Abstract
The objective of this study was to investigate the biochemical difference of pork under high oxygen modified atmosphere packaging and their contribution to meat tenderness and water holding capacity of pork during postmortem storage. Twelve longissimus dorsi muscles were randomly assigned to either high oxygen modified atmosphere packaging or vacuum packaging and stored for 1, 4, and 6 days at 4 °C. The carbonyl content, protein surface hydrophobicity, protein solubility, calpain activity, desmin degradation, tenderness, and water loss of pork were determined. Results showed that carbonyl content, protein surface hydrophobicity, and protein solubility were significantly affected (P < 0.05) by packaging method, while storage time did not significantly influence protein surface hydrophobicity and the solubility of sarcoplasmic protein (P > 0.05). Samples from high oxygen modified atmosphere packaging at 1 day showed greater intensity of intact 80 KDa calpain and lower intensity of autolyzed 76 KDa calpain product compared to samples from vacuum packaging (P < 0.05). Desmin degradation was significantly affected (P < 0.05) by packaging method and storage time, while their interaction presented no significance (P > 0.05). Higher intensity of intact desmin was observed in samples from high oxygen modified atmosphere packaging than vacuum packaging samples from 1 day of postmortem storage. Both packaging method and storage time showed significant effects (P < 0.05) on tenderness and water loss of pork muscle during postmortem storage. Changes in protein oxidation, calpain activation, and protein proteolysis of postmortem pork under high oxygen modified atmosphere packaging could help to explain decreased meat tenderness and increased centrifuge loss of pork.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High oxygen modified atmosphere packaging (HiOx) system with the gas bulk of 80 % O2 and 20 % CO2 is widely used as it preserves the flushing pink appearance of fresh meat and prolongs shelf life by inhibiting microbial growth. However, the oxygen-enrichment atmosphere inevitably accelerated oxidative reactions in meat which have been shown for lipids to cause rancidity in meat (Kim et al., 2010) and flavors deterioration (Jongberg et al. 2014) through the breakdown of lipid hydroperoxides into volatile ketones and aldehydes (Ladikos and Lougovois 1990). Oxidation of lipids, which results in the production of free radicals, has been extensively studied and widely believed as a promoter of protein oxidation. Protein oxidation is manifested as a loss of enzyme activity, modification of peptide chains and functional groups, changes in proteolytic susceptibility, and formation of intra- and intermolecular crosslinks (Lund et al. 2011; Zhang et al. 2013b). It is generally accepted that protein oxidation could lead to undesirable texture changes including tenderness (Zakrys-Waliwander et al. 2012; Lund et al. 2007) and water retention properties (Delles and Xiong 2014; Traore et al. 2012a) in fresh meat and processed muscle foods. Meat tenderness and water holding capacity (WHC) are the most commonly parameters used for the evaluation of meat quality by consumers, while the role of protein oxidation on the regulation of tenderness and WHC of fresh pork during postmortem aging has not been well defined.
Multiple factors have been conducted to identify the causes of the variations in both meat tenderness and water loss during postmortem storage, among which the degradation of muscle myofibrillar proteins is believed to play key roles in meat tenderization and water retention (Huff -Lonergan and Lonergan 2005; Rowe et al. 2004; Zhang et al. 2006). Previous studies have demonstrated that many myofibrillar proteins involved in protein proteolysis are largely regulated by the cysteine protease calpain (Goll et al. 2003; Koohmaraie and Geesink 2006). Although calpain has been extensively studied over the past few decades, the mechanisms that control its activity have not been fully elucidated (Goll et al. 2003; Carlin et al. 2006). Among calpain system, μ-calpain instead of m-calpain is generally believed to be the main protease for the majority of myofibril protein proteolysis associated with meat texture properties during postmortem storage (Cheng and Sun 2008; Huff-Lonergan et al. 1996). Calpain requires the reactive sulfhydryl groups located in the active site to be reduced and functional. However, the 80 KDa of μ-calpain contains an active cysteine residue at position 115 of catalytic domain II whereas cysteine is highly susceptible to oxidation and is easy to be inactivated through disulfide bond formation during protein oxidative modifications (Lametsch et al. 2008; Zhang et al. 2013b). Oxidation of proteins has been shown to change their functional properties and thereby concomitant oxidation of calpain and of their myofibrillar substrates can perform how those proteases and proteins act in the control of postmortem aging (Zhang et al. 2013b; Rowe et al. 2004; Xue et al. 2012). To our knowledge, the effects of protein oxidation under HiOx on calpain activation, protein proteolysis, and then meat texture quality of pork are rather poorly studied. Therefore, our objective was to investigate the biochemical changes under HiOx and determine their contribution to meat tenderization and WHC of pork during postmortem storage.
Materials and Methods
Sample Preparation
Six Duroc × Landrace × Yorkshire crossbred pork of 6 month old with live weight of 95–105 kg were slaughtered humanly on the same day at a commercial slaughter plant in Sushi Meat Co. Ltd. (Huaian, Jiangsu, China) under standard commercial conditions according to National Standard of China. The six Duroc × Landrace × Yorkshire crossbred pork grew up with the same feeding ingredients (basal soybean meal, Table 1) and the sample heterogeneity was inconsiderable. Both sides of the longissimus dorsi (LD) muscles from rib 4 to rib 13 of the carcasses were immediately excised after precooled at 4 °C for 24 h. Subcutaneous fat and fascia tissues were trimmed free, and then, each LD muscle was sliced into three equal chops of 150 g (2–2.5 cm thickness). The three chops were numbered from the anterior to the posterior part irrespective of which side of carcass they came from. Treatments were distributed along the chops locations according to the Latin Square design with randomized order of locations and animal.
HiOx was accomplished with a gas flushing tray sealer (Smart 500, ULMA Packaging, Onati, Spain); polypropylene-polyethylene (PP) trays (6.0 × 23 × 13 cm in dimension; Cryovac Packaging, NJ, USA) allowed oxygen to evenly surround the sample and wrapped with PP film (O2 transmission rate <1 cm3/m2/24 h/atm; Cryovac Packaging, NJ, USA). For vacuum packaging (VP), samples were packed by using Cryovac stretchable vacuum bags (O2 transmission rate <30 cm3/m2/24 h/atm) upon infra-bar depression machine (Cryovac Packaging, NJ, USA). The minimum pressure condition of vacuum procedure was 10 mbar. All samples were stored at 4 °C for 1, 4, and 6 days with display light on (constantly 24 h/day) providing approximately 500 lx fluorescent source to simulate commercial conditions of fresh meat storage. Samples were exposed to air and cut into suitable size for measuring the index of tenderness and water loss at each storage point. The remained meat was dissected into small pieces and preserved in antifreeze tubes, immediately frozen in liquid nitrogen and kept at −80 °C until further biochemical analysis. Except for chemicals labeled, a known source, other unlabeled chemicals used in our experiment were obtained from the pilot lab of National Center of Meat Quality and Safety Control of China, Nanjing Agricultural University.
Determination of Purge Loss
All samples were dried using absorbent towel and noted the initial weight prior to being put into vacuum bags or PP trays. After storage, samples removed from packaging were immediately towel dried and reweight. Purge loss was measured by calculating the percentage of weight loss during storage time.
Determination of Centrifuging Loss
An ultra-centrifugal method was also used to investigate the water loss as reviewed by Trout (1988) with slight modifications. Ten gram of minced samples were gently filled into a 10-mL ultracentrifuge tube and centrifuged for 15 min at 40,000g. The liquid at the top of tubes was removed, and the meat samples were weighed. The percentage of liquid loss at each specific storage time was calculated.
Low-Field Nuclear Magnetic Resonance (LF-NMR) Measurements
Samples (1 × 1 × 1.5 cm) were collected by cutting along the muscle fiber direction and then gently placed in cylindrical glass tube (1.5 cm in diameter, 5 cm in height) which matched with the inner diameter of the 1.8 cm temperature variable probe tube. Samples collection avoided overexertion and cavity existence. All samples were equilibrated for 30 min at 25 °C. Five measurements were made for each sample.
The LF-NMR measurements were performed on a Maran Benchtop Pulsed NMR analyzer (Resonance Instruments, Witney, UK), operating at a stable frequency of 22.6 MHz at 32 °C. Transverse relaxation time (T2) was performed with the Carr-Purcell-Meiboom-Gill sequence (Carr and Purcell 1954; Meiboom and Gill 1958) with four scan repetitions and a 2-s relaxation delay between successive pulse scans. T2 was measured with a τ-value of 200 μs between pulses of 90 and 180°. Data were expressed by using the MultiExp Inv Analysis software (Niumag Electric Corporation, Shanghai, China). The transverse relaxation time of bound water (T2B), immobilized water (T21), and free water (T22) and their corresponding water populations (P2B, P21, P22) were recorded as water mobility and distribution, respectively.
Determination of Warner-Bratzler Shear Force Values
Warner-Bratzler shear force (WBSF) was measured by the method of Lindahl et al. (2010). Raw pork samples (2.54 cm in thickness) were wrapped in individual retort pouches and broiled at 80 °C water until the internal temperature reaching 72 °C. Cooking samples were cooled down upon running water until samples reached room temperature. Six cores (1 × 1 × 5 cm) from each pork sample were cut parallel to the muscle fibers orientation and sheared perpendicular to the fiber direction using a texture analyzer (Stable Micro System, England) equipped with a shear force blade. The probe was lowered 20 mm from the point of resistance, and the penetration speed was 1.5 mm/s when cutting through. All results were expressed using Newtons.
Determination of Carbonyl Content
The content of carbonyl was evaluated by incubating with 2,4-dinitrophenylhydrazine (DNPH, Sigma-Aldrich, USA) as described by Zhang et al. (2013a, b) with slight modification. Half gram finely minced LD muscle was homogenized in 5 mL pyrophosphate butter (2 mM sodium pyrophosphate, 10 mM Tris-maleate, 100 mM potassium chloride, and 2 mM ethylene glycol tetraacetic acid, pH 7.4) using Ploytron (IKA T25 digial ultraturrox, IKA, German) at the speed of 15,000 rpm for 30 s (10 s × 3). Two equal aliquots of homogenate (0.5 mL) were precipitated with 20 % (w/v) trichloroacetic acid (0.5 mL) and centrifuged at 12,000g for 5 min at 4 °C (the following centrifuge conditions were the same). The supernatant was removed, and 10 % (w/v) trichloroacetic acid (0.5 mL) was added to the precipitant for further washing. After centrifugation, one pellet was incubated with 10 mM DNPH dissolved in 2 M hydrochloric acid (HCl, 0.5 mL) and the same volume of 2 M HCl was added to the other pellet as a blank. Both samples reacted in darkness for 30 min (vortex for 10 s every 10 min). Precipitate was purified with 20 % (w/v) trichloroacetic acid and centrifuged again. DNPH was washed three times with 10 mM HCl dissolved in 1:1 (v/v) ethanol/ethyl acetate (2 mL). Finally, the pellet reacted with 6 M guanidine hydrochloride dissolved in 20 mM potassium dihydrogen phosphate (1 mL, pH 2.3) and then shaken at 4 °C overnight. The absorbance was detected at 370 nm using a UV-2540 UV–visible spectrophotometer (Shi-madzu, Kyoto, Japan). The protein concentration of supernatant was measured by Biuret method at 540 nm using bovine serum albumin as a standard curve. The carbonyl content was expressed as nmol carbonyls per mg of protein. This experiment was performed in triplicate for each sample, and the results were obtained to determine an average value.
Myofibrillar and Sarcoplasmic Protein Extraction
Myofibrillar protein was isolated after 1, 4, and 6 days of chilled storage using isolation buffer (10 mM phosphate buffer, 100 mM sodium chloride, 2 mM magnesium chloride, and 1 mM ethylene glycol tetraacetic acid, pH 7.0) as described by Xiong (2005). Protein content was determined by BCA Protein Assay Kit (Thermo, RD, USA). The myofibrillar samples were frozen at −80 °C until used for assessing protein surface hydrophobicity and electrophoresis.
Sarcoplasmic protein was prepared following the description of Veiseth et al. (2001). All samples were homogenized with extraction solutions (10 mM ethylene diamine tetraacetic acid, 100 mM Tris–HCl, and 0.1 % β-mercaptoethanol (v/v), pH 8.3). The same polytron was used twice at 15,000 rpm for 30 s. After centrifugation under 4 °C at the speed of 15,000g for 30 min, the concentration of supernatant was determined and uniformly adjusted to 6.4 mg/mL with double distilled water. The samples of the sarcoplasmic protein were kept in frozen storage.
Determination of Protein Surface Hydrophobicity
The hydrophobic ability of myofibril proteins was determined by the method of Chelh et al. (2006) with minor changes. Forty microliter of 1 mg/mL bromophenol blue (BPB) was added to 2 mL myofibril suspension (2 mg/mL). All myofibril solutions were thoroughly mixed and centrifuged at 10,000g for 15 min at 4 °C. Control sample without myofibrils was also performed. Supernatant absorbance corresponding to free BPB of sample was detected at 595 nm against a phosphate buffer blank. The amount of bound BPB was calculated as: bound BPB (μg) = 40 μg × (OD control − OD sample)/OD control.
Determination of Protein Solubility
Protein solubility was performed corresponding to the method of Joo et al. (1999a). The total protein was extracted from 1-g minced muscle using 20 mL of ice-cold 1.1 M potassium iodide in 0.1 M phosphate buffer (pH 7.2). The sample was homogenized with the lowest speed and kept extracting at 4 °C overnight. Three equal aliquots of each homogenate were centrifuged at 2000g for 30 min, and then supernatant protein concentration was determined by Biuret method. The solubility of sarcoplasmic proteins was acquired similarly except the extraction buffer (10 mL ice-cold 25 mM potassium phosphate buffer, pH 7.2). The difference between total protein and sarcoplasmic protein solubility was calculated as myofibrillar protein solubility.
Determination of Western Blotting
Sarcoplasmic protein samples for detecting calpain were thawed and mixed with tracking dye buffer (2:1, v/v) containing 30 mM Tris–HCl, 20 % glycerol (v/v), 3 % sodium dodecyl sulfate (w/v), 0.02 % BPB (w/v), 3 mM ethylene diamine tetraacetic acid, and 10 % β-mercaptoethanol (v/v) (pH 8.0). As for myofibrillar proteins, samples used for detecting desmin were uniformly adjusted to a concentration of 7 mg/mL and then 1:1 (v/v) diluted by dispersing in loading buffer (125 mM Tris-base, 4 % sodium dodecyl sulfate (w/v), 20 % glycerol (v/v), 0.5 % β-mercaptoethanol (v/v), 0.1 % BPB (w/v), pH 6.8). The mixtures were denaturized in 95 °C water bath for 5 min.
The 10 % polyacrylamide separating gels were used for both calpain and desmin electrophoresis (Nanjing Jiancheng, Nanjing, China). For μ-calpain and desmin, the amount of protein samples per lane was 60 and 28 μg, respectively. The 5 μL standard protein marker was used as reference to determine the molecular weight of proteins. A constant voltage of 120 V for approximately 1.5 h was set on the gels. After electrophoresis, gels were immediately transferred to Bio Trace NT nitrocellulose transfer membranes (Pall, FL, USA) and then run on a Mini-Protean II machine (Bio-Rad Laboratories, CA, USA) at 90 V for 90 min. The temperature of the transfer procedure was operated under 4 °C. The membrane imprinted proteins were blocked at room temperature for 1.5 h using 5 % (w/v) skim fat dry milk powder (Beijing Dingguo, Beijing, China). The μ-calpain primary antibody (monoclonal anti-μ-calpain antibody, MA3-940; Affinity Bioreagents, Golden, CO) was diluted 1:10,000 in mixture of Tris buffer saline and Tween-20 (TBST, pH 7.4). The desmin primary antibody (monoclonal anti-desmin antibody produced in mouse, Abcam, Cambrigde, UK) was 1:500 diluted in TBST. They were both incubated overnight at 4 °C. Membrane rinsed with TBST (6 min × 5) before secondary antibody (conjugated affinity purified) at a dilution of 1:5000 was added. After 1.5 h incubation and six washes with TBST, the membrane was stained with Pierce electrochemiluminescence (ECL) Western blotting Substrate (Thermo Fisher Scientific, NY, USA) for 6 min and detected by ImageQuant LAS 4000 imaging analyzer (GE, CT, USA).
Statistical Analysis
The experimental data analysis was carried out with the Statistical Analysis System version 8.1 (SAS Institute, NC, USA). The mixed model of ANOVA procedure was applied to calculating significance of packaging method, storage time, and their interaction. In mixed model design, type-3 tests of fixed effects for packaging method, storage time and their interaction, and random effects for animal were determined. Least squares mean differences were assessed by the Bonferroni t test and expressed as mean ± SD. The data of calpain activation was expressed by one-way ANOVA mainly focusing on the effect of packaging method. Six repetitions were assayed for all experiments. The experiment was conducted in triplicate for each LD muscle, and results were obtained to determine an average value. In Figures and Tables, values with different letters indicated significant difference at the level of P < 0.05.
Results and Discussion
Carbonyl Content
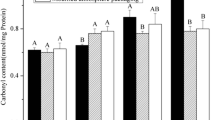
Muscle proteins are potentially highly susceptive to oxidation during postmortem storage leading to protein modification and carbonyl derivative generation (Zhang et al. 2013a, b). The carbonyl derivatives mainly result from direct or indirect oxidation of amino acids in side chain including arginine, lysine, proline, and threonine, fragmentation of protein backbone through α-amidation and β-scission and reactions of lipid peroxidation such as hexanal, semialdehyde, or malondialdehyde binding with proteins (Amici et al. 1989; Burcham and Kuhan 1996). The carbonyl content was significantly affected by packaging method (PM), storage time (ST), and their interaction (PM × ST) (P < 0.05; Fig. 1; Table 2). Pork samples from HiOx showed significantly higher carbonyl content than VP samples after 4 and 6 days of postmortem storage (P < 0.05), while no significant difference (P > 0.05) were found between packaging treatments after 1 day of storage. The level of protein oxidation of HiOx packaging samples significantly increased (P < 0.05) as the storage time extended. However, pork samples from VP showed similar carbonyl content during 6 days of display. These results indicate that high level of available oxygen in meat packaging system is associated with decreased ability to maintain its antioxidant system and increased level of protein oxidation (Astruc et al. 2007). Similar findings were presented by Zakrys-Waliwander et al. (2012) who showed that the carbonyl content was significantly higher after 8 and 14 days of storage at 4 °C in high oxygen packaged beef compared to vacuum packaged samples. In lamb LD and chicken meat, Santé-Lhoutellier et al. (2008) and Xiao et al. (2011) both reported that samples wrapped with air-preamble film showed increased carbonyl content during 7 days of chilled storage
Protein carbonyl content of pork stored in high oxygen modified atmosphere packaging and vacuum packaging during postmortem storage. HiOx high oxygen modified atmosphere packaging with a gas mixture of 80 % O2 and 20 % CO2; VP vacuum packaging; a–c values with different letters indicate significantly different at P < 0.05 (n = 6). Bars indicate standard deviations
Protein Surface Hydrophobicity
The protein surface hydrophobicity was significantly (P < 0.05) affected by PM, while ST and their interaction (PM × ST) showed no significant effect (P > 0.05) on protein surface hydrophobicity (Fig. 2; Table 2). At 4 and 6 days, the protein surface hydrophobicity from HiOx samples was significantly (P < 0.05) higher than samples treated by VP. However, no significant difference (P > 0.05) was found between packaging treatments after 1 day of postmortem display. Changes in protein conformations are usually associated with changes in protein surface hydrophobicity. In addition, biophysical and biochemical properties of proteins including solubility, hydrophobicity can be modified by protein oxidation (Liu and Xiong 2000). Chao et al. (1997) examined the effects of oxygen radical-dependent oxidation on the surface hydrophobicity of rat liver proteins. They reported that oxidative modification of proteins may be responsible for the increase of protein surface hydrophobicity and the modification of protein conformation. Protein denaturation is a process during which the intrinsic conformation of protein molecules such as the unfolding of myofibrillar protein and the rearrangement of protein molecules were opened and non-polar amino acids (mostly hydrophobic groups) buried in protein inside were subsequently exposed to surface (Traore et al. 2012b). Previous studies proposed that higher levels of protein surface hydrophobicity were associated with increased drip loss (Joo et al. 1999a; Joo et al. 1999b). Santé-Lhoutellier et al. (2007) showed that the proteolysis susceptibility was also negatively correlated with carbonyl contents and protein surface hydrophobicity which were explained by the formation of amide bonds and activation of endogenous hydrolytic enzymes. Hence, the role of protein oxidation on the regulation of texture properties through influencing conformational alterations, proteolysis, and activation of proteases needed to be further studied.
Protein surface hydrophobicity of pork stored in high oxygen modified atmosphere packaging and vacuum packaging during postmortem storage. HiOx high oxygen modified atmosphere packaging with a gas mixture of 80 % O2 and 20 % CO2; VP vacuum packaging; a, b values with different letters indicate significantly different at P < 0.05 (n = 6). Bars indicate standard deviations
Protein Solubility
PM and ST significantly affected the solubility of total protein and myofibrillar protein, while sarcoplasmic protein solubility was only significantly influenced by PM (P < 0.05; Table 3). No significant effect (P > 0.05) of PM × ST was found in current study on the solubility of total and sarcoplasmic proteins, whereas significant effect (P < 0.05) of PM × ST was found in solubility of myofibrillar proteins. Compared to samples from VP, samples treated by HiOx showed lower solubility in total proteins at 1 and 4 days of storage (P < 0.05). Pork samples from HiOx showed significantly (P < 0.05) lower myofibrillar protein solubility than samples from VP during postmortem storage. As for sarcoplasmic proteins, significant difference (P < 0.05) was detected between samples from HiOx and VP at 6 days of storage period. Protein solubility was used as an indicator of protein oxidation and denaturation, which in turn results in lower protein solubility by protein aggregation and the formation of insolubility condensate (Joo et al. 1999a). Sayre and Briskey (1963) reported that denatured proteins could precipitate and cover on myosin heavy chain and lower the solubility through influencing the extractability of myofibrillar proteins. In addition, proteins are more prone to denaturation when facing the conformational changes including alterations in secondary and tertiary structures of oxidized proteins. All these changes provide explanation regarding the association between protein solubility and meat texture properties. Dikeman et al. (1971) found a combination of protein solubility, texture score and sarcomere length accounted for 88 and 72 % of the variation in shear force and taste panel tenderness, respectively. Wilson and van Laack (1999) reported that there was a significant correlation between protein solubility and water loss. Similarly, Choi et al. (2010) used two-dimensional electrophoresis to characterize the solubility in total proteins and found that decreased protein solubility was accompanied by increased water loss. All these results indicated that differences between protein solubility and their susceptibility to denaturation may result in changes regarding to meat texture properties.
Calpain Activity
Autolysis of μ-calpain during the conversation of muscle to meat is considered as the index for its history of activation. Once the μ-calpain is activated, intact 80 KDa calpain could be autolyzed to 76 KDa μ-calpain product via an intermediate 78 KDa product. The current study revealed that HiOx samples showed lower intensity of autolyzed 76 KDa subunit and greater intensity of intact 80 KDa compared to VP samples after 1 day of storage (P < 0.05; Table 4). The greater proportion of 76 KDa at 1 day postmortem indicates that μ-calpain was activated earlier which indicated that HiOx could negatively regulate the μ-calpain activity of pork LD muscle. As for greater degree of protein oxidation evidenced by higher content of protein carbonyl, proteases are also the target of free radical attacks, especially cysteine-proteases. Calpain is a family of cysteine-proteases and disulfide bond formed between Cys 108 and Cys 115 within the active cite of calpain could decrease its activity in vitro (Lametsch et al. 2008). Previous studies have shown that calpain system plays a key role in beef tenderness and pork water retention properties through regulating the rate and extent of protein proteolysis (Huff-Lonergan and Lonergan 2005; Huff-Lonergan et al. 2010). The proteolysis of key myofibrillar proteins such as desmin, troponin T, titin, and nebulin helps to effectively keep integrity of muscle and transfer the shrinkage to the entire cell ( Huff-Lonergan et al. 1996; Kristensen and Purslow 2001). Therefore, the postmortem protein degradation can damage the integral structure of muscle cells, increase protein fragmentation, and potentially improve the tenderness (Taylor et al. 1995). Besides, the shrinkage could result in higher water loss from intracellular spaces as reported by Zhang et al. (2006) who found significant correlation between autolysis of μ-calpain at 1 day with drip or purge loss. Similarly, Yin et al. (2014) found decreased calpain activity and less degradation of proteins could contribute to the lower water binding capacity of PSE pork at 1 and 5 days.
Protein Degradation
Desmin is the key protein for structural and functional integrity of muscle through connecting adjacent myofibrils at the Z-lines and integrating myofibrils and cell membrane skeleton ( Huff-Lonergan et al. 1996). Both PM and ST significantly affected the content of desmin (P < 0.05; Table 2), while the interaction between PM and ST showed no significant effect on the extent of desmin degradation (P > 0.05; Table 2). The degradation of desmin from HiOx samples was significantly slowed by treatment of HiOx in comparison with samples treated by VP (P < 0.05; Fig. 3). The interaction between protein oxidation and proteolysis has been extensively studied, whereas few studies focused on their influence on pork texture properties during postmortem aging. In consistent with current results, Zakrys-Waliwander et al. (2012) reported that the postmortem degradation of desmin could damage the orderliness and the integral structure of muscle cells and potentially improve the tenderness of beef samples during postmortem storage. Moreover, because the majority of water is physically or chemically held within the myofibrils or between the muscle bundles and fibers (Kristensen and Purslow 2001), limited degradation of desmin during early postmortem aging could transfer the shrinkage of myofibrils to muscle cells and muscle bundles (Kristensen and Purslow 2001; Melody et al. 2004). A higher level of desmin degradation is believed to be associated with improved water binding capacity during postmortem aging (Kristensen and Purslow 2001; Yin et al. 2014; Zhang et al. 2006). Desmin degradation normally results from the calcium dependent proteolytic enzyme system, especially μ-calpain activity (Yin et al. 2014). Calpain is largely, if not solely, responsible for meat tenderization and water retention properties after postmortem (Huff-Lonergan et al. 1996; Kim et al. 2010). Therefore, concomitant oxidation of proteases and of their myofibrillar substrates can act in synergy to influence the meat texture properties.
a Desmin degradation between high oxygen modified atmosphere packaging (HiOx) and vacuum packaging (VP) during postmortem storage. Twenty eight microgram of total proteins was loaded into each lane. One protein sample whose band presents clear and stable in the page was loaded into each gel as a standard reference (Std, Lane 1). The intact desmin bands of HiOx at 1, 4, and 6 days of postmortem storage were on lane 2, 4, 6, respectively, and the intact desmin bands of VP at 1, 4, and 6 days of postmortem storage were on lane 3, 5, 7, respectively. b Ratios were calculated as the blot intensity of desmin in each gel over the intensity of standard band. a–d values with different letters indicate significantly different at P < 0.05 (n = 6). Bars indicate standard deviations
WBSF Level and Water Loss
The PM, ST, and their interaction showed significant effects on the tenderness of pork LD muscles (P < 0.05; Table 5). The WBSF level of samples from HiOx was significantly higher (P < 0.05) compared to samples treated by VP at 4 and 6 days of storage, while no significance (P > 0.05) was found at 1 day of storage. This is in agreement with the study of Fu et al. (2015) who showed that modified atmosphere packaging decreased beef tenderness after 7 and 10 days of postmortem storage. For the index of water loss, both PM and ST had significant effects on the purge loss and centrifuge loss of pork LD muscles (P < 0.05; Table 5). The purge loss from VP samples was significantly higher (P < 0.05) than samples from HiOx during postmortem storage. However, the VP samples showed lower centrifuge loss compared to HiOx samples at 4 and 6 days of storage (P < 0.05). The results of LF-NMR help to deeply analyze water distribution and behavior of water mobility. Previous studies proposed that P21 corresponded to water located within a highly organized intra-myofibrillar protein matrix and mainly determined by the ability of water binding within myofilament lattice. However, negatively correlation was found between P22 and drip loss, suggesting that the loss of extra-myofibrillar water was easily influenced by structural organizations and modal features within the muscle tissue (Bertram et al. 2002; McDonnell et al. 2013; Pearce et al. 2011). There were significant PM and ST effect (P < 0.05) on P21 and P22 of pork LD muscles and no significant difference (P > 0.05) was observed in P2B. At 4 and 6 days, the P21 levels were significantly (P < 0.05) lower than samples from VP, while the P22 of samples from HiOx showed higher values compared to samples treated by VP (P < 0.05). No PM effect (P > 0.05) was observed on T2B, T21, and T22.
Oxidative environment has been reported to result in increased protein oxidation and decreased myofibril fragmentation in beef (Lindahl et al. 2010) and in pork (Delles and Xiong 2014). These studies suggest that the oxidative conditions could damage the orderliness and integrity of muscle cells, restrict the ability of myofibrils to imbibe water, and thwart water retention of fresh meat through increased protein oxidation or reduced proteolysis or both. In the current study, HiOx could induce protein oxidation and denaturation, evidenced by increased formation of carbonyl compounds, protein surface hydrophobicity, and decreased protein solubility compared with samples stored in VP during postmortem storage (Tables 2 and 3). All these changes between proteins and their susceptibility to denaturation therefore result in variations with regard to meat texture properties. Furthermore, the results showed that samples with the highest shear force and centrifuge loss had the least proportion of calpain autolysis and desmin degradation, respectively. The autolysis of calpain at 1 day is a potential indicator to predict the degree of desmin degradation, which explained the limited proteolytic process under HiOx.
Interestingly, the purge loss with regard to its ability to water holding ability during postmortem storage which could be mainly caused by physical changes in squeezed pressure that samples undergo during packaging procedure. Evidences that support this have been proposed by previous studies (Cayuela et al. 2004; Schluter et al. 1994) where the less purge loss showed that samples from HiOx was found to be inapplicable for the proper relation between increased protein oxidation and the reduction in water binding capacity. HiOx impaired the ability that water bind with myofibrillar proteins of pork, the less amount of purge loss in HiOx muscle compared with VP muscle was largely responsible for water retention properties of fresh pork during postmortem storage. The results were also verified by the distribution of P22 who presented a significant lower ability to withhold mostly free water.
Conclusion
The present results reveal that LD muscle of pork treated by HiOx presented increased carbonyl content, protein surface hydrophobicity, and decreased protein solubility due to higher levels of protein oxidation compared to samples from VP. Increased protein oxidation may negatively regulate the activation of μ-calpain and slow the proteolysis of desmin. The HiOx system is known to improve the fresh meat color, while changes in protein degradation and calpain system through protein oxidation could decrease tenderness and water binding capacity of pork.
Abbreviations
- HiOx:
-
High oxygen modified atmosphere packaging
- VP:
-
Vacuum packaging
- WHC:
-
Water holding capacity
- WBSF:
-
Warner-Bratzler shear force
- LD:
-
Longissimus dorsi
- PM:
-
Packaging method
- ST:
-
Storage time
- PM × ST:
-
Interaction of packaging method and storage time
- LF-NMR:
-
Low-field nuclear magnetic resonance
- DNPH:
-
2,4-Dinitrophenylhydrazine
- BPB:
-
Bromophenol blue
References
Amici, A., Levine, R. L., Tsai, L., & Stadtman, E. R. (1989). Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. Journal of Biological Chemistry, 264(6), 3341–3346.
Astruc, T., Marinova, P., Labas, R., Gatellier, P., & Santé-Lhoutellier, V. (2007). Detection and localization of oxidized proteins in muscle cells by fluorescence microscopy. Journal of Agricultural and Food Chemistry, 55(23), 9554–9558.
Bertram, H. C., Purslow, P. P., & Andersen, H. J. (2002). Relationship between meat structure, water mobility, and distribution: a low-field nuclear magnetic resonance study. Journal of Agricultural and Food Chemistry, 50(4), 824–829.
Burcham, P. C., & Kuhan, Y. T. (1996). Introduction of carbonyl groups into proteins by the lipid peroxidation product, malondialdehyde. Biochemical and Biophysical Research Communications, 220(3), 996–1001.
Carlin, K. R., Huff-Lonergan, E., Rowe, L. J., & Lonergan, S. M. (2006). Effect of oxidation, pH, and ionic strength on calpastatin inhibition of μ-and m-calpain. Journal of Animal Science, 84(4), 925–937.
Carr, H. Y., & Purcell, E. M. (1954). Effects of diffusion on free precession in nuclear magnetic resonance experiments. Physical Review, 94(3), 630–638.
Cayuela, J. M., Gil, M. D., Bañón, S., & Garrido, M. D. (2004). Effect of vacuum and modified atmosphere packaging on the quality of pork loin. European Food Research and Technology, 219(4), 316–320.
Chao, C. C., Ma, Y. S., & Stadtman, E. R. (1997). Modification of protein surface hydrophobicity and methionine oxidation by oxidative systems. Proceedings of the National Academy of Sciences, 94(7), 2969–2974.
Chelh, I., Gatellier, P., & Santé-Lhoutellier, V. (2006). Technical note: a simplified procedure for myofibril hydrophobicity determination. Meat Science, 74(4), 681–683.
Cheng, Q., & Sun, D. W. (2008). Factors affecting the water holding capacity of red meat products: a review of recent research advances. Critical Reviews in Food Science and Nutrition, 48(2), 137–159.
Choi, Y. M., Lee, S. H., Choe, J. H., Rhee, M. S., Lee, S. K., Joo, S. T., & Kim, B. C. (2010). Protein solubility is related to myosin isoforms, muscle fiber types, meat quality traits, and postmortem protein changes in porcine longissimus dorsi muscle. Livestock Science, 127(2), 183–191.
Delles, R. M., & Xiong, Y. L. (2014). The effect of protein oxidation on hydration and water-binding in pork packaged in an oxygen-enriched atmosphere. Meat Science, 97(2), 181–188.
Dikeman, M. E., Tuma, H J. & Beecher G. R. (1971). Bovine muscle tenderness as related to protein solubility. Journal of Food Science, 36(2), 190-193.
Fu, Q. Q., Liu, R., Zhang, W. G., Li, Y. P., Wang, J., & Zhou, G. H. (2015). Effects of different packaging systems on beef tenderness through protein modifications. Food and Bioprocess Technology, 8, 580–588.
Goll, D. E., Thompson, V. F., Li, H., Wei, W. E. I., & Cong, J. (2003). The calpain system. Physiological Reviews, 83(3), 731–801.
Huff-Lonergan, E., & Lonergan, S. M. (2005). Mechanisms of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Science, 71(1), 194–204.
Huff-Lonergan, E., Mitsuhashi, T., Beekman, D. D., Parrish, J. F. C., Olson, D. G., & Robson, R. M. (1996). Proteolysis of specific muscle structural proteins by mu-calpain at low pH and temperature is similar to degradation in postmortem bovine muscle. Journal of Animal Science, 74(5), 993–1008.
Huff-Lonergan, E., Zhang, W. G., & Lonergan, S. M. (2010). Biochemistry of postmortem muscle - lessons on mechanisms of meat tenderization. Meat Science, 86(1), 184–195.
Jongberg, S., Wen, J. Z., Tørngren, M. A., & Lund, M. N. (2014). Effect of high-oxygen atmosphere packaging on oxidative stability and sensory quality of two chicken muscles during chill storage. Food Packaging and Shelf Life, 1(1), 38–48.
Joo, S. T., Kauffman, R. G., Kim, B. C., & Park, G. B. (1999a). The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle. Meat Science, 52(3), 291–297.
Joo, S. T., Kauffman, R. G., Laack, R., Lee, S., & Kim, B. C. (1999b). Variations in rate of water loss as related to different types of post-rigor porcine musculature during storage. Journal of Food Science, 64(5), 865–868.
Kim, Y. H., Huff-Lonergan, E., Sebranek, J. G.. & Lonergan, S. M. (2010). High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Science, 85(4), 759-767.
Koohmaraie, M., & Geesink, G. H. (2006). Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Science, 74(1), 34–43.
Kristensen, L., & Purslow, P. P. (2001). The effect of ageing on the water-holding capacity of pork: role of cytoskeletal proteins. Meat Science, 58(1), 17–23.
Ladikos, D., & Lougovois, V. (1990). Lipid oxidation in muscle foods: a review. Food Chemistry, 35(4), 295–314.
Lametsch, R., Lonergan, S., & Huff-Lonergan, E. (2008). Disulfide bond within μ-calpain active site inhibits activity and autolysis. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1784(9), 1215–1221.
Lindahl, G., Lagerstedt, Å., Ertbjerg, P., Sampels, S., & Lundström, K. (2010). Ageing of large cuts of beef loin in vacuum or high oxygen modified atmosphere—effect on shear force, calpain activity, desmin degradation and protein oxidation. Meat Science, 85(1), 160–166.
Liu, G., & Xiong, Y. L. (2000). Electrophoretic pattern, thermal denaturation, and in vitro digestibility of oxidized myosin. Journal of Agricultural and Food Chemistry, 48(3), 624–630.
Lund, M. N., Heinonen, M., Baron, C. P., & Estevez, M. (2011). Protein oxidation in muscle foods: a review. Molecular Nutrition & Food Research, 55(1), 83–95.
Lund, M. N., Lametsch, R., Hviid, M. S., Jensen, O. N., & Skibsted, L. H. (2007). High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Science, 77(3), 295–303.
McDonnell, C. K., Allen, P., Duggan, E., Arimi, J. M., Casey, E., Duane, G., & Lyng, J. G. (2013). The effect of salt and fibre direction on water dynamics, distribution and mobility in pork muscle: a low field NMR study. Meat Science, 95(1), 51–58.
Meiboom, S., & Gill, D. (1958). Modified spin-echo method for measuring nuclear relaxation times. Review of Scientific Instruments, 29(8), 688–691.
Melody, J. L., Lonergan, S. M., Rowe, L. J., Huiatt, T. W., Mayes, M. S., & Huff-Lonergan, E. (2004). Early postmortem biochemical factors influence tenderness and water-holding capacity of three porcine muscles. Journal of Animal Science, 82(4), 1195–1205.
Pearce, K. L., Rosenvold, K., Andersen, H. J., & Hopkins, D. L. (2011). Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—a review. Meat Science, 89(2), 111–124.
Rowe, L. J., Maddock, K. R., Lonergan, S. M., & Huff-Lonergan, E. (2004). Oxidative environments decrease tenderization of beef steaks through inactivation of μ-calpain. Journal of Animal Science, 82(11), 3254–3266.
Santé-Lhoutellier, V., Aubry, L., & Gatellier, P. (2007). Effect of oxidation on in vitro digestibility of skeletal muscle myofibrillar proteins. Journal of Agricultural and Food Chemistry, 55(13), 5343–5348.
Santé-Lhoutellier, V., Engel, E., Aubry, L., & Gatellier, P. (2008). Effect of animal (lamb) diet and meat storage on myofibrillar protein oxidation and in vitro digestibility. Meat Science, 79(4), 777–783.
Sayre, R. N., & Briskey, E. J. (1963). Protein solubility as influenced by physiological conditions in the muscle. Journal of Food Science, 28(6), 675–679.
Schluter, A. R., Miller, M. F., Jones, D. K., Meade, M. K., Ramsey, C. B., & Patterson, L. L. (1994). Effects of distribution packaging method and storage time on the physical properties and retail display characteristics of pork. Meat Science, 37(2), 257–269.
Taylor, R. G., Geesink, G. H., Thompson, V. F., Koohmaraie, M., & Goll, D. E. (1995). Is Z-disk degradation responsible for postmortem tenderization? Journal of Animal Science, 73(5), 1351–1367.
Traore, S., Aubry, L., Gatellier, P., Przybylski, W., Jaworska, D., Kajak-Siemaszko, K., & Santé-Lhoutellier, V. (2012a). Higher drip loss is associated with protein oxidation. Meat Science, 90(1), 917–924.
Traore, S., Aubry, L., Gatellier, P., Przybylski, W., Jaworska, D., Kajak-Siemaszko, K., & Santé-Lhoutellier, V. (2012b). Effect of heat treatment on protein oxidation in pig meat. Meat Science, 91(1), 14–21.
Trout, G. R. (1988). Techniques for measuring water-binding capacity in muscle foods—a review of methodology. Meat Science, 23(4), 235–252.
Veiseth, E., Shackelford, S. D., Wheeler, T. L., & Koohmaraie, M. (2001). Effect of postmortem storage on mu-calpain and m-calpain in ovine skeletal muscle. Journal of Animal Science, 79(6), 1502–1508.
Wilson, G. G., & van Laack, R. L. J. M. (1999). Sarcoplasmic proteins influence water-holding capacity of pork myofibrils. Journal of the Science of Food and Agriculture, 79(13), 1939–1942.
Xiao, S., Zhang, W. G., Lee, E. J., Ma, C. W., & Ahn, D. U. (2011). Lipid and protein oxidation of chicken breast rolls as affected by dietary oxidation levels and packaging. Journal of Food Science, 76(4), C612–C617.
Xiong, Y. L. (2005). Role of myofibrillar proteins in water-binding in brine-enhanced meats. Food Research International, 38(3), 281–287.
Xue, M., Huang, F., Huang, M., & Zhou, G. H. (2012). Influence of oxidation on myofibrillar proteins degradation from bovine via μ-calpain. Food Chemistry, 134(1), 106–112.
Yin, Y., Zhang, W. G., Zhou, G. H., & Guo, B. (2014). Comparison of protein degradation, protein oxidation, and μ-calpain activation between pale, soft, and exudative and red, firm, and nonexudative pork during postmortem aging. Journal of Animal Science, 92(8), 3745–3752.
Zakrys-Waliwander, P. I., O’Sullivan, M. G., O’Neill, E. E., & Kerry, J. P. (2012). The effects of high oxygen modified atmosphere packaging on protein oxidation of bovine M. longissimus dorsi muscle during chilled storage. Food Chemistry, 131(2), 527–532.
Zhang, W. G., Lonergan, S. M., Gardner, M. A., & Huff-Lonergan, E. (2006). Contribution of postmortem changes of integrin, desmin and μ-calpain to variation in water holding capacity of pork. Meat Science, 74(3), 578–585.
Zhang, W. G., Marwan, A. H., Samaraweera, H., Lee, E. J., & Ahn, D. U. (2013a). Breast meat quality of broiler chickens can be affected by managing the level of nitric oxide. Poultry Science, 92, 3044–3049.
Zhang, W. G., Xiao, S., & Ahn, D. U. (2013b). Protein oxidation: basic principles and implications for meat quality. Critical Reviews in Food Science and Nutrition, 53(11), 1191–1201.
Acknowledgments
The authors would like to thank The National Natural Science Foundation of China (31271899) and The Ministry of Science and Technology of China (2012BAD28B03) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Zhou, Gh. & Zhang, Wg. Effects of High Oxygen Packaging on Tenderness and Water Holding Capacity of Pork Through Protein Oxidation. Food Bioprocess Technol 8, 2287–2297 (2015). https://doi.org/10.1007/s11947-015-1566-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1566-0