyo, Japan) and a 1-mm-path length quartz cell, following the procedure of Glories (1984a, b). Both colour intensity and hue were recorded. The total polyphenol index (TPI) was determined spectrophotometrically by measuring the absorbance at 280 nm in a 1-cm-path length quartz cell after a 1:100 dilution with Milli-Q water.
Analytical Determinations of Amino Acids
Amino acid contents were determined using a Jasco (Tokyo, Japan) UHPLC chromatograph series X-LCTM, equipped with a 3120-FP fluorescence detector. Gradients of solvent A (methanol/acetonitrile, 50:50, v/v) and solvent B (sodium acetate/tetrahydrofuran, 99:1, v/v) were used in a C18 (Halo, USA) column (100 mm × 2.1 mm; particle size 2.7 μm) as follows: 90 % B (0.25 mL/min) from 0 to 6 min, 90–78 % B linear (0.2 mL/min) from 6 to 7.5 min, 78 % B from 7.5 to 8 min, 78–74 % B linear (0.2 mL/min) from 8 to 8.5 min, 74 % B (0.2 mL/min) from 8.5 to 11 min, 74–50 % B linear (0.2 mL/min) from 11 to 15 min, 50 % B (0.2 mL/min) from 15 to 17 min, 50–20 % B linear (0.2 mL/min) from 17 to 21 min, 20–90 % B linear (0.2 mL/min) from 21 to 25 min and re-equilibration of the column from 25 to 26 min. Detection was performed by scanning in the 340–455 nm range. Quantification was performed by comparison against external standards of the studied amino acids. The different amino acids were identified by their retention times.
Sensorial Analysis
Wines were evaluated by an experienced panel of nine judges according to Loira et al. (2013). Briefly, the panellists used a scale from 0 to 10 to rate the intensity of different attributes (0 = attribute not perceptible, 10 = attribute strongly perceptible). Each panellist also provided an overall impression of the wines produced, taking into account olfactory and taste features, including any defects.
Statistical Analysis
Means and standard deviations were calculated, and differences were examined using ANOVA and the least significant difference (LSD) test. All calculations were made using PC Statgraphics v.5 software (Graphics Software Systems, Rockville, MD, USA). Significance was set at p < 0.05.
Results and Discussion
Effects of E-Beam Irradiation on Grape Structure
None of the irradiation doses had any effect on berry shape, external appearance or colour (Fig. 1a). There were no any noticeable changes observed in the pulp of the peeled grapes (Fig. 1b). In contrast, pigment migrations from the skins towards the pulp and seeds have been recorded in grapes treated with high hydrostatic pressure (200 MPa) (another emerging cold pasteurisation technique) (Morata et al. 2015). Irradiation was, however, associated with some release of juice (seen in the bags), especially the 10 kGy treatment. This might be due to softening of the fruit texture (due to the softening of the cell walls), as reported for other e-beam-irradiated fruits (Yu et al. 1995; Niemira 2003; Palekar et al. 2004; Moreno et al. 2007). At the low radiation doses (0.5 and 1 kGy), no modifications in firmness were appreciable, as reported in other studies on fruit irradiation (Zhao et al. 1996; D’Innocenzo and Lajolo 2001). An interesting finding was that the irradiated grapes were less aromatic than the controls.
Post E-Beam Irradiation Microbial Populations of the Must and Wines
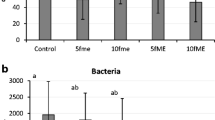
Yeast counts for the must of control grapes returned an initial population of 106 cfu/mL (Fig. 2a). E-beam irradiation at 0.5 kGy reduced the initial population by 0.5 log cycles, while the 1 kGy treatment reduced it by 1 log cycle. Under 10 cfu/mL of yeasts, these were detected, however, in the must from grapes irradiated at 10 kGy (Fig. 2a). Non-Saccharomyces yeast species, estimated by plating the must on a lysine medium, were found to make up some 50 % of the initial yeasts. The effects of the radiation treatment on the Saccharomyces and non-Saccharomyces yeasts were similar at each dose (Fig. 2a).
The bacterial population in the must from control grapes was 102 cfu/mL. The 0.5 kGy treatment reduced this by 0.5 log cycles and the 1 kGy treatment by 1 log cycle. Only a few colonies were observed in the must from grapes irradiated at 10 kGy (<10 cfu/mL) (Fig. 2b). After inoculation of the must with S. cerevisiae 7VA, followed by fermentation for 15 days at 25 °C, the yeast count reached 107 cfu/mL on average. Bacterial counts remained at residual levels (<10 cfu/mL) for all fermentations.
The final wines made from irradiated and control grapes showed very low residual sugar concentrations (Table 2), indicating that fermentation had been completed correctly. The acetic acid concentrations of the wines made from control and 0.5 and 1 kGy-treated grapes were similar, but significantly lower in the wine made from 10 kGy-irradiated grapes (Table 2); this was probably a consequence of the latter’s very low bacterial count. The wines made from irradiated grapes had a lower alcohol content (1 % v/v approximately; Table 2) than that made from control grapes. This cannot be related to fermentation being incomplete due to the absence of residual sugars.
The above results show that e-beam irradiation would allow the amount of SO2 added to fermentations to be reduced—a goal sought in modern winemaking. Moreover, the reduction in the initial wild yeast and bacteria populations would allow winemakers to use new biotechnological strategies such as sequential or mixed fermentations with non-Saccharomyces yeasts or co-inoculation with LAB to achieve simultaneous alcoholic and malolactic fermentation. The elimination or strong reduction of the wild population by irradiation facilitates the development and fermentation with low implantation non-Saccharomyces. Also, it is necessary to have less SO2 to control wild microorganisms.
Anthocyanins
Before fermentation, the mean total anthocyanin content of the must from control grapes was 72.3 mg/L, and 62.8–124.8 mg/L for that was from the e-beam-treated grapes. Irradiation led to a dose-dependent increase in the extraction of these compounds (Fig. 3a). The differences between the control and 0.5 and 1 kGy treatments were not significant, but the 10 kGy dose increased the total anthocyanin extraction more than 70 % compared to the controls. A similar trend was seen for acylated anthocyanins (acetylated and cinnamoyl derivatives) with the 10 kGy treatment, leading to significantly larger extractions. The anthocyanin content of the final control wines was slightly but significantly higher (p < 0.05) than in any wine made from irradiated grapes, a consequence of radiation-associated oxidation (Fig. 3b). This agrees with that reported by Ayed et al. (1999) and Alothman et al. (2009). Pyranoanthocyanins (vitisins and vinylphenol adducts) showed a more stable behaviour, with similar contents seen in wines made from both control and irradiated grapes (p < 0.05; Fig. 3b). Perhaps, the double heteroaromatic ring of these compounds is more stable under the oxidative conditions of irradiation.
Anthocyanin content (mg/L) of total anthocyanins, anthocyanin-3-O-glucosides (a3G), acetylated derivatives (a3GAc), cinnamoyl derivatives (a3GCin), vinylphenol pyranoanthocyanins (A3GVPh) and vitisins (Vit) a in the crushed grape must after irradiation and b in wines. Values are the means ± standard deviations of four replicates. Different letters in the same series indicate significant differences between means (p < 0.05)
Colour and Total Polyphenol Index
The TPI was significantly higher for musts from grapes irradiated at 10 kGy than that for those of the control and low radiation dose-treated grapes, showing a trend similar to that seen for total anthocyanins (p < 0.05; Fig. 4a). Colour intensity was similar in the musts of control and irradiated grapes. The hue of a must indicates its degree of oxidation, and the value for the must from the 10 kGy-irradiated grapes was lower than that of the control grapes.
The colour intensity and TPI of the wines, however, showed no significant differences (Fig. 4b). Irradiation did not, therefore, affect the final wine colour or phenolic compound content.
Volatile Compounds
Most of the fermentative volatile compounds (carbonyl compounds, higher alcohols and esters) were at similar concentrations in the wines made from control and irradiated grapes (Table 3). E-beam radiation does not, therefore, affect the aromatic profile afforded by the yeasts during fermentation. However, the concentration of certain compounds, such as methanol, was significantly higher (p < 0.05) in wines made from grapes treated at 10 kGy than that in those made from control or low-dose (0.5 and 1 kGy) irradiated grapes. During maceration, methanol is extracted from the methoxylated acids of the pectin. Normally, its concentration increases during the making of red wines due to mechanical effects (punch downs and pump overs), but in this work, this was likely related to the softening of the cell walls seen at the high radiation dose (10 kGy). Comparable effects have been observed during grape pressurisation (Morata et al. 2015). Modifications to the degree of pectin esterification following radiation treatment have also been reported (Munarin et al. 2013). However, the concentration reached in the 10 kGy treatment is below of the maximum acceptable limit of 400 mg/L for red wines (EC Regulation 1999).
Amino Acids
The radiation treatment slightly but dose dependently increased the release of amino acids in the wine, especially of alanine and arginine (Table 4). However, the differences in the concentration of total amino acids between wines made from control and irradiated grapes were insufficient to affect the formation of biogenic amines (Anli and Bayram 2009).
Sensory Analysis
Figure 5 shows the panel which recorded a more fruity profile in the wines produced from the irradiated grapes (p < 0.05). They also detected lower reductive notes in these wines than in those made from control grapes (p < 0.05). The greater extraction of phenolic compounds from grapes irradiated at 10 kGy was not perceived by the tasters as an increase in either astringency or bitterness or tannicity.
Conclusions
E-beam irradiation can be used for the cold pasteurisation of grapes; this removes wild yeast and bacteria very effectively without affecting the sensory quality of the final wine. Such treatment could help in the use of non-competitive yeasts and bacteria in new winemaking biotechnologies, such as mixed and sequential fermentations with non-Saccharomyces yeasts. It could also possibly reduce the microbial risks involved in co-inoculations of yeast and bacteria designed to achieve simultaneous alcoholic and malolactic fermentations.
References
Abalos, D., Vejarano, R., Morata, A., González, C., & Suárez-Lepe, J. A. (2011). The use of furfural as a metabolic inhibitor for reducing the alcohol content of model wines. European Food Research and Technology, 232, 663–669.
Alothman, M., Bhat, R., & Karim, A. A. (2009). Effects of radiation processing on phytochemicals and antioxidants in plant produce. Trends in Food Science & Technology, 20, 201–212.
Anli, R. E., & Bayram, M. (2009). Biogenic amines in wines. Food Reviews International, 25, 86–102.
Ayed, N., Yu, H. L., & Lacroix, M. (1999). Improvement of anthocyanin yield and shelf-life extension of grape pomace by gamma irradiation. Food Research International, 32, 539–543.
D’Innocenzo, M., & Lajolo, F. M. (2001). Effect of gamma irradiation on softening changes and enzyme activities during ripening of papaya fruit. Journal of Food Biochemistry, 25, 425–438.
EC Regulation. (1999). Code of oenological practices and processes. Commission Regulation (EC) No 1493/1999 and No 423/2008.
Farkas, J., & Mohácsi-Farkas, C. (2011). History and future of food irradiation. Trends in Food Science & Technology, 22, 121–126.
Farkas, J., Ehlermann, D. A. E., & Mohácsi-Farkas, C. (2014). Food technologies: food irradiation. Encyclopedia of Food Safety, 3, 178–186.
Fugelsang, K.C. (1989). Recent research on sulfur dioxide. Wines & Vines, August.
Glories, Y. (1984a). La couleur des vins rouges I. Connaissance de la vigne et du vin, 18, 195–217.
Glories, Y. (1984b). La couleur des vins rouges II. Connaissance de la vigne et du vin, 18, 253–271.
Guerrero, R. F., & Cantos-Villar, E. (2014). Demonstrating the efficiency of sulphur dioxide replacements in wine: a parameter review. Trends in Food Science & Technology, 42, 27–43.
Kong, Q., Wu, A., Qi, W., Qi, R., Carter, J. M., Rasooly, R., & He, X. (2014). Effects of electron-beam irradiation on blueberries inoculated with Escherichia coli and their nutritional quality and shelf life. Postharvest Biology and Technology, 95, 28–35.
Korkmaz, M., Polat, M. (2005). Irradiation of fresh fruit and vegetables. In: Jongen W (Ed.) Improving the safety of fresh fruit and vegetables. Woodhead Publishing Series in Food Science, Technology and Nutrition, 13, pp. 387–428
Loira, I., Vejarano, R., Morata, A., Ricardo-da-Silva, J. M., Laureano, O., González, C., & Suárez-Lepe, J. A. (2013). Effect of Saccharomyces strains on the quality of red wines aged on lees. Food Chemistry, 139, 1044–1051.
Loira, I., Vejarano, R., Bañuelos, M. A., Morata, A., Tesfaye, W., Uthurry, C., Villa, A., Cintora, I., & Suárez-Lepe, J. A. (2014). Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. LWT–Food Science and Technology, 59, 915–922.
Morata, A., Benito, S., Loira, I., Palomero, F., González, M. C., & Suárez-Lepe, J. A. (2012). Formation of pyranoanthocyanins by Schizosaccharomyces pombe during the fermentation of red must. International Journal of Food Microbiology, 159, 47–53.
Morata, A., Loira, I., Vejarano, R., Bañuelos, M. A., Sanz, P. D., Otero, L., & Suárez-Lepe, J. A. (2015). Grape processing by high hydrostatic pressure: effect on microbial populations, phenol extraction and wine quality. Food and Bioprocess Technology, 8, 277–286.
Moreno, M. A., Castell-Perez, M. E., Gomes, C., Da Silva, P. F., & Moreira, R. G. (2007). Quality of electron beam irradiation of blueberries (Vaccinium corymbosum L.) at medium dose levels (1.0–3.2 kGy). LWT–Food Science and Technology, 40, 1123–1132.
Munarin, F., Bozzini, S., Visai, L., Tanzi, M. C., & Petrini, P. (2013). Sterilization treatments on polysaccharides: effects and side effects on pectin. Food Hydrocolloids, 31, 74–84.
Niemira, B.A. (2003). Irradiation of fresh and minimally processed fruits and vegetables. In: J.S. Novak, G.M. Sapers, V.K. Juneja (Eds.) Microbial safety of minimally processed foods, chapter 13. CRC, pp. 279–299.
Palekar, M. P., Cabrera-Diaz, E., Kalbasi-Ashtari, A., Maxim, J. E., Miller, R. K., Cisneros-Zevallos, L., & Castillo, A. (2004). Effect of electron beam irradiation on the bacterial load and sensorial quality of sliced cantaloupe. Journal of Food Science, 69, M267–M273.
Suárez-Lepe, J. A., & Morata, A. (2012). New trends in yeast selection for winemaking. Trends in Food Science & Technology, 23, 39–50.
Yu, L., Reitmeier, C. A., Gleason, M. L., Nonnecke, G. R., Olson, D. G., & Gladon, R. J. (1995). Quality of electron beam irradiated strawberries. Journal of Food Science, 60, 1084–1087.
Zhao, M., Moy, J., & Paul, R. E. (1996). Effect of gamma-irradiation on ripening papaya pectin. Postharvest Biology and Technology, 8, 209–222.
Acknowledgments
This work was funded by the Ministerio de Economía y Competitividad (AGL2013-40503-R). J. A. Sánchez (Departamento de Química y Tecnología de Alimentos) and P. Santos and B. Diez de Tejada (Departamento de Biotecnología-Biología Vegetal) are thanked for providing excellent technical assistance. S. Toledo (Ionisos, Tarancon, Spain) is thanked for assistance in grape irradiation. The authors are very grateful to the Estación Enológica de Haro Laboratory for the amino acid analyses, especially to Montserrat Iñiguez and Elena Melendez.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morata, A., Bañuelos, M.A., Tesfaye, W. et al. Electron Beam Irradiation of Wine Grapes: Effect on Microbial Populations, Phenol Extraction and Wine Quality. Food Bioprocess Technol 8, 1845–1853 (2015). https://doi.org/10.1007/s11947-015-1540-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1540-x