Abstract
Wine grape pomace, a by-product known for its high polyphenolic content, was used as a source of anthocyanins in the acquisition of active biodegradable films, which were produced with anthocyanins encapsulated with different wall materials endowed with film-forming properties. Gum arabic and maltodextrin were used in different proportions during the encapsulation process, and the anthocyanin contents of the powders obtained were quantified by high-efficiency liquid chromatography (HPLC), resulting in up to 91.5 % retention. The antioxidant properties, solubility, and color stability of powders were analyzed. Because the microcapsules were added in the highest proportion, it was considered the major component in the formation of films. Microcapsules produced with gum arabic showed higher antioxidant activity as compared to microcapsules produced with maltodextrin. Nevertheless, based on a comparison of the films obtained in this study, the films made with anthocyanins encapsulated with maltodextrin showed better mechanical properties and higher protective effect on oil oxidation, which makes maltodextrin a promising material enabling the formation of films with satisfactory mechanical properties endowed with anthocyanins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthocyanins are one of the largest and most widespread groups of plant constituents, known as flavonoids, and are responsible for the bright attractive colors of most fruits, vegetables, flowers, and some cereal grains. In addition to its coloring properties, anthocyanins are known for their possible health benefits as dietary antioxidants, which help to prevent neuronal diseases, cardiovascular illnesses, cancer, diabetes, inflammation, and many other diseases (Yousuf et al. 2015).
The high antioxidant activity of anthocyanins is generally attributed to their peculiar structure, which allows the easy donation of H atoms from aromatic hydroxyl groups, thus improving the capacity to bear the impaired electron via conjugation with the π-electron system (Tsuda et al. 1996). Hence, anthocyanins have been described as effective scavengers of reactive oxygen species, thus avoiding the propagation of new free radical species, which catalyze lipid oxidation and may cause damage in several systems (Van Acker et al. 1996).
Wine grape pomace (WGP), which is contained in skins, seeds, and the remainder of the pulp, is rich in many bioactive compounds that are left after the winemaking process, including a considerable amount of anthocyanins (Fontana et al. 2013). While grape seed is rich in extractable phenolic antioxidants such as phenolic acid, flavonoids, procyanidins, and resveratrol, grape skins contain abundant anthocyanins, compounds that make WGP a by-product of high interest in the pursuit of bioactive compounds (Melo et al. 2015).
The incorporation of anthocyanins into food and medical products is a challenging task due to their low stability toward environmental conditions during processing and storage; however, these compounds can endow antioxidant properties to the products in which they are added. Encapsulation is an efficient way to introduce such compounds into these products. Wall materials, also called encapsulating agents, act as a protective coating against ambient adverse conditions such as light, humidity, and oxygen. Encapsulated bioactive compounds are easier to handle and offer improved stability (Yousuf et al. 2015).
Methods of encapsulation of food ingredients include spray-drying, freeze-drying, fluidized bed coating, extrusion, cocrystallization, molecular inclusion, and coacervation (Shahidi and Han 1993). Freeze drying, despite of its long dehydration period, is an efficient method for anthocyanin encapsulation to produce a porous, nonshrunken structure especially useful for temperature-sensitive active agents like anthocyanins (Zuidam and Shimoni 2010; Mahdavi et al. 2014.
Wall materials used in microencapsulation include gums, polysaccharides, lipids, proteins, fibers, and mixtures of these materials (Davidov-Pardo et al. 2013). The selection of an adequate wall material is important for the stability of the compounds, and several authors have shown that the effective usage of maltodextrin, gum arabic, and the combination of both can provide the formation of more homogeneous particles to encapsulate anthocyanins (Idham et al. 2012; Souza et al. 2015; Silva et al. 2014).
Gum arabic, an exudate from Acacia trees, is principally a mixture of polysaccharides and proteoglycans, the latter being arabinogalactan proteins (Gharsallaoui et al. 2007). Maltodextrins are formed by partially hydrolyzing corn flour with acids or enzymes, and they are supplied as dextrose equivalents (DE), a measure of the degree of starch polymer hydrolysis, where maltodextrins with degrees of DE between 10 and 20 are widely used in the encapsulation of anthocyanins (Madene et al. 2006). Both materials are commonly used as wall materials due to favorable properties of emulsification, water solubility, low viscosity at high concentrations, biodegradability, and film formation (Gharsallaoui et al. 2007; Silva et al. 2014).
Considering the properties of encapsulation and film formation of gum arabic and maltodextrin, it is possible and convenient to produce active biodegradable films with bioactive compounds microencapsulated with these materials. Active films can be described as auxiliary systems for food preservation, where substances are released from the film to the food surface with which they are in contact (Bodaghi et al. 2013).
To minimize the consumption of conservative chemical additives and, therefore, the adverse health effects, the technology of active packaging films with natural antioxidants presents an alternative (Júnior et al. 2015). Several natural antioxidants have been incorporated into films in order to promote greater stability to lipid oxidation of fatty products and to extend the shelf life of highly perishable products (Gomez-Estaca et al. 2014).
Cassava starch, which is known to form odorless, tasteless, colorless, nontoxic, and biologically degradable films (Flores et al. 2007), was selected to produce films with anthocyanins encapsulated with different wall materials. The objective of this study was to analyze the influence of wall materials, gum arabic, and maltodextrin, on the microencapsulation of anthocyanins extracted from WGP and their effect on active biodegradable film properties.
Materials and Methods
The Cabernet Sauvignon grape pomace (79.98 ± 0.40 % moisture; 0.15 ± 0.00 % acidity in tartaric acid; pH 3.41 ± 0.02; total soluble solids 6.35 ± 0.01°Brix; 1.18 ± 0.04 % ash; 2013–2014 vintage) derived from the wine-making process was provided by Vanmarino Winery (Pinto Bandeira, RS). The seeds were manually removed, and the skins and the remainder of the pulp were stored at −18 °C until the time of analysis.
Preparation of Grape Pomace Extracts
The anthocyanin extraction followed the procedure of Srivastava and Vankar (2010) with modifications. The food-grade anthocyanin extraction was performed using a hydroalcoholic solvent composed of 70 % ethanol, acidified with HCl (0.1 %), in a proportion of 1:80 (grape pomace/solvent). The extraction occurred in two stages of 1 h each in a water bath (40 ° C) under stirring and protected from light.
Microcapsule Formation
The freeze-drying process (Freeze Dryer Liotop, L101, Brazil) was used to microencapsulate the extracted compounds. Extracts were concentrated in a Fisatom rotaevaporator (M802, Brazil) at 40 °C until the alcoholic fraction was completely removed and were then re-diluted in acidified water (0.1 % HCl) until achieving one tenth of the original extract volume. Maltodextrin DE 20 (Vallen, Brazil) and gum arabic (Dinamica, Brazil) were used as wall materials, where solutions were prepared at a final concentration of 30 % (w/v) prior to the addition of the pomace extracts (Silva et al. 2013). Three types of microencapsulated powders (MPs) were obtained, differing from each other by the wall material solutions utilized: (i) 30 % maltodextrin (MD), (ii) a mixture of 15 % gum arabic and 15 % maltodextrin (GA/MD), and (iii) 30 % gum arabic (GA). The samples were freeze-dried for 72 h under protection from light, manually macerated, and sieved (mesh 35). The freeze-drying of pure wall material solutions was performed under the same conditions to obtain empty capsules (empty MP). The powders were stored under vacuum in a nylon multilayer packaging under protection from light.
Anthocyanin Content
To identify and quantify the anthocyanins present in the pomace extract, the extract was dried in nitrogen and stored at −18 ° C under protection from light until the time of chromatographic analysis, when it was re-diluted in acidified methanol (HCl 0.1 %). The anthocyanin retention (AR) of the MP was measured by extraction with acidified methanol/water (50:50), followed by centrifugation (3500×g, 10 min). High-efficiency liquid chromatography (HPLC) was used to quantify the anthocyanins, and the AR was represented as the percentage of anthocyanins retained in the powder when compared to the extract. The sum of individually assessed anthocyanins was considered as the total anthocyanins (AT).
Chromatographic Conditions
Anthocyanin quantification was performed via HPLC. For this analysis, an HPLC chromatograph (Agilent 1100 Series, Santa Clara, CA, USA) equipped with a quaternary pump system solvent and a UV–visible detector was used with a C18 Shim-Pak CLC-ODS column (5 μm, 250 × 4.6 mm). The mobile phase consisted of a linear gradient elution of 5 % aqueous formic acid/methanol 85:15 (v/v) to 20:80 over 25 min, and this isocratic ratio was maintained for 15 min. The mobile phase flow was 0.8 mL/min, the injection volume was 5 μL, and the column temperature was maintained at 29 °C. The chromatograms were processed at a fixed wavelength of 520 nm.
This analysis identified and quantified the anthocyanins most often found in Vitis vinifera grapes (Fraige et al. 2014), of which standards were purchased from Sigma-Aldrich (USA): malvidin-3-glucoside (CAS 7228-78-6, ≥90.0 %), delphinidin-3-glucoside (CAS 6906-38-3, ≥97.0 %), petunidin-3-glucoside (CAS 6988-81-4, ≥95.0 %), and cyanidin-3-glucoside (CAS 7084-24-4, ≥95.0 %). The identification and quantification of compounds were performed by comparing retention times and peak areas of the samples and their respective standards under the same chromatographic conditions. For quantification, a standard curve was constructed in the following concentration ranges for the anthocyanins: malvidin-3-glucoside, 5–50 mg/mL; delphinidin-3-glucoside, 5–100 mg/mL; petunidin-3-glucoside, 3–40 mg/mL; and cyanidin-3-glucoside, 5–40 mg/mL.
The limits of detection (LOD) and quantification (LOQ) were as follows: malvidin-3-glucoside, 2.07 × 10−2 and 3.45 × 10−2 μg/mL; delphinidin-3-glucoside, 1.16 × 10−1 and 1.93 × 10−1 μg/mL; petunidin-3-glucoside, 0.66 × 10−2 and 1.1 × 10−2 μg/mL; and cyanidin-3-glucoside, 1.69 × 10−2 and 2.81 × 10−2 μg/mL.
Microencapsulated Powder Characterization
Microencapsulation Efficiency
The microencapsulation efficiency (ME) was defined as the difference between the AR and the anthocyanins located in the microcapsule surface (AS). To quantify the AS, the procedure of Saénz et al. (2009) was followed: 200 mg of each MP was treated with 1 mL of a mixture of ethanol and methanol (1:1). These dispersions were stirred in a Vortex at room temperature for 1 min and then centrifuged (3500×g, 10 min). The anthocyanins were quantified by HPLC. The ME percentage was calculated according to Eq. (1). All analyses were performed in triplicate.
2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid Radical Scavenging Activity
The 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) scavenging capacity of the MP samples was determined according to a modified method of Re et al. (1999). ABTS+· radical cations were produced by reacting 5 mL of ABTS (7 mM) stock solution with 88 μL of potassium persulfate (140 mM) and allowing the mixture to stand in the dark at room temperature for 16 h before use.
To analyze the antioxidant activity of the pomace extract, the ABTS+ solution was diluted with ethanol to an absorbance of 0.700 ± 0.050 at 734 nm (Spectrophotometer Shimadzu CPS-240A, Japan). After the addition of 30 μL of sample or trolox standard to 3 mL of diluted ABTS·+ solution, the absorbance values were recorded 6 min after mixing.
The antioxidant activity of the MP and the empty MP in aqueous solution was analyzed. The MPs were solubilized in acidified water, sonicated for 10 min, and centrifuged (3000×g, 10 min; Sigma Centrifugal 4K15, Germany), and the supernatant was collected and analyzed. The ABTS assay was measured under the same conditions described for the grape pomace extract, but the ethanol was substituted for an aqueous buffer solution, pH 3.
Solutions of known trolox concentrations were used for calibration, and the results were expressed as 1 M trolox/g dry grape pomace and 1 M trolox/g MP for the grape pomace extract and MP, respectively.
Moisture and Water Solubility Index
The moisture content was determined using the AOAC method (2006), where mass loss was determined after 1 g of MP was placed in an oven dryer at 105 °C for 3 h. The water solubility index (WSI) was determined according to the procedure of Anderson (1982) with modifications. MP (2.5 g) and water (30 mL) were vigorously mixed in a centrifuge tube, incubated in a 25 °C water bath for 30 min, and then centrifuged (3500×g, 15 min). The supernatant was collected in a preweighed Petri dish, and the residue was weighed after oven-drying overnight at 105 °C (DeLeo DL-SE-X, Brazil). The amount of solids in the dried supernatant as a percentage of the total dry solids in the original 2.5 g sample was an indicator of WSI.
Color Stability
The MPs were stored at 25 °C under vacuum in a nylon multilayer packaging that was protected from light. MP color was analyzed on the day of acquisition (day 0) and after 21 and 42 days, in triplicate. The color was measured using a colorimeter (Hunter Lab system, model Miniscan XE, USA) using the CIELab color parameters. The parameters L*(luminosity), a* (red–green), and b* (yellow–blue) were determined. A white disk (L 0*, 94.97; a 0*, 0.12; and b 0*, 1.7) was used as a standard.
Film Formation
The films were produced by a casting technique in which three films were developed with the MP: with GA powder (F.GA), MD powder (F.MD), and GA/MD powder (F.GA/MD). Film-forming solutions were prepared with a suspension of cassava starch (Stival, Brazil) and glycerol (Dinamica, Brazil). The solutions were kept at 80 °C for 5 min with constant stirring in a water bath (De Leo B450) to promote starch gelatinization. The gelatinized solution was cooled to 40 °C, and then, the MPs were added.
Films were prepared by weighing an amount of film solution that provided 0.02 g/cm2 of solids (which comprise starch and MP) on an acrylic plate resting on a leveled surface. A fixed proportion of starch/MP was utilized (0.625:1). Preliminary tests made with different concentrations of glycerol indicated that formulations containing GA required extra glycerol; otherwise, the films would easily break when peeled from the plates. This explains the different proportions of glycerol used in each formulation (25.2, 22, and 15.4 % in F.GA, F.GA/MD, and F.MD, respectively) relative to the solids. The films were dried in an oven with forced air circulation (DeLeo B5AFD) at 40 °C for 7 h and conditioned at 25 °C and 58 % relative humidity (RH) in desiccators for 24 h prior to analysis, when they were peeled off the plates.
Film Characterization
Mechanical Properties
Before analysis, the films were stored for 48 h under conditions of controlled moisture (58 % RH) and temperature (25 °C). The films were cut into strips (70–25 mm), and the strip thickness was measured using a micrometer (MDC-25, Mitutoyo Corp, Japan, precision 0.001 mm, resolution/0~25 mm) at five random positions on each strip. A texture analyzer (TA.XT2i e Stable Micro Systems, UK) with a load cell of 5 kg and an A/TGT self-tightening roller grips fixture was used to evaluate the percentage elongation at break (E) and the tensile strength at break (TS). These parameters were determined from stress–strain curves obtained from uniaxial tensile tests to film failure and were calculated according to the ASTM D882 standard method. The strips were mounted individually between the grips of the equipment with an initial grip separation of 50 mm and a test speed of 0.8 mm · s−1. At least ten replications of each test sample were run.
Water Vapor Permeability
Water vapor permeability (WVP) was determined gravimetrically according to the method described by Talja et al. (2008) with some modifications. The samples were placed in permeation cells (inner diameter 63 mm, height 25 mm) that were filled with anhydrous CaCl2 and covered with the sample; the cells were then hermetically sealed. The permeation cells were placed in a glass chamber with saturated sodium chloride solution, providing RH gradients of 0/74 % at 25 °C. The mass gain of the cell permeation was determined by weighing the cell on an analytical balance (AY 220, Shimadzu) after 24 h. The WVP of the samples was determined in triplicate using Eq. 2.
where “w” is the weight of water permeated through the film (g), “L” is the thickness of the film (m), “A” is the permeation area (m2), T is the time of permeation (s), and “Δp” is the water vapor pressure difference (at 25 °C) between the two sides of the film (Pa).
Opacity
The opacity of the films was determined by measuring their absorbance spectra at 600 nm using a UV spectrophotometer (Shimadzu UV-1800). The films were cut into rectangle pieces and directly placed in a spectrophotometer test cell. An empty quartz test cell was used as the reference. The opacity of the films was calculated, dividing the values of absorbance by the thickness of the film (mm) (Wang et al. 2013).
Effect of Films on Retardation of Sunflower Oil Oxidation
The films were wrapped to form bags (100 mm × 75 mm) to which 15 mL of sunflower oil was added, and the films were sealed and placed into a camera. Experiments were performed for 9 days under the following conditions: 40 °C, exposure to fluorescent lights with an intensity of 900–1000 lux, and a RH of 54 %, which results in an accelerated environment of degradation. The peroxide value (PV) of the samples was determined according to the IUPAC method on days 0, 3, 6, and 9. An open glass Petri dish containing sunflower oil was used as control. The samples were evaluated in triplicate.
Films and MP Surface Characteristics
The surface morphology of MP and films was observed under a scanning electron microscope (SEM) (Model JSM 6060, JEOL). The samples were fixed on aluminum stubs, coated with gold, and scanned with an accelerating voltage of 5.0 kV and a magnification of 500×.
Statistical Analysis
The results were evaluated by analysis of variance (ANOVA) and a Tukey test at significance level of 5 % (p < 0.05) using the software Statistica 7.1. (STATSOFT Inc.), corresponding to the average of three replicates (n = 3) ± standard deviation.
Results and Discussion
Anthocyanin Retention and Microencapsulation Efficiency
Comparing the amount of individual anthocyanins from the pomace extract to the MP, the freeze-drying process showed no significant reduction for most of the analyzed compounds, where malvidin-3-glucoside was the major anthocyanin in the extract (4601 mg / kg db), showing a retention between 92.16 and 97.78 %. The AR of ATs when GA and MD were used as wall materials was 91.49 %, and no significant difference was observed compared to the other powders (Table 1). These results show similar ARs compared to studies in which the encapsulation of anthocyanins was performed by spray dryer: Tonon et al. (2008) obtained ARs of approximately 77–86 % from açaí (Euterpe oleracea) juice, Silva et al. (2013) obtained ARs of 79–100 % from jaboticaba (Myrciaria cauliflora) extracts, and Souza et al. (2015) obtained ARs of 88–97 % from Bordo grape (V. vinifera) wine pomace.
Microencapsulation efficiency (ME) refers to the potential of the wall material to encapsulate or hold the core material inside the microcapsule. Encapsulation efficiencies are also related to the shelf life of the anthocyanin content in the powder (Idham et al. 2012). In this study, the usage of different wall materials did not cause differences in the AR and ME, where the three formulations presented a high percentage (above 88 %) of ME, indicating that the largest portion of the anthocyanins is located inside the core of the capsules, while the minority is on its surface. Many authors have studied the usage of MD and GA as wall materials to encapsulate anthocyanins (Idham et al. 2012; Souza et al. 2015; Silva et al. 2013), and there is no conclusive information about the best materials to optimize the AR and ME of anthocyanins. The concomitant usage of these materials was more frequently reported as one of best choices, although the disagreement of results found in many studies demonstrates the complexity that involves the encapsulation of these compounds.
2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid, Solubility, and Moisture of the Microencapsulated Powders
The GA powder showed the highest antioxidant activity (Table 2), followed by GA/MD and MD powders. Because Rodrigues et al. (2012) suggested antioxidant properties of GA, the present study analyzed the antioxidant activity of empty MP. However, no significant difference between the wall materials was verified concerning this property (2.2 ± 0.1, 2.0 ± 0.2, and 1.8 ± 0.1 Trolox equivalents per gram of empty GA, empty MD, and empty GA/MD, respectively).
Considering that the powders showed similar AR and ME and that the wall materials utilized presented the same antioxidant activity, no difference between the antioxidant properties of the powders was expected. However, GA presented higher antioxidant activity than the other powders. Once the ABTS was measured in water, the solubility of the powder in water became an important parameter, as the release of encapsulated anthocyanins is highly dependent on the dissolution of the wall material (Berg et al. 2012). Because GA and GA/MD presented higher solubility in water (Table 2), it is possible to suggest that higher amounts of anthocyanins were released in water when these materials were used as wall materials, promoting a higher antioxidant activity of the capsules in the aqueous environment.
Color Stability of Powders
During 42 days of storage (25 °C, packaged under vacuum and protection from light), the most affected parameter was a*, where positive values are an indication of the red tonality (Table 3). The powders all presented a high value of a*, which can be attributed to a high anthocyanin content (Jiménez-Aguilar et al. 2011). This parameter remained stable during 21 days to all MP, suggesting that the encapsulation with GA, MD, and GA/MD promoted the stability of anthocyanins during this period. The three formulas of MP analyzed showed a reduction of this parameter after 42 days, which demonstrates a loss of red color related to the anthocyanin degradation. The decrease in the parameter a* was also reported by Idham et al. (2012), who evaluated the color stability of anthocyanins encapsulated with GA, MD, and starch during storage at 4, 25, and 37 °C in the absence of light, and Souza et al. (2015), who evaluated color stability of anthocyanins encapsulated with different concentrations of MD during storage at 25 °C, 38 % RU, without protection from light.
Except for some fluctuations, an increase in lightness (L*) was observed in all powders. The increase of parameter L* during the storage of encapsulated anthocyanins has been found in other studies, where it is interpreted as an indication of discoloration due to degradation of anthocyanins (Jiménez-Aguilar et al. 2011).
Only MD suffered a reduction in parameter b* (a decrease in b* is related to the loss of blue), while the other powders presented oscillating values of this parameter. According to Schwartz et al. (2010), malvidin-3-glucosideo, the major anthocyanin found in this study, is the anthocyanin contributing the most to the blue color; hence, the decrease in b* would indicate its degradation (Table 3).
Film Characterization
The film properties are presented in Table 4, where the three formulations presented the same measure of thickness. The film formed with MD (F.MD) presented the lowest opacity, indicating a higher transparency of films and, thus, a less protective barrier against light.
One of the main functions of a film for food packaging is often to impede moisture transferring between food and the surrounding atmosphere, so the WVP of the film should be as low as possible (Wang et al. 2015). Comparing the films developed in this study, F.MD presented the lowest WVP, which also had lower moisture (Table 4). The rate of migration of water molecules in a biopolymer film decreases with a decrease in the size of the biopolymers in a film matrix due to an increase in the tortuosity of the pathway of water molecules (Kim et al. 2012). This resulted in a decrease of WVP in the film made with MD (F.MD) because MD DE 20 presents smaller particle size than GA. Additionally, the lower WVP and moisture can be attributed to the lower percentage of glycerol used during F.MD formulation, which was 15.4 % (related to solids), compared to 25.2 % in F.GA and 22 % in F.GA/MD. The presence of glycerol, a hydrophilic plasticizer, favors water molecule adsorption (Yoshida et al. 2003), thus provoking a higher WVP on the formulations containing higher concentrations of this component (Müller et al. 2008). The WVP results found on the present study were lower than cassava starch-based films (40 g m−1 s−1 Pa−1 × 10−11) and starch–polyethylene films (120 to 220 g m−1 s−1 Pa−1 × 10−11) and comparable to cassava starch-based film with bentonite nanoparticles (8.3 40 g m−1 s−1 Pa−1 × 10−11) (Jiménez et al. 2012).
In the present study, the film formulation with better resistance (higher TS) was obtained when MD powders were used (F.MD); thus, it is possible to presume that the usage of GA has weakened other formulations (F.GA and F.GA/MD) as can be observed at Table 4. The high addition of GA in films has been reported to reduce the intermolecular force between the chains of adjacent macromolecules and, consequently, to reduce the tensile strength (LI et al. 2015). Additionally, the presence of glycerol is related to a reduction in tensile strength due to the decrease in intermolecular interactions between film matrix molecules, which can explain the lower tensile strength from the formulations F.GA and F.GA/MD (Table 4). These results are similar to those from the study of Souza et al. (2012), where the addition of glycerol caused a decrease in TS in cassava starch films.
Considering that mechanical properties may vary with specimen thickness, method of preparation, speed of testing, type of grips used, and manner of measuring extension (Souza et al. 2012), it is difficult to compare these properties with literature data. Despite these difficulties, F.MD resistance is comparable with those reported in literature for biodegradable films, whereas F.GA and F.GA/MD present low TS results.
Elongation (%) represents the maximum change in length of the test specimen before breaking, thus reflecting the ability of films to strain and adapt into packaged products. The formulations containing MD (F.MD and F.GA/MD) presented outstanding elongation ability (higher than 100 %) when compared with other biodegradable films. This property can be attributed to the plasticization effect caused by the higher mobility promoted by the relatively low molecular weight of MDs (Embuscado and Huber 2009) combined with the usage of glycerol.
Effect of Films on Retardation of Sunflower Oil Oxidation (Peroxide Value)
The peroxide index (PV) was used to monitor the effect of the films on the oxidation of sunflower oil. Figure 1 shows the evolution of this index for the different samples over time. Once all samples exceeded the limit of 10 meq peroxides/kg (maximum value permitted by Brazilian legislation to oil commercialization), the test was suspended.
Effect of films with anthocyanins encapsulated with maltodextrin (F.MD), gum arabic (F.GA), and gum arabic/maltodextrin (F.GA/MD) on sunflower oil oxidation at 40 °C, 54 % RH, and 950 ± 50 lux of luminosity, measured by peroxide formation over time. An open glass Petri dish containingsunflower oil was used as control
Edible films and coatings that include antioxidant agents in their formulation protect food both by the antioxidant effect of compounds and by the air barrier that they represent, which results in a better preservation of quality (Bonilla et al. 2012). Because oils and fats are easily broken down during storage, the PV tends to increase during early stages of oxidation when the formation rate of hydroperoxides is higher than the rate of decomposition (Pereira de Abreu et al. 2010). The control sample, which was not coated and thus was directly exposed to the air and light, reached 65.8 meq peroxides/kg after 3 days of storage, while the samples coated with films presented lower values (28.7, 18.5, and 4.7 meq peroxides/kg to F.GA, F.GA/MD, and F.MD, respectively). Regarding the established PV limit, only oil samples protected with F.MA were appropriate to consume after 3 days, being under the limit until the sixth day of accelerated storage environment. Considering the protective effect of films on sunflower oil, F.MD showed the best results, where it reduced the rate of the primary oxidation process and therefore kept the oil in appropriate conditions for commercialization for a longer time. Upon exposing sunflower oil to light and heat, the PVs obtained showed that the films actively protected the oil.
A later decrease in PV is expected as a result of the lower substrate availability and the instability of peroxide molecules, which leads to a formation rate lower than that of decomposition (Pereira de Abreu et al. 2010). The increase and subsequent decrease in the PV value observed in F.GA and F.GAMD samples are a consequence of lower substrate availability, which limited formation of new peroxides. During the time analyzed, this behavior was not observed at the control sample because its direct exposure to the air allows an abundant substrate availability that induces a high formation rate of new hydroperoxides.
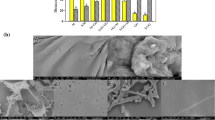
Surface Characteristics Powders and Film
The analysis of the surface of MP particles obtained with different encapsulating agents and the films obtained from them was performed using SEM. The MPs (Fig. 2a–c) showed smooth surfaces and irregular, flake-like structures of different sizes, eventually forming aggregates, which was expected because the powders were freeze-dried and ground in mortar (Gurak et al. 2013). Figure 2b shows that MD was the encapsulating agent that enabled the formation of capsules with fewer wrinkles on their surfaces, which is in accord with the research of Silva et al. (2014). The adherence of small particles to the surface of larger particles was observed in the three treatments, which was also observed in the studies of Cano-Chauca et al. (2005) and Silva et al. (2014).
On the film’s surface (Fig. 2d–f), it is possible to observe that the particles were partially dissolved in the films, where small dispersed fractions can be visualized. Films made with MD (Fig. 2e) enabled the formation of a smoother surface with fewer wrinkles, which can be explained by the lower molecular weight of MD in comparison with GA, thus forming a denser structure. F.GA presented an irregular surface with some disruptions, what helps to clarify its poor mechanical properties.
Conclusions
The wall materials utilized in this study showed effectiveness in encapsulating the anthocyanins extracted from WGP, achieving up to 91.5 % of AR and 89.6 % of ME, where no difference was found between the treatments. A higher antioxidant activity was found in GA powders, which was attributed to its higher solubility in water and consequent liberation of anthocyanins to the aqueous environment. Films prepared with the different microencapsulated powders showed different mechanical properties, and films made with MD powder (F.MD) presented the best results for tensile strength and a high elongation. The F.MD also presented a higher protective effect on peroxide formation in sunflower oil, which makes MD a promising material for the acquisition of films with satisfactory mechanical properties endowed with encapsulated anthocyanins. Further research will be conducted in order to understand the mechanism of release of anthocyanins from films and its activity on reducing the presence of radical oxidative species in food.
References
Anderson, R. A. (1982). Water absorption and solubility and amylograph characteristics of roll-cooked small grain products. Cereal Chemistry (USA), 59, 265–269.
Association of Official Analytical Chemists. (2006). Official methods of analysis (18th ed.). Maryland: AOAC.
Berg, S., Bretz, M., Hubbermann, E. M., & Schwarz, K. (2012). Influence of different pectins on powder characteristics of microencapsulated anthocyanins and their impact on drug retention of shellac coated granulate. Journal of Food Engineering, 108(1), 158–165.
Bodaghi, H., Mostofi, Y., Oromiehie, A., Zamani, Z., Ghanbarzadeh, B., Costa, C., Conte, A., & Del Nobile, M. A. (2013). Evaluation of the photocatalytic antimicrobial effects of a TiO2 nanocomposite food packaging film by in vitro and in vivo tests. LWT - Food Science and Technology, 50(2), 702–706.
Bonilla, J., Atarés, L., Vargas, M., & Chiralt, A. (2012). Edible films and coatings to prevent the detrimental effect of oxygen on food quality: possibilities and limitations. Journal of Food Engineering, 110(2), 208–213.
Cano-Chauca, M., Stringheta, P. C., Ramos, A. M., & Cal-Vidal, J. (2005). Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innovative Food Science & Emerging Technologies, 6(4), 420–428.
Davidov-Pardo, G., Arozarena, I., & Marín-Arroyo, M. (2013). Optimization of a wall material formulation to microencapsulate a grape seed extract using a mixture design of experiments. Food and Bioprocess Technology, 6(4), 941–951.
Embuscado, M. E., & Huber, K. C. (2009). Edible films and coatings for food applications. Springer.
Flores, S., Famá, L., Rojas, A. M., Goyanes, S., & Gerschenson, L. (2007). Physical properties of tapioca-starch edible films: influence of filmmaking and potassium sorbate. Food Research International, 40(2), 257–265.
Fontana, A. R., Antoniolli, A., & Bottini, R. (2013). Grape pomace as a sustainable source of bioactive compounds: extraction, characterization, and biotechnological applications of phenolics. Journal of Agricultural and Food Chemistry, 61(38), 8987–9003.
Fraige, K., Pereira-Filho, E. R., & Carrilho, E. (2014). Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC–DAD–MS and exploratory analysis by principal component analysis. Food Chemistry, 145, 395–403.
Gharsallaoui, A., Roudaut, G., Chambin, O., Voilley, A., & Saurel, R. (2007). Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Research International, 40(9), 1107–1121.
Gomez-Estaca, J., Lopez-de-Dicastillo, C., Hernandez-Munoz, P., Catala, R., & Gavara, R. (2014). Advances in antioxidant active food packaging. Trends in Food Science & Technology, 35(1), 42–51.
Gurak, P. D., Cabral, L. M. C., & Rocha-Leão, M. H. (2013). Production of grape juice powder obtained by freeze-drying after concentration by reverse osmosis. Brazilian Archives of Biology and Technology, 56(6), 1011–1017.
Idham, Z., Muhamad, I. I., & Sarmidi, M. R. (2012). Degradation kinetics and color stability of spray-dried encapsulated anthocyanins from hibiscus sabdariffa L. Journal of Food Process Engineering, 35(4), 522–542.
Jiménez, A., Fabra, M. J., Talens, P., & Chiralt, A. (2012). Edible and biodegradable starch films: a review. Food and Bioprocess Technology, 5(6), 2058–2076.
Jiménez-Aguilar, D. M., Ortega-Regules, A. E., Lozada-Ramírez, J. D., Pérez-Pérez, M. C. I., Vernon-Carter, E. J., & Welti-Chanes, J. (2011). Color and chemical stability of spray-dried blueberry extract using mesquite gum as wall material. Journal of Food Composition and Analysis, 24(6), 889–894.
Júnior, A., Fronza, N., Foralosso, F., Dezen, D., Huber, E., dos Santos, J., Machado, R., & Quadri, M. (2015). Biodegradable duo-functional active film: antioxidant and antimicrobial actions for the conservation of beef. Food and Bioprocess Technology, 8(1), 75–87.
Kim, I.-H., Yang, H.-J., Noh, B.-S., Chung, S.-J., & Min, S. C. (2012). Development of a defatted mustard meal-based composite film and its application to smoked salmon to retard lipid oxidation. Food Chemistry, 133(4), 1501–1509.
Li, C., Zhu, W., Xue, H., Chen, Z., Chen, Y., & Wang, X. (2015). Physical and structural properties of peanut protein isolate-gum Arabic films prepared by various glycation time. Food Hydrocolloids, 43, 322–328.
Madene, A., Jacquot, M., Scher, J., & Desobry, S. (2006). Flavour encapsulation and controlled release—a review. International Journal of Food Science & Technology, 41(1), 1–21.
Mahdavi, S. A., Jafari, S. M., Ghorbani, M., & Assadpoor, E. (2014). Spray-drying microencapsulation of anthocyanins by natural biopolymers: a review. Drying Technology, 32(5), 509–518.
Melo, P. S., Massarioli, A. P., Denny, C., dos Santos, L. F., Franchin, M., Pereira, G. E., Vieira, T. M. F. d. S., Rosalen, P. L., & Alencar, S. M. d. (2015). Winery by-products: extraction optimization, phenolic composition and cytotoxic evaluation to act as a new source of scavenging of reactive oxygen species. Food Chemistry, 181, 160–169.
Müller, C. M. O., Yamashita, F., & Laurindo, J. B. (2008). Evaluation of the effects of glycerol and sorbitol concentration and water activity on the water barrier properties of cassava starch films through a solubility approach. Carbohydrate Polymers, 72(1), 82–87.
Pereira de Abreu, D. A., Losada, P. P., Maroto, J., & Cruz, J. M. (2010). Evaluation of the effectiveness of a new active packaging film containing natural antioxidants (from barley husks) that retard lipid damage in frozen Atlantic salmon (Salmo salar L.). Food Research International, 43(5), 1277–1282.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9–10), 1231–1237.
Rodrigues, E., Mariutti, L. R. B., Faria, A. F., & Mercadante, A. Z. (2012). Microcapsules containing antioxidant molecules as scavengers of reactive oxygen and nitrogen species. Food Chemistry, 134(2), 704–711.
Saénz, C., Tapia, S., Chávez, J., & Robert, P. (2009). Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chemistry, 114(2), 616–622.
Schwartz, S. J., Von Elbee, J. H., & Giusti, M. M. (2010). Corantes. In S. Damodaran, K. L. Parkin, & O. R. Fennema (Eds.), Química de Alimentos de Fennema (4th ed., pp. 445–498). Porto Alegre: Artmed.
Shahidi, F., & Han, X. Q. (1993). Encapsulation of food ingredients. Critical Reviews in Food Science & Nutrition, 33(6), 501–547.
Silva, P. I., Stringheta, P. C., Teófilo, R. F., & de Oliveira, I. R. N. (2013). Parameter optimization for spray-drying microencapsulation of jaboticaba (Myrciaria jaboticaba) peel extracts using simultaneous analysis of responses. Journal of Food Engineering, 117(4), 538–544.
Silva, V. M., Vieira, G. S., & Hubinger, M. D. (2014). Influence of different combinations of wall materials and homogenisation pressure on the microencapsulation of green coffee oil by spray drying. Food Research International, 61, 132–143.
Souza, A. C., Benze, R., Ferrão, E. S., Ditchfield, C., Coelho, A. C. V., & Tadini, C. C. (2012). Cassava starch biodegradable films: influence of glycerol and clay nanoparticles content on tensile and barrier properties and glass transition temperature. LWT - Food Science and Technology, 46(1), 110–117.
Souza, V. B., Thomazini, M., Balieiro, J. C. d. C., & Fávaro-Trindade, C. S. (2015). Effect of spray drying on the physicochemical properties and color stability of the powdered pigment obtained from vinification byproducts of the Bordo grape (Vitis labrusca). Food and Bioproducts Processing, 93, 39–50.
Srivastava, J., & Vankar, P. S. (2010). Canna indica flower: new source of anthocyanins. Plant Physiology and Biochemistry, 48(12), 1015–1019.
Talja, R. A., Helén, H., Roos, Y. H., & Jouppila, K. (2008). Effect of type and content of binary polyol mixtures on physical and mechanical properties of starch-based edible films. Carbohydrate Polymers, 71(2), 269–276.
Tonon, V., Brabet, C., & Hubinger, M. D. (2008). Influence of process conditions on the physicochemical properties of acai (Euterpe oleraceae Mart.) powder produced by spray drying. Journal of Food Engineering, 88(3), 411–418.
Tsuda, T., Shiga, K., Ohshima, K., Kawakishi, S., & Osawa, T. (1996). Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochemical Pharmacology, 52(7), 1033–1039.
Van Acker, S. A. B. E., Van Den Berg, D.-j., Tromp, M. N. J. L., Griffioen, D. H., Van Bennekom, W. P., Van Der Vijgh, W. J. F., & Bast, A. (1996). Structural aspects of antioxidant activity of flavonoids. Free Radical Biology and Medicine, 20(3), 331–342.
Wang, L., Dong, Y., Men, H., Tong, J., & Zhou, J. (2013). Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocolloids, 32(1), 35–41.
Wang, Q., Tian, F., Feng, Z., Fan, X., Pan, Z., & Zhou, J. (2015). Antioxidant activity and physicochemical properties of chitosan films incorporated with Lycium barbarum fruit extract for active food packaging. International Journal of Food Science & Technology, 50(2), 458–464.
Yoshida, C. M. P., Antunes, A. C. B., Antunes, L. J., & Antunes, A. J. (2003). An analysis of water vapour diffusion in whey protein films. International Journal of Food Science & Technology, 38(5), 595–601.
Yousuf, B., Gul, K., Wani, A. A., & Singh, P. (2015). Health benefits of anthocyanins and their encapsulation for potential use in food systems: a review. Critical Reviews in Food Science and Nutrition.
Zuidam, N. J., & Shimoni, E. (2010). Overview of microencapsulates for use in food products or processes and methods to make them. In Encapsulation technologies for active food ingredients and food processing (p. 3–29). Springer: New York.
Acknowledgments
The authors are grateful to CAPES and CNPq for the financial support provided for this research and Electronic Microscope Center (CME) of Federal University of Rio Grande do Sul (UFRGS) for technical assistance. The authors thankfully acknowledge Vanmarino winery, from Rio Grande do Sul, Brazil, for supplying the raw material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stoll, L., Costa, T.M.H., Jablonski, A. et al. Microencapsulation of Anthocyanins with Different Wall Materials and Its Application in Active Biodegradable Films. Food Bioprocess Technol 9, 172–181 (2016). https://doi.org/10.1007/s11947-015-1610-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1610-0