Abstract
The main purpose of this study was to investigate effects of three packaging methods on beef tenderness via protein oxidative modifications. Tenderness, protein oxidation, μ-calpain activation, and protein proteolysis of bovine longissimus dorsi (LD) muscle were measured during 10 days (d) of chilled storage. Compared with beef samples from vacuum packaging (VP), beef samples from air packaging (AP) and modified atmosphere packaging (MAP) showed significantly lower free thiol group and higher carbonyl group content, while tenderness was lower after 7 and 10 d of chilled storage, respectively (p < 0.05). μ-Calpain activity was less in beef samples from AP and MAP compared with VP at d 4 and 7 of postmortem storage (p < 0.05). Desmin showed decreased proteolysis in beef samples from AP and MAP compared with VP after 4 and 7 d of storage (p < 0.05). The results above indicated that the reduction in tenderness of beef samples from AP and MAP compared to VP was closely associated with protein modifications including oxidation, proteolysis, and enzyme activation of bovine LD muscle during postmortem storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air packaging (AP), vacuum packaging (VP), and modified atmosphere packaging (MAP) have been widely used in retail meat market during chilled storage, especially longissimus lumborum, psoas major, and longissimus thoracis are packaged due to its high price. However, different packaging has its advantage and disadvantage. AP can cause quick myoglobin oxidation and gets desirable red color in a short time, but discoloration usually occurs after 6–9 days (d) of storage (Sanz et al. 2011). VP keeps a stable purple color due to deoxymyoglobin formation and increases the shelf life of fresh meat (Jeremiah 2001; Li et al. 2012). However, VP can cause liquid exudation and product deformation in soft meat products (Santos et al. 2005). Fresh beef from MAP with 70–80 % oxygen can maintain desirable and stable cherry red color of fresh meat (José and María 2012; McMillin 2008) and extend the shelf life of fresh beef (Arvanitoyannis and Stratakos 2012; Santos et al. 2005). Nevertheless, it can possibly cause meat discoloration and deteriorates the sensory quality due to protein oxidation and lipid oxidation (Clausen et al. 2009) during chilled storage. Lipid oxidation in meat caused the rapid development of undesirable rancid odors and potential toxic flavor compounds resulting in quality loss and further negatively affected nutritive values and color of fresh meat (Kanner 1994). Protein oxidation in meat caused protein modification and negatively affected texture and tenderness of fresh meat.
As one of the most important quality attributes of fresh meat, beef tenderness greatly affects consumer choices (Li et al. 2010). The major factor affecting meat tenderness is closely related to the degradation of key myofibrillar proteins during postmortem maturation (Taylor et al. 1995; Koohmaraie 1996). The postmortem degradation of myofibrillar structural proteins is a complex process of enzyme in nature (Sentandreu et al. 2002). The calpain proteinase system especially μ-calpain is considered as key enzyme for protein proteolysis which is closely associated with meat tenderization during postmortem aging (Huff-Lonergan and Lonergan 2005; Huff-Lonergan et al. 1996; Zhang et al. 2006). μ-Calpain is known to be cysteine enzyme which is especially sensitive to oxidation environment (Zhang et al. 2013). Protein oxidation is ubiquitous throughout postmortem storage of fresh meat which can modify protein structure and function leading to deteriorated fresh meat quality. The most common consequences of protein oxidation include protein cross-linking, amino acid side chain modification, and the formation of protein fragmentation (Lund et al. 2011). Previous studies have demonstrated that protein oxidation could negatively impact enzyme activity which in turn influences the rate and degree of protein degradation (Lametsch et al. 2008).
Previous researches have shown that MAP and AP can influence the tenderness of meat samples with increased carbonyl concentration and decreased μ-calpain activity compared with VP during chilled storage (Lund et al. 2011; Rowe et al. 2004; Zakrys-Waliwander et al. 2012; Xiao et al. 2011). However, the behind mechanism about how different packaging conditions influence fresh meat quality is still not clear so far. This study aims to evaluate the effects of three different packaging methods on protein oxidative modification of bovine longissimus dorsi (LD) muscle and its subsequent influence on μ-calpain activity and the proteolysis of skeletal key protein during chilled storage in simulated retail conditions.
Materials and Methods
Preparation of Beef Samples
Four 20-month-old Chinese Northeast female yellow cattle with live weight of 235–260 kg were slaughtered humanely on the same day at a commercial slaughter plant in Yurun Food Co. Ltd (Fuyang, Anhui, China) under standard commercial conditions according to the National Standard of China. After the carcass was weighed (the carcass weight was 105–110 kg), LD muscle on each cattle was immediately excised from the left half of carcasses and placed in darkness at 4 °C overnight. Prior to packaging, color and pH (5.62 ± 0.20) of all LD muscle were measured to guarantee that we chose the samples with normal quality.
Packaging of Beef Samples
After 24-h postmortem, 2.54-cm-thick beef samples were trimmed with a stainless steel knife from each LD muscle, and then, all beef samples were mixed and randomly divided into three groups equally. One group was packaged in air-permeable film (10,000 cm3 O2/m2/24 h polyvinyl chloride film). The other two groups of muscle samples were packed under MAP (80 % O2:20 % CO2) or VP (nylon and polyvinyl chloride) conditions, respectively. The beef samples were stored for 4, 7, and 10 d in a dark refrigeration house at 4 °C to mimic commercial storage conditions. At 4, 7, and 10 d, instrumental Warner-Bratzler shear force (WBSF) was determined. After the remaining beef samples were trimmed of external fat and visible connective tissue, around 50-g samples were cut at each time point, packed under vacuum condition, and immediately frozen in liquid nitrogen for at least 30 s and stored at refrigerator of −80 °C for posterior biochemical analysis.
Isolation of Myofibrillar Protein
Myofibrils from LD muscle at 0, 4, 7, and 10 d postmortem were prepared using the method of Park et al. (2006). An aliquot of 5 g of minced frozen muscle was finely chopped and homogenized in 25 mL of cold isolation buffer (2 mM MgCl2, 100 mM NaCl, 1 mM ethylene glycol tetraacetic acid (EGTA), 10 mM phosphate buffer, pH 7.0) with Polytron (Ultra Turrax, IKA, Germany) at a speed of 12,000 rpm twice with 15 s for each time. The above homogenate was centrifuged at 2,000×g for 10 min at 4 °C (Avanti J-E, Beckman Coulter, USA). The pellet was treated with 20 mL of the same isolation buffer two times and blended followed by centrifugation as the above condition. Then, 20 mL of 100 mM NaCl was utilized to wash the pellet three times. The pellet was finally dissolved in phosphate buffer (20 mM, pH 6.5). The final myofibril concentration was adjusted to 2 and 4 mg/mL. The myofibrillar protein concentration was determined by Biuret method (Gornall et al. 1949). The 2 mg/mL of myofibril protein solutions was used for the determination of protein thiol groups, while 4 mg/mL of myofibril solutions was used for the measurement of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
Sarcoplasmic Protein Preparation
Sarcoplasmic proteins were extracted using the method described by Veiseth et al. (2001) with some modification. At 0, 4, 7, and 10 d of cold storage, 1 g of ground beef samples was homogenized in 5 mL of cold buffer (pH 8.3) which contained 100 mM Tris-base, 10 mM EDTA-Na2, and 0.1 % β-mercaptoethanol (MCE), using a Polytron at 15,000 rpm for 15 s three times. The samples were further centrifuged at 20,000×g at 4 °C (30 min). The supernatant of all beef samples was filtered on gauze to eliminate collagen and other impurities. The filtrate was collected and the final concentration of sarcoplasmic proteins was adjusted to 6 mg/mL for determining the μ-calpain activity by casein zymography. The protein concentration was determined by BCA protein assay kit (Pierce Biotechnology, USA).
Measurement of Protein Carbonyl Group Content
Protein carbonyl groups were measured by derivatization with 2,4-dinitrophenylhydrazine (DNPH) at 0, 4, 7, and 10 d postmortem following the method of Zhang et al. (2011). One and half gram of frozen beef samples was minced and homogenized in 15 mL pyrophosphate buffer solution (pH 7.4) which contained 2 mM Na2P4O7, 10 mM Tris-maleate, 2 mM MgCl2, 100 mM KCl, and 2 mM EGTA, using a Polytron for 15 s three times at 12,000 rpm. After homogenization, the homogenate was under centrifugation for 5 min (4 °C, 12,000×g). Two 3-mL solutions were precipitated using 3 mL of 20 % trichloroacetic acid (TCA). The precipitate was further centrifuged at 12,000×g for 5 min. The supernatants were removed and the precipitants were washed by 10 % TCA. After washing, the samples were centrifuged at 12,000×g for 5 min. Pellets were either incubated with 3 mL of 10 mM DNPH dissolved in 2 M HCl or 3 mL of 2 M HCl as blank. Samples were shaken for 15 s every 10 min during reaction. Twenty percent of TCA (3 mL) was utilized to precipitate the protein samples. After precipitation, samples were centrifuged at room temperature at 12,000×g (5 min). After washing with 10 mM HCl dissolved in 1:1 (v/v) ethyl acetate/ethanol to remove DNPH, samples were under centrifugation at 12,000×g for 5 min after each wash. The pellet was put in 20 mM phosphate buffer containing 6 M guanidine hydrochloride (3 mL). All samples were kept shaking with a shaker at 4 °C, and the samples were centrifuged for 5 min at 12,000×g the next day. The content of carbonyls was measured at the wavelength of 370 nm. Each sample was analyzed in triplicate. The content of carbonyl used the unit of nanomoles of carbonyl per milligram of protein.
Determination of Free and Total Protein Thiol Group Content
Free and total protein thiol groups were monitored at 0, 4, 7, and 10 d postmortem after derivatization with 5,5′-dithiobis-2-nitrobenzoate (DTNB) as described by Yongsawatdigul et al. (2003) with slight modifications. One milliliter of myofibrillar protein solutions (2 mg/mL) was put in 50 mM phosphate buffer at pH 7.0 (9 mL) which contained 8 M urea, 10 mM EDTA, and 0.6 M KCl with 0.4 mL of 10 mM DTNB, and the mixture solutions were incubated for 25 min at 40 °C. To calculate the total sulfhydryl groups, the sample absorbance was detected at 412 nm with molar extinction coefficient of 13,600 M−1/cm−1. Free thiol group contents were performed with incubation of the reaction mixtures without urea for 1 h at 4 °C. Results were expressed in nanomole thiol groups per milligram myofibrillar protein.
Casein Zymography
The relative μ-calpain activity in each beef sample at 0, 4, 7, and 10 d postmortem was determined by using casein zymography procedure (Melody et al. 2004) with slight modification. One milliliter of extraction supernatant was mixed with the same volume of sample buffer at pH 6.8 which contained 150 mM Tris-base, 20 % glycerol, 0.75 % MCE, and 0.05 % bromophenol blue (BB). Then, target samples were loaded into native 12.5 % PAGE casein gels and run on electrophoresis units (Bio-Rad Laboratories, Hercules, CA). The native gels were kept for 15 min at 100 V in advance and then run in a constant voltage of 100 V condition at 4 °C for approximately 6 h. The gel was immediately incubated in buffer solutions at pH 7.8 which contained 50 mM Tris–HCl, 5 mM CaCl2, and 0.1 % MCE for 20 min three times. The native gels were then incubated for 16 h in the same buffer solutions above. Gel staining was using solution of 7 % glacial acetic acid, 40 % methanol, and 0.1 % Coomassie brilliant blue R-250 for 50 min and was then destained using solution of 53 % water, 7 % glacial acetic acid, and 40 % methanol.
Western Blotting Analysis
Western blotting was performed at 0, 4, 7, and 10 d postmortem as previously described by Davis et al. (2004) with some modifications. An aliquot of 4 mg/mL of myofibril solutions was added to an equal volume of sample buffer solutios (20 % glycerol, 0.75 % MCE, 0.05 % BB, 10 % SDS, and 150 mM Tris-base, pH 6.8) with heating at 95 °C and 5 min. After cooling, above samples were used to separate myofibril proteins by SDS-PAGE and examine troponin T and desmin changes of myofibril proteins with 4 % of stacking gel and 12.5 % of separate gel, respectively. Precision plus 4-μL standard protein maker was used to determine the molecular weight and the location of the target protein and precision plus 20 and 30 μg of protein per well for each sample for analysis of troponin T and desmin degradation. Running buffer contained 0.1 % [w/v] SDS, 192 mM glycine, and 25 mM Tris-base. The stacking gel was run in 60 V for 30 min and the separating gel was run in 110 V for 70 min. Troponin T and desmin of the target protein were at once moved to nitrocellulose membranes by Bio-Rad System (Bio-Rad, Hercules, CA, USA) at a constant voltage of 70 V for 50 min and 90 V for 70 min at 4 °C respectively. The transfer buffer was made up of 10 % (v/v) methanol, 192 mM glycine, and 25 mM Tris-base. The electroblotted membranes were then kept for 2 h in blocking 137 mM NaCl, 20 mM Tris-base, 0.05 % Tween 20, 5 mM KCl, and 5 % nonfat dry milk powder (TTBS buffer) at 25 °C. The membranes were immediately incubated for 15 h at 4 °C after blocking using primary antibody, namely an anti-troponin T mouse monoclonal antibody (Sigma-Aldrich, Chemie GmbH, Germany) and an anti-desmin mouse monoclonal antibody (Abcam, Cambridge, USA) at a dilution of 1:500 in TTBS buffer, respectively. After washing six times with TTBS for 5 min each, the membranes were incubated for 120 min at 20 °C with goat anti-rabbit IgG antibody (Chemicon, Temecula, USA) diluted by 1:5,000 in TTBS buffer. The membranes were washed six times with TTBS for 5 min each and stained with milliter ECL reagents (Pierce, Rockford, USA) for 5 min. The membrane was electronically scanned (GT-800F, EPSON, USA) using 300 dpi resolution, and the image was captured with identical exposure time, and then band intensities were quantified by Quantity One software. The result of troponin T and desmin degradation was calculated by using the intensity of immunoreactive troponin T and desmin band over the intensity of the immunoreactive troponin T and desmin band in a reference sample of 0 d to determine percentage change.
Measurement of Warner-Bratzler Sheer Force
The 2.54-cm-thick beef samples were used to determine WBSF at each time point. The fresh beef samples treated in open bags at 0, 4, 7, and 10 d postmortem were boiled at 72 °C until reaching 70 °C (internal temperature). Temperature was monitored using an electrothermo digital probe (Testo105 Testo AG, Germany). Beef samples were chilled using running cold tap water for 10 min. After cooling, six rectangular cores (1 cm × 1 cm × 6 cm) were taken from each beef sample parallel to the muscle fiber. Each core was sheared perpendicular to muscle fiber orientation in four replicates by the texture analyzer (Stable Micro System, England). The penetration speed was 1.5 mm/s using Warner-Bratzler probe. Data was analyzed by Texture Expert software V1.0 and date acquisition rate was 200 pps.
Statistical Analysis
One-way analysis of variance (ANOVA) using the SAS version 8.0 software was utilized to analyze the data. All the data was expressed as mean ± standard deviation of triplicate determinations of each sample. Comparison of means was carried out by the Duncan’s multiple range rest. Significant differences were identified by means of Tukeys’ test (p < 0.05).
Result and Discussion
Protein Carbonyl Content
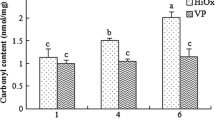
The formation of protein carbonyl derivatives from amino acid side chains was used as a general and reliable indicator for assessing protein oxidation. Particularly, cysteine, tyrosine, phenylalanine, tryptophan, histidine, proline, arginine, lysine, and methionine have been reported to be susceptible to reactive oxygen species and easily converted to carbonyl derivatives (Davies and Dean 2003). The major pathways of carbonyl formation in oxidized protein include peptide backbone scission, lipid peroxidation such as 4-hydroxy-2-nonenal and malondialdehyde (Xiong 2000), direct oxidation of amino acid side chains (Amici et al. 1989), and carbonyl derivatives generated by reducing sugars (Xiong 2000). Postmortem changes in carbonyl content of beef samples from AP, VP, and MAP during 10 d of chilled storage are shown in Fig. 1. Carbonyl content of beef samples from VP and MAP was significantly higher than that from AP after 4 d of chilled storage, and no significant differences were observed between beef samples from AP and MAP (p > 0.05). Beef samples from AP showed higher carbonyl values than that from VP at d 7 (p < 0.05). Carbonyl content of beef samples from AP was significantly higher than those from VP and MAP after 10 d of chilled storage (p < 0.05). However, no significant difference in carbonyl content of beef samples from VP and MAP was found at 10 d of chilled storage (p > 0.05). This result showed the same trends as Lund et al. (2007) who reported that there were no significant differences in carbonyl content among beef samples from VP and MAP (O2 70 %:CO2 30 %) at 4 °C during 4, 8, and 14 d of cold storage (p > 0.05). Lagerstedt et al. (2011) also found that there were no significant differences in carbonyl content among beef samples from VP and MAP (O2 80 %:CO2 20 %) at 4 °C during 5 and 15 d of cold storage (p > 0.05). In chicken meat under different packaging, Xiao et al. (2011) observed that the content of carbonyl groups of raw broiler thigh meat was dramatically higher at 4 °C for 7 d of cold storage under air-packaged condition compared with equivalent samples under vacuum-packaged condition (p < 0.05).
Effect of three packaging methods (AP, VP, and MAP) on protein carbonyl group contents of beef samples during 10 d of chilled storage at 4 °C. Means of different capital letters A, B, and C are significantly different at the same storage time (p < 0.05). Vertical bars represent the standard deviation of the means
Free and Total Protein Thiol Content
Free thiol group of cysteine residues is extremely susceptible to oxidation (Neumann 1972), and therefore thiol group content is regarded as a marker of oxidation (Hofmann and Reiner 1978). In this study, the content of free thiol groups of beef samples from VP was significantly higher than those from AP and MAP at 4, 7, and 10 d of chilled storage period (p < 0.05, Fig. 2a). Free thiol group content of beef samples from AP was lower than that from MAP at 4 d (p < 0.05). At d 7 and 10, beef samples from AP and MAP did not significantly differ in free thiol content (p > 0.05). As for total thiol group content, VP samples were significantly higher than those from MAP at d 4 and greater than samples from AP at d 7 and 10 (p < 0.05, Fig. 2b). No significant differences were detected in total thiol content of beef samples from VP and MAP at d 4 and 7 (p > 0.05), while MAP showed higher total thiol content compared with AP at 10 d (p < 0.05). The decreased level of free and total thiol may be involved in the formation of disulfide bond. The oxidative conversion of free thiol groups into S–S bonds mainly forms dimerization and polymerization of myosin (Xiong et al. 2009). The result was in agreement with Lund et al. (2007) who showed that the content of free thiol groups was significantly decreased at 4 °C during d 8 of cold storage in vacuum skin-packaged pork samples compared with pork samples in high oxygen-modified atmosphere condition.
a, b Effect of three packaging methods (AP, VP, and MAP) on free SH group contents and total SH group contents of beef samples during 10 d of chilled storage at 4 °C, respectively. Means of different capital letters A, B, and C are significantly different at the same storage time (p < 0.05). Vertical bars represent the standard deviation of the means
Calpain Activity
The calpain proteolytic system is composed of μ-calpain and m-calpain and their specific inhibitor calpastatin (Huff-Lonergan et al. 2010). μ-Calpain has been regarded as a key factor for the improvement of beef quality during postmortem aging (Pulford et al. 2009). There was significant difference in μ-calpain activity under three packaging conditions during 10 d of chilled storage (Fig. 3). Beef samples had two large and brighter clear zones at 0 d which showed higher μ-calpain activity and autolysis. There were significant differences in μ-calpain activity of beef samples under three packaging conditions examined by the lightness of clear zone after 4 d of cold storage (p < 0.05). The differences among three packaged beef samples were even more obvious after 7 d of cold storage. However, there was no detectable μ-calpain activity at 10 d of cold storage. The μ-calpain activity of beef samples from MAP was highest followed by air-packaged beef samples, and vacuum-packaged beef samples was weakest regardless of 4 or 7 d. The result was in accordance with the report of Rowe et al. (2004) which showed irradiated samples obviously had more μ-calpain activity compared with nonirradiated samples at 3 and 7 d irradiation, and there was no detectable μ-calpain activity in nonirradiation samples. It would be explained that the μ-calpain activity after oxidation was less active in beef samples and autolysis was decreased. Nevertheless, the μ-calpain activity was reversibly restored when the reducing agent was used in the experiment (Lindahl et al. 2010). No significant changes were observed in m-calpain activity regardless of cold storage time and packaging method during chilled storage (Ducastaing et al. 1985; Koohmaraie, et al. 1987).
Effect of three packaging methods (AP, VP, and MAP) on μ-calpain activity of beef samples during 10 d of chilled storage at 4 °C. Means of different capital letters A, B, and C are significantly different at the same storage time of three packages (p < 0.05). Vertical bars represent the standard deviation of the means. Sixty micrograms of sarcoplasmic protein was loaded for each lane. Lane 1 is beef samples at 0 d and it represents control. Lanes 2, 3, and 4 are beef samples from AP, VP, and MAP at 4 d, respectively. Lanes 5, 6, and 7 are beef samples from AP, VP, and MAP at 7 d, respectively. Lanes 8, 9, and 10 are beef samples from AP, VP, and MAP at 10 d, respectively. The upper band represents μ-calpain activity, while the lower band represents m-calpain activity
Degradation of Troponin T and Desmin
The 30-kD polypeptide which resulted from the degradation of troponin T is considered as an indicator of calpain activation in postmortem muscle (Penny and Dransfield 1979). In our study, the degradation of troponin T from beef samples during 10 d of chilled storage is shown in Fig. 4. The extent of troponin T degradation was quantified as a percentage of the relative content of the intact troponin T band during chilled storage. The degradation rate of troponin T of beef samples from VP and MAP was significantly higher than that from AP at d 4 (p < 0.05), while no significant differences in the degradation rate of troponin T of beef samples from VP and MAP were found (p > 0.05). At d 7, VP beef samples showed greater degradation rate of troponin T than MAP beef samples (p < 0.05). However, beef samples from AP and MAP did not differ significantly in the rate of troponin T degradation (p > 0.05).
Effect of three packaging methods (AP, VP, and MAP) on troponin T degradation of beef samples during 7 d of chilled storage at 4 °C. Means of different capital letters A, B, and C are significantly different at the same storage time (p < 0.05). Vertical bar represents the standard deviation of the mean. Twenty micrograms of myofibril protein was loaded for each lane. Lane 1 was beef samples at 0 d. Lanes 2, 3, and 4 were beef samples from AP, VP, and MAP at 4 d, respectively. Lanes 5, 6, and 7 were beef samples from AP, VP, and MAP at 7 d, respectively
Desmin is a predominant protein in the intermediate filament structure which interlinks Z-disk of the sarcomere and sarcolemma in skeletal muscle (Balogh et al. 2005). Desmin plays a key role in maintaining the function and the structure integrity of the muscle cell (Hynes 1992). The degradation of desmin has a significant effect on improvement of meat quality during postmortem aging (Taylor et al. 1995). The extent of desmin degradation was quantified as a percentage of the relative content of the intact desmin band during chilled storage (Fig. 5). The degradation rate of desmin of beef samples from VP was faster than those from AP and MAP (p < 0.05), while the degradation rate of desmin of beef samples from AP was higher than that from MAP at d 4 and d 7 of storage (p < 0.05). These results could be explained by the extent of protein oxidation and μ-calpain activity of beef samples from three packaging methods. Higher levels of protein oxidation might inactivate μ-calpain leading to decreased degradation of desmin and troponin T in the case of oxygen packaging.
Effect of three packaging methods (AP, VP, and MAP) on desmin degradation of beef samples during 7 d of chilled storage at 4 °C. Means of different capital letters A, B, and C are significantly different at the same storage time (p < 0.05). Vertical bars represent the standard deviation of the means. Twenty micrograms of myofibril protein were loaded for each lane. Lane 1 was beef samples at 0 d. Lanes 2, 3, and 4 were beef samples from AP, VP, and MAP at 4 d, respectively. Lanes 5, 6, and 7 were beef samples from AP, VP, and MAP at 7 d, respectively
Warner-Bratzler Shear Force Values
Meat tenderness is considered as key quality attribute of fresh meat which is directly associated with consumer satisfaction (Paredi et al. 2012; Shackelford et al. 2001). At d 4, the tenderness of beef samples from VP was lower than those from AP and MAP (Fig. 6; p < 0.05), while the tenderness of beef samples from AP and MAP was not significantly different (p > 0.05). At d 7 and 10, the tenderness of beef samples from VP was greater than those from AP and MAP (p < 0.05), whereas the tenderness of beef samples from AP and MAP was significantly different at d 7 and 10 (p < 0.05). Zakrys-Waliwander et al. (2012) reported the similar results that the tenderness of beef steaks from MAP was lower in comparison with VP at 8 and 14 d of chilled storage (p < 0.05). Lund et al. (2007) also observed that the tenderness of beef samples from VP tended to be higher than that from MAP at 8 and 14 d of chilled storage aging (p < 0.05). However, Lagerstedt et al. (2011) observed that there was no significant difference in tenderness of beef samples from VP compared with MAP at 5 d of chilled aging (p > 0.05).
Conclusion
In short, incensement of carbonyl values, reduction of free thiol group content, and total thiol group content in AP and MAP beef samples indicated the higher levels of protein oxidation compared to VP during chilled storage. Protein oxidation might negatively regulate the activation of μ-calpain and slow the degradation rate of troponin T and desmin during chilled storage. Less degradation of key myofibrillar proteins might be associated with decreased tenderness of beef samples during postmortem aging. Therefore, protein oxidation may be the behind mechanism to regulate the structure changes and tenderization of beef samples under different packaging conditions. VP could be the better method for beef tenderness to serve the retail compared with AP and MAP methods.
Abbreviations
- LD:
-
Longissimus dorsi
- VP:
-
Vacuum packaging
- AP:
-
Air packaging
- MAP:
-
Modified atmosphere packaging
- MCE:
-
β-Mercaptoethanol
- TCA:
-
Trichloroacetic acid
- DNPH:
-
2,4-Dinitrophenylhydrazine
- DTNB:
-
5,5′-Dithiobis-2-nitrobenzoate
- BB:
-
Bromophenol blue
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- d:
-
Day or days
References
Amici, A., Levine, R. L., Tsai, L., & Stadtman, E. R. (1989). Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed reactions. The Journal of Biological Chemistry, 264(6), 3341–3346.
Arvanitoyannis, I. S., & Stratakos, A. C. (2012). Application of modified atmosphere packaging and active/smart technologies to red meat and poultry: a review. Food and Bioprocess Technology, 5, 1423–1446.
Balogh, J., Li, Z., Paulin, D., & Arner, A. (2005). Desmin filaments influence myofilament spacing and lateral compliance of slow skeletal, muscle fibers. Biophisical Journal, 88(2), 1156–1165.
Clausen, I., Jakobsen, M., Ertbjerg, P., & Madsen, N. T. (2009). Modified atmosphere packaging affects lipid oxidation, myofibrillar fragmentation index and eating quality of beef. Packaging Technology and Science, 22, 85–96.
Davies, M. J., & Dean, R. T. (2003). Radical-mediated protein oxidation. (Eds.) Oxford Science Publications, Oxford.
Davis, K. J., Sebranek, J. G., Huff-Lonergan, E., & Lonergan, S. M. (2004). The effects of aging on moisture-enhanced pork loins. Meat Science, 66(3), 519–524.
Ducastaing, A., Valin, C., Schollmeyer, J., & Cross, R. (1985). Effects of electrical stimulation on postmortem changes in the activities of two Ca2+ dependent neutral proteinases and their inhibitor in beef muscle. Meat Science, 15(4), 193–202.
Gornall, A. G., Bardawill, C. J., & David, M. M. (1949). Determination of serum proteins by means of the biuret reaction. Journal of Biological Chemistry, 177(2), 751–766.
Hofmann, K., & Reiner, H. (1978). Sulfhydryl and disulfide groups in meats. Advances in Food Research, 24(2), 1–111.
Huff-Lonergan, E., & Lonergan, S. M. (2005). Mechanism of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Science, 71(1), 194–204.
Huff-Lonergan, E., Mitsuhashi, T., Beekman, D. D., Parrish, J. F. C., Olson, D. G., & Robson, R. M. (1996). Proteolysis of specific muscle structural proteins by μ-calpain at low pH and temperature is similar to degradation in postmortem bovine muscle. Journal of Animal Science, 74, 993–1008.
Huff-Lonergan, E., Zhang, W. G., & Lonergan, S. M. (2010). Biochemistry of postmortem muscle—lessons on mechanisms of meat tenderization. Meat Science, 86(1), 184–195.
Hynes, R. O. (1992). Integrins: versatility, modulation, and signalling in cell adhesion. Cell, 69(1), 11–25.
Jeremiah, L. E. (2001). Packaging alternatives to deliver fresh meats using short- or long-term distribution. Food Research International, 34, 749–772.
José, M. L., & María, G. (2012). Shelf life of fresh foal meat under MAP, overwrap and vacuum packaging conditions. Meat Science, 92, 610–618.
Kanner, J. (1994). Oxidative processes in meat and meat products: quality implications. Meat Science, 36, 169–189.
Koohmaraie, M. (1996). Biochemical factors regulating the toughening and tenderization processes of meat. Meat Science, 43(Supplement 1), 193–201.
Koohmaraie, M., Seideman, S. C., Schollmeyer, J. E., Dutson, T. R., & Crouse, J. D. (1987). Effect of postmortem storage on Ca2+-dependent proteases, their inhibitor and myofibril fragmentation. Meat Science, 19, 187–196.
Lagerstedt, Å., Lundström, K., & Lindahl, G. (2011). Influence of vacuum or high-oxygen modified atmosphere packaging on quality of beef M. longissimus dorsi steaks after different ageing times. Meat Science, 87(2), 101–106.
Lametsch, R., Lonergan, S., & Huff-Lonergan, E. (2008). Disulfide bond with mμ-calpain active site inhibits activity and autolysis. Biochimica et Biophysica Acta, 1784(9), 1215–1221.
Li, C. B., Zhou, G. H., & Xu, X. L. (2010). Dynamic changes of beef intramuscular connective tissue and muscle fiber during heating and their effects on beef shear force. Food and Bioprocess Technology, 3, 521–527.
Li, X., Lindahl, G., Zamaratskaia, G., & Lundström, K. (2012). Influence of vacuum skin packaging on color stability of beef longissimus lumborum compared with vacuum and high-oxygen modified atmosphere packaging. Meat Science, 92, 604–609.
Lindahl, G., Lagerstedt, Å., Ertbjerg, P., Sampels, S., & Lundström, K. (2010). Ageing of large cuts of beef loin in vacuum or high oxygen modified atmosphere—effect on shear force, calpain activity, desmin degradation and protein oxidation. Meat Science, 85, 160–166.
Lund, M. N., Lametsh, R., Hviid, M. S., Jensen, O. L., & Skibsted, L. H. (2007). High oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Science, 77(3), 295–303.
Lund, M. N., Heinonen, M., Boron, C. P., & Este´vez, M. (2011). Protein oxidation in muscle foods: a review. Molecular Nutrition and Food Research, 55(1), 83–95.
McMillin, K. W. (2008). Where is MAP going? A review and future potential of modified atmosphere packaging for meat. Meat Science, 80, 43–65.
Melody, J. L., Lonergan, S. M., Rowe, L. J., Huiatt, T. W., Mayes, M. S., & Huff-Lonergan, E. (2004). Early postmortem biochemical factors influence tenderness and water holding capacity of three porcine muscles. Journal of Animal Science, 82(4), 1195–1205.
Neumann, N. P. (1972). Oxidation with hydrogen peroxide. Methods in Enzymology, 25, 393–400.
Paredi, G., Raboni, S., Bendixen, E., Almeida, A., & Andrea, M. (2012). “Muscle to meat” molecular events and technological transformations: the proteomics insight. Journal of Proteomics, 75(14), 4275–4289.
Park, D., Xiong, Y. L., & Alderton, A. L. (2006). Concentration effects of hydroxyl radical oxidizing systems on biochemical properties of porcine muscle myofibrillar protein. Food Chemistry, 101(3), 1239–1246.
Penny, I. F., & Dransfield, E. (1979). The relationship between toughness and troponin-T in conditioned beef. Meat Science, 3(4), 135–138.
Pulford, D. J., Dobbie, P., Fraga Vazquez, S., Fraser-Smith, E., Frost, D. A., & Morris, C. A. (2009). Variation in bull beef quality due to ultimate muscle pH is correlated to endopeptidase and small heat shock protein levels. Meat Science, 83(1), 1–9.
Rowe, L. J., Maddock, K. R., Lonergan, S. M., & Huff-Lonergan, E. (2004). Oxidative environments decrease tenderization of beef steaks through inactivation of m-calpain. Journal of Animal Science, 82(11), 3254–3266.
Santos, E. M., Jaime, I., Rovira, J., Lyhs, U., KoRkeala, U., & Bjorkroth, J. (2005). Characterization and identification of lactic acid bacteria in “morcila de burgos”. International Journal of Microbiology, 97, 285–296.
Sanz, A., Ripoll, G., Blasco, I., Alvarez-Rodríguez, J., & Albertí, P. (2011). Performance of yearling, bull and steer of serrana de teruel breed. In A. Sanz, I. Casasús, M. Joy, J. Álvarez-Rodriguez, J. H. Calvo, B. Panea, P. Muñoz, & J. Balcells (Eds.), XIV Jornadas sobre Producción Animal (Vol. II, pp. 745–747). Zaragoza: AIDA.
Sentandreu, M. A., Coulis, G., & Ouali, A. (2002). Role of muscle endopeptidases and their inhibitors in meat tenderness. Trends in Food Science and Technology, 13(12), 400–421.
Shackelford, S. D., Wheeler, T. L., Meade, M. K., Reagan, J. O., Byrnes, B. L., & Koohmaraie, M. (2001). Consumer impressions of tender select beef. Journal of Animal Science, 79(10), 2605–2614.
Taylor, R. G., Geesink, G. H., Thompson, V. F., Koohmaraie, M., & Goll, D. E. (1995). Is Z-disk degradation responsible for postmortem tenderization? Journal of Animal Science, 73(5), 1351–1367.
Veiseth, E., Shackelford, S. D., Wheeler, T. L., & Koohmaraie, M. (2001). Effect of postmortem storage on μ-calpain and m-calpain in ovine skeletal muscle. Journal of Animal Science, 79(6), 1502–1508.
Xiao, S., Zhang, W. G., Lee, E. J., Ma, C. W., & Ahn, D. U. (2011). Effects of diet, packaging, and irradiation on protein oxidation, lipid oxidation, and color of raw broiler thigh meat during refrigerated storage. Poultry Science, 90(6), 1348–1357.
Xiong, Y. L. (2000). Protein oxidation and implications for muscle food quality. In E. A. Decker, C. L. Faustman, & C. J. Lopez-Bote (Eds.), Antioxidants in muscle foods (pp. 85–111). New York: John Wiley & Sons, Inc.
Xiong, Y. L., Park, D., & Ooizumi, T. (2009). Variation in the cross-linking pattern of porcine myofibrillar protein exposed to three oxidative environments. Journal of Agricultural and Food Chemistry, 57(1), 153–159.
Yongsawatdigul, J., & Park, J. W. (2003). Thermal denaturation and aggregation of threadfin bream actomyosin. Food Chemistry, 83(3), 409–416.
Zakrys-Waliwander, P. I., O’Sullivan, M. G., O’Neill, E. E., & Kerry, J. P. (2012). The effects of high oxygen modified atmosphere packaging on protein oxidation of bovine M. longissimus dorsi muscle during chilled storage. Food Chemistry, 131, 527–532.
Zhang, W. G., Lonergan, S. M., Gardner, M. A., & Huff-Lonergan, E. (2006). Contribution of postmortem changes of integrin, desmin and μ-calpain to variation in water holding capacity of pork. Meat Science, 74(3), 578–585.
Zhang, W. G., Xiao, S., Lee, E. J., & Ahn, D. U. (2011). Consumption of oxidized oil increases oxidative stress in broilers and affects the quality of breast meat. Journal of Agricultural and Food Chemistry, 59(3), 969–974.
Zhang, W. G., Xiao, S., & Ahn, D. U. (2013). Protein oxidation: basic principles and implications for meat quality. Critical Reviews in Food Science and Nutrition, 53(11), 1191–1201.
Acknowledgments
The authors would like to thank the general manager and technical workers in Yurun Food Co. Ltd. (Fuyang, Anhui, China) for their cooperation and their assistance in the collection and packaging of beef samples. This study was supported by the National Natural Science Foundation of China (31271899) and the Ministry of Science and Technology of China (2012BAD28B03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, Qq., Liu, R., Zhang, Wg. et al. Effects of Different Packaging Systems on Beef Tenderness Through Protein Modifications. Food Bioprocess Technol 8, 580–588 (2015). https://doi.org/10.1007/s11947-014-1426-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-014-1426-3