Abstract
This study describes novel formulations and approaches to produce reconstitutable (in water) spray dried kiwifruit milk powders that possess the intrinsic fruit colour and antioxidants of kiwifruits. Novel feed formulations, containing the aqueous fraction of green or gold kiwifruit puree, skim milk, fortifying zinc (in the form of zinc citrate) and a minimal amount of maltodextrin (3 % w/w), were spray dried at a relatively low inlet temperature (150 °C) to produce kiwifruit juice–milk powders with good dissolution efficiency (complete dissolution in 21–28 s), low water activity (0.22–0.28) and high retention of vitamin C, phenolics and carotenoid antioxidants. Both the green and gold kiwifruit juice–milk powders were pseudoplastic-like materials. Their reconstituted aqueous solutions had viscosity of 2–23 mPa · s and exhibited a shear rate dependence. Adding kiwifruit pulp residue to the feed solution at a mass ratio of 25:75 significantly enhanced the dissolution efficiency and the natural kiwifruit colour of the product powders without affecting the total phenolic and vitamin C contents. Preheating the feed solution to 50 °C for 1 min had a positive impact on the physicochemical attributes and bioactive profile of kiwifruit juice–milk powders, including a greater retention of initial kiwifruit juice colour, lower water activity, better dissolution efficiency and a higher total phenolic content. The powders obtained in this study contain multiple health-promoting nutrients and bioactives including kiwifruit antioxidants and milk proteins, and can be used in beverages or as functional ingredients for nutraceutical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Milk powder is one of the most widely consumed dairy products worldwide due to its excellent nutritional value and handling convenience. With the growing public awareness of the benefits of healthy eating, considerable opportunities also exist in the fruit juice sectors where fortified juice products are gaining increasing market share. Nowadays, fruit- and dairy-based ingredients are increasingly being used together to produce consumer products because of the broad range of intrinsic nutrients and bioactives contained therein, such as proteins, dietary fibres, antioxidants and minerals (Shinde et al. 2014; Sun-Waterhouse et al. 2013a; Rashidinejad et al. 2013). However, such products are technically challenging to manufacture because fruit acids can cause disintegration of casein micelles, precipitation of caseins and peptides and aggregation of whey proteins (Sun-Waterhouse et al. 2013a; Pearce 1983). Also, fruit phenolics can interact with milk protein via complexation to decrease the antioxidant content (Rawel et al. 2001; Perez-Jimenez and Saura-Calixto 2006). Overcoming these challenges to produce a kiwifruit juice–milk powder that can be reconstituted in water, which contains the bioactives and colour of kiwifruit and milk, is the focus of this current study.
Kiwifruit (Actinidia sp.) has many appealing consumer attributes including its colour, flavour and nutrients (Nishiyama 2007). Regular consumption of kiwifruit promotes human well-being, via improving immune system, digestive and eye health (Montoya et al. 2014; Parkar et al. 2010; Tan et al. 2008; Philpott et al. 2007) and protecting against respiratory problems, DNA damage and oxidative stress (Chan et al. 2007; Rush et al. 2006; Duttaroy and Jorgensen 2004; Forastiere et al. 2000). However, whole kiwifruits are highly perishable and their juice products are unstable during processing or storage due to enzymatic/nonenzymatic mechanisms (Sun-Waterhouse et al. 2009a, b; Lamikanra 2002; Richard-Forget and Gauillard 1997; Wong and Stanton 1989). Spray drying of kiwifruit beverage powders is generally difficult because of the considerable loss of intrinsic nutrients and colour that occurs during the process, and the poor processibility resulting from its calcium oxalate, sugars and organic acids (Cassano et al. 2006; Rasam and Laing 2005; Lodge and Robertson 1990).
Spray drying is an established cost-effective preservation method (Cano-Chauca et al. 2005; Reineccius 2004) and an efficient microencapsulation approach for bioactives (Soukoulis et al. 2014; Sun-Waterhouse et al. 2013b; Bakar et al. 2013). Stickiness is a major problem in spray drying of fruit juice-containing products due to the high concentrations of low molecular-weight sugars and organic acids (Bhandari et al. 1993, 1997). High molecular weight drying aids such as maltodextrin are often added to the feed before spray drying to overcome this problem whilst reducing powder hygroscopicity (Saénz et al. 2009; Righetto and Netto 2005). Maltodextrins, comprising D-glucose units linked mainly via (1 → 4) glycosidic bonds, are considered safe by the US Food and Drug Administration (FDA) and have been used to produce powders from Andes berry (Villacrez et al. 2014), red pitaya (Bakar et al. 2013), Gac fruit (Kha et al. 2010), cactus pear (Obon et al. 2009) and black carrot (Ersus and Yurdagel 2007). However, the FDA does not necessarily equate ‘safe’ with ‘healthy’. The healthiness of maltodextrins has been a subject of recent concern, due to its high glycemic index (>130) and potential unhealthy effects on humans upon frequent consumption (Brand-Miller et al. 2013; Whelan 2004; http://www.bloodindex.org/glycemic_index.php). Mixing a high concentration of maltodextrin with fruit juice/puree may cause undesirable flavour. Thus, minimising maltodextran usage is an important consideration for powdered beverages that target wellness or ‘everyday consumption’.
In this study, the feasibility of spray drying kiwifruit juice–milk mixtures, using minimal maltodextrin, is systematically explored. To our knowledge, no previous work has been reported examining spray-dried kiwifruit juice–milk powders. The proposed combined use of kiwifruit juice and milk has the potential to deliver a spray dried powder product that conveys the intrinsic bioactives of kiwifruit and milk and the natural kiwifruit colour. Further, by using naturally occurring fibre polysaccharides in the kiwifruit juice and proteins in milk as encapsulants, the addition of maltodextrin could be minimised. This study aimed to fill an existing knowledge gap and to synergistically manipulate the interactions amongst different food components to produce kiwifruit juice–milk powders containing the natural goodness of both kiwifruit and milk and also minimal maltodextrin.
Materials and Methods
Chemicals and Materials
Skim milk (HOMEBRAND, containing fat 0.4 g, protein 3.7 g and carbohydrate or sugars 4.9 g per 100 mL milk), green or gold kiwifruit and baking soda (Edmonds, from Goodmans Fielder Ltd., Auckland, New Zealand) were purchased from the Countdown supermarket, Newmarket, Auckland. Maltodextrin 12 DE (Glucidex®) was from Roquette, France. Zinc citrate was from Jungbunzlauer Ladenburg, Ladenburg, Germany. HPLC grade acetonitrile was purchased from J. T. Baker (Phillipsburg, NJ, USA). Methanol, n-hexane and formic acid were purchased from Ajax Finechem (Auckland, New Zealand). Acetone was from Burdick & Jackson (Muskegon, MI, USA). Folin–Ciocalteu phenol reagent, catechin, epicatechin, kaempferol, phlorizin, phloretin, procyanidin B1, quercetin, rutin, quercetin-glucoside, quercetin-rhamnoside, gallic acid, caffeic acid, chlorogenic acid, 3,4-dihydroxybenzoic acid, p-hydroxybenzoic acid, p-coumaric acid, protocatechuic acid, beta-carotene, lutein, antheraxanthin, violaxanthin, zeaxanthin and chlorophylls were purchased from Sigma-Aldrich (St. Louis, MO, USA). Milli-Q Plus water (i.e. the deionized water after further purification and with a resistivity of 18.2 MΩ/cm at 25 °C) was used for all the reagent preparation.
Spray Drying of Kiwifruit Juice–Milk Powders
Green or gold kiwifruits with a firmness range of 2.85–3.25 kgf (determined using a hand penetrometer; Model FT-327, Effegi, Alphonsine, Italy) were firstly washed with water to remove any external contaminants. Their pHs and soluble solid contents were measured. Both ends of each fruit were cut off and the skin was removed with a sterilized stainless steel peeler (APS001; Andefia, Zhongshan, China). The kiwifruits were pureed using a Juice Fountain Juicer (Model BJE 200C; Breville, Sydney, New South Wales, Australia), centrifuged at a speed of 5,000×g for 25 min at 4 °C using a Sorvall Instrument RC5C (DuPont, Michigan, WI, USA) and a Fibrelite F10s rotor (Piramoon Technologies Inc, Santa Clara, CA, USA). The resultant aqueous fraction and pulp solid residue were collected separately. The aqueous fraction was filtered through a sterilized 0.8-mm mesh cloth to eliminate suspended solids. The mass yield of puree and derived aqueous fraction were 51.06 g and 43.76 g wet weight per 100 g fresh weight green kiwifruit, and 44.07 g and 36.03 g wet weight per 100 g fresh weight gold kiwifruit, respectively.
The aqueous fraction of kiwifruit puree and skim milk were blended in a volume ratio of 40:60 before Maltodextrin 12 DE was added at 3 % w/w with gentle stirring. The mixture (‘feed solution’) was homogenized using a shear homogenizer (500 rpm × 1 min × 3 bursts, Silverson L4R, Buckinghamshire, UK). All the feed solutions were kept at room temperature (i.e. 20 ± 1 °C) under magnetic stirring and in the dark (wrapped in aluminium foil) during spray drying. Spray drying was carried out using a spray dryer (Büchi mini B-290, Büchi Labortechnik AG, Switzerland) equipped with a 0.7-mm standard diameter nozzle and operated under these conditions: inlet temperature 150 ± 1 °C, air flow rate 100 L/h, feed flow rate 10 mL/min, atomization pressure 20 psi (~0.138 MPa) and pump speed 30 %. The resultant powder is referred to ‘kiwifruit juice–milk powder’ in the text below. The spray drying conditions were determined after preliminary trials and selected as the ‘optimal and constant variables’ for all of the spray drying experiments conducted in this study, in order to focus on the effects of formulation (including the addition of kiwifruit pulp residue) and pre-treatment (i.e. pre-heating the feed solution).
In a separate set of experiments, the feed solutions were preheated at 50 °C for 1 min prior to spray drying (i.e. the conditions remained the same as described above for the kiwifruit juice–milk powder). The resultant powder is termed as ‘preheated kiwifruit juice–milk powder’.
In another set of experiments, the pulp residue collected from centrifugation of the kiwifruit puree was firstly warmed to 50 °C before baking soda was added to give a pH of 5.5, to which zinc citrate (a final concentration, 15 ppm) was added with gentle stirring. The obtained mixture was immediately blended with the feed solution of the ‘kiwifruit juice–milk powder’ at a 15:85 mass ratio, followed by homogenisation using the Silverson homogenizer (1,000 rpm × 1 min × 3 bursts) and spray drying (i.e. the conditions remained the same as described above for the kiwifruit juice–milk powder). The resultant powder is termed as ‘kiwifruit juice–milk-pulp residue powder’ in the text below.
All the obtained spray dried powders were transferred to high-density polyethylene zip lock plastic bags for temporary storage and immediate repeated use (due to the convenience of zip lock feature). A portion of each freshly prepared spray-dried powder was subjected to product evaluation including measurement of colour, water activity, bulk density and/or surface area/porosity. A second portion of the freshly prepared powder was reconstituted in water for dissolution efficiency and viscosity determination. A third portion of the freshly prepared powder was subjected to storage trials at 4 ± 1 °C (for 30 days) and 20 ± 2 °C (for 30 and 60 days). Any remaining powder was transferred to a food grade aluminum foil pouch and stored in the dark at −20 ± 2 °C until chemical analyses.
Physicochemical Analyses
Measurement of the Total Soluble Solid Content and pH
The total soluble solid content of fresh kiwifruit puree and derived aqueous fractions was determined in triplicate at 20 °C using a handheld refractometer (Pocket Pal-1, Atago, Tokyo, Japan) and expressed as °Brix (which is based on the relationship between refractive index and the total soluble solid content of a pure aqueous sucrose solution). The pH was determined in triplicate using a pH meter (CG837, Schott Instruments, Germany) equipped with a glass electrode (850, Schott Instruments, Mainz, Germany).
Bulk Density, Water Activity, Hygroscopicity and Yield of Spray Dried Powders
The bulk density of the spray-dried powders was determined by accurately measuring the sample weight using an analytical balance (Mettler Toledo, XS205 dual range, Mettler-Toledo International Inc., Greifensee, Switzerland) and the bulk volume using a Stec Pycnometer (VM 100 STEC Volumeter, Florida, USA).
The water activity (a w ) of the spray-dried powders was measured at room temperature using a water activity meter (AQUA LAB CX-2, Decagon Devices Inc., Pullman, WA, USA). The hygroscopicity of the spray-dried powder was evaluated by spreading each powder (~1 g, weighed accurately, termed ‘Winitial’) evenly on Petri dishes (9 cm diameter), placing these Petri dishes in a desiccator at 20 ± 1 °C and 75 ± 1 % over a saturated solution of NaCl for 7 days, then recording the final weight of powder (Wfinal), and calculating using the following equation:
The yield (%) of the spray drying process was evaluated as the ratio of the weight of the powder produced and the feed mixture consumed on a dry weight basis. The weight of the powder produced was defined as the solid powder collected in the drying chamber and the cyclone (which was the difference between the weight of the dry mass in the feed consumed and the weight the powder deposited/left on the walls of spray dryer). The following equation was used for calculating the yield:
Colour Measurement
The colour of spray dried powders was measured using a Minolta CR-300 colorimeter (Konica Minolta Sensing Inc., Osaka, Japan) and expressed as L*, a* and b* values. L* value defines the lightness (L* = 0 for black and L* = 100 for white), and a* and b* values define the red-greenness (green [−] to red [+]) and yellow-blueness (blue [−] to yellow [+]), respectively. Triplicate measurements were taken on each sample. Total colour difference (ΔE*) between the ‘kiwifruit juice–milk’ powder and the ‘preheated kiwifruit juice–milk’ powder or ‘kiwifruit juice–milk–pulp residue’ powder was calculated using the following equation:
N2 Physisorption Measurements of Powder Surface Area and Mesoporosity
N2 physisorption measurements were carried out at liquid nitrogen temperature (−195 °C) using an analyzer (Tristar 3000 instrument, Micromeritics, Georgia, USA). Samples were degassed in a Heraeus vacuum chamber at 40 °C for 1 h prior to the N2 physisorption measurements. Brunauer–Emmett–Teller (BET) specific surface areas and Barrett–Joyner–Halenda (BJH) adsorption average pore diameters were determined from N2 physisorption isotherms taken at −195 °C (Condon 2006).
Phenolic Analyses
Extraction of Phenolics
Extraction of phenolics from kiwifruit purees, derived aqueous fractions and spray dried powders was performed in triplicate via accelerated solvent extraction (ASE) (ASE300, Dionex Corp., Sunnyvale, CA, USA). The test sample (3 g) was mixed with diatomaceous earth (Celite®, Maville Service Corporation, USA) (4 g) to facilitate homogenous mixing. The resultant mixture was packed into 34 mL extraction cells. Three cycles of extraction were carried out using acetone, methanol and hexane, respectively under N2 (at 40 °C and 1,500 psi, with 5 min heating and 10 min static time). The obtained extracts were collected separately, concentrated using a Labconco RapidVap® concentrator (Model 790000, Labconco Corp., Kansas City, Missouri, USA) under N2 (at 40 °C and 10 KPa for 50 min) and a Ultra-Low Cold Trap Centrivap® concentrator (Model 78100–01, Labconco Corp., Kansas City, MO; 30 °C for 3 h), then freeze-dried at −10 °C (Telstar Cryodos-80 Freeze Drier, Telstar Industrial, SL, Terrassa, Spain), and stored at −80 °C until analysis of total phenolic content or HPLC profiling.
Determination of Total Extracted Phenolic Content (TEPC)
TEPC was determined via the Folin-Ciocalteu assay (Singleton et al. 1997). The extracts obtained above were pretreated before the Folin–Ciocalteu assay using a solid phase extraction (SPE) column, to minimise the interference of sugar and ascorbic acid. The first eluate from SPE was used for the analysis of vitamin C content (see the ‘Determination of Vitamin C Content’ section). The eluates resulting from the subsequent flushes with 95 % methanol (5 mL per flush) were collected for TEPC analysis. Three measurements were carried out on each test sample using a microplate reader (SpectraMax Plus 384, Molecular Devices, Sunnyvale, USA; absorbance at 760 nm), and the TEPC results are expressed as mg catechin equivalent (CtE) per gram or mL test sample.
High Pressure Liquid Chromatography (HPLC) Analysis of Individual Phenolics
Freeze-dried phenolic extracts were dissolved in 25 % methanol at a 10-mg/mL concentration, vortexed and centrifuged at 3,000 rpm for 10 min (Eppendorf Centrifuge 5702, Hamburg, Germany) to recover any insoluble solids. The resultant supernatant was analysed (in duplicate, over the wavelength range of 200–530 nm) following the method of Stevenson et al. (2006) and using a Shimadzu analytical HPLC equipped with a column oven (C40-10ASVP), auto-sampler (SIL-10AF), vacuum solvent degas module and diode-array detector (SPD-M10AVP), and fitted with a Synergi® Polar-RP ether-linked column (250 × 4.6 mm, 4 μm particle size, 80 Å ether-linked column, at 45 °C, Phenomenex, Auckland, New Zealand). The mobile phases consisting of (A) acetonitrile + 0.1 % formic acid and (B) acetonitrile/water/formic acid (5:92:3) were pumped at a rate of 1.0 mL/min and at 45 °C. The injection volume was 30 μL. Individual phenolics were identified through comparing their retention time and absorbance maximum (λmax) with those of external standards (catechin, caffeic acid, chlorogenic acid, gallic acid, epicatechin, 2,4-dihydroxybenzoic acid, 3,4-dihydroxybenzoic acid, ferulic acid, p-hydroxybenzoic acid, protocatechuic acid, salicylic acid, syringic acid, p-/m-/o-coumaric acids, phloretin, phlorizin, procyanidin B1, quercetin, rutin, quercetin-glucoside and quercetin-rhamnoside), and based on our previous HPLC analysis of kiwifruit products (Sun-Waterhouse et al. 2009a, b, 2013a). Most of the individual phenolics were quantified using the internal standard phlorizin at a wavelength of 280 nm while the flavonols were quantified using kaempferol at 370 nm.
Analysis of Carotenoids and Chlorophylls
Extraction of Carotenoids and Chlorophylls
Three cycles of extraction of the carotenoids and chlorophylls in the spray dried powder were performed (in triplicate) with acetone in an environment with no direct light and minimised remote lighting, using the ASE method (purging time, 90 s; flush volume, 60 %; static time, 10 min for the extraction at 40 °C and 2 min for the extraction at 100 °C). The spray dried powders (4 g) were mixed with diatomaceous earth (Celite®) at a ratio of 1:1. The obtained extracts were collected and dried using the Ultra-Low Cold Trap Centrivap® concentrator prior to HPLC analysis.
HPLC Profiling of Carotenoids and Chlorophylls
The extracts obtained above were analyzed in duplicate using the Shimadzu analytical HPLC described in the ‘Phenolic Analyses’ section and using the gradient system described in Ampomah-Dwamena et al. (2009). The HPLC profiles were monitored at wavelengths of 300–700 nm by a photo diode array 996 Waters detector. The chromatographic peaks at 450 nm were identified according to their retention times and UV–vis spectra, and through comparing with the authentic standards of beta-carotene, lutein, antheraxanthin, violaxanthin, zeaxanthin and chlorophylls and published data (Sun-Waterhouse et al. 2013a; Ampomah-Dwamena et al. 2009; Rodriquez-Amaya 1999; Jeffrey et al. 1997). The concentrations of the identified carotenoids and chlorophylls were evaluated using a lutein calibration curve.
Determination of Vitamin C Content
The vitamin C content in the puree, derived aqueous fraction and spray dried powder from each replicate group of the green or gold kiwifruits was analysed (in duplicate) using the titration method of the Association of Official Analytical Chemists (AOAC 1990).
Determination of Uronic Acid (UA) Content
The pectin content of a test sample was expressed as galacturonic acid (GalA) equivalents. The amount of its building block, uronic acid (UA), was determined (in duplicate) following the procedures of Sun-Waterhouse et al. (2010). The UA content of test samples hydrolysed with concentrated sulphuric acid was measured colorimetrically at 525 nm using the method of Filisetti-Cozzi and Carpita (1991).
Reconstitution of Spray Dried Powder and Associated Analyses
Dissolution Efficiency Test
Dissolution efficiency was evaluated in triplicate through reconstituting spray-dried powder (5 g) in Milli-Q water (100 mL) at 20 ± 2 °C. The obtained mixture was vortexed (MT19 vortex mixer, Chiltern International, Slough, UK; 3 × 30 s at ‘speed 4’ setting, i.e. speed scale 1–8 with 1 the lowest and 8 the highest). The time taken to fully reconstitute the powders was recorded.
Viscosity of the Reconstituted Aqueous Solutions
The viscosity of the reconstituted aqueous solutions (25 mL) was examined at 20 ± 1 °C, using a stress-controlled rheometer (StressTech, Reologica, Lund, Sweden) equipped with a bob (diameter 25 mm) and cup (diameter 27.5 mm) geometry and a humidity chamber (to prevent water loss) and set up with a measurement gap distance of 1 mm. The viscosity was measured as a function of shear rate over the range of 1–100 s−1 including those simulating the effective shear rate range (40–50 s−1) in the mouth (Wood and Goff 1973). All tests were performed in triplicate.
Statistical Analysis
All data are expressed as ‘mean ± standard deviation’ from at six replicates (i.e. processing experiments were run in duplicate and analysis of the resultant test samples were performed in triplicate). Analyses were carried out using MINITAB 15 (Minitab Inc., Pennsylvania, USA) statistical software with one-way ANOVA procedure followed by Tukey’s multiple comparison test at p < 0.05.
Results and Discussion
Physicochemical Attributes of the Kiwifruit Juice–Milk Spray-Dried Powders
The green or gold kiwifruit juice–milk powders produced in this study were free of scorched particles, brown specks and lumps, and had a yield of 58.8 % and 53.2 %, respectively. The kiwifruit juice–milk powders had uniform and appealing light green or light yellow colour, and the pleasant aroma of green or gold kiwifruit, respectively. Milk and maltodextrin ingredients that were included in the feed formulations for spray drying diluted the colour intensity of the final kiwifruit juice–milk powders. Conventional spray drying parameters (typically inlet temperatures of 150–200 °C) could be expected to impact negatively on powder colour. During spray drying, water is removed in the form of vapour from the sprayed particles, and the hot air in the vicinity of the particles takes up such moisture. The green pigments (chlorophyll a and b) are thermally unstable and readily lose Mg2+ to form brown pheophytin under acidic conditions (Cassano et al. 2006). Carotenoids are susceptible to heat damage and oxidation due to their multiple unsaturated bonds (Stefanovich and Karel 1982). Phenolics and vitamin C are also sensitive to environmental conditions such as pH, water activity, light exposure, oxygen and temperature, and are likely to degrade under detrimental conditions causing changes in product colour (Cilliers and Singleton 1989; Richard-Forget and Gauillard 1997; Kennedy et al. 1989). In this study, a relatively low inlet temperature (150 ± 1 °C) was employed for spray drying, which minimised the degradation of these pigments and bioactives.
The two types of kiwifruit juice–milk powders derived from green or gold kiwifruit juice, had almost the same water activity (i.e. 0.26–0.28; Table 1). Such a low water activity range is consistent with the visual observations, i.e. all the obtained spray-dried powders were free flowing powders which is typical for products with water activity < 0.5. Water activity predicts the interactions between water and powder components and is used to gauge product storage stability. The low water activities of the kiwifruit juice–milk powders produced here indicate a low risk of microbial spoilage and a good expected shelf life.
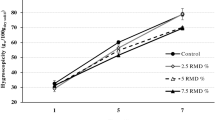
There were differences in powder hygroscopicity and surface area between the two types of kiwifruit juice–milk powders. The green kiwifruit juice–milk powder had a greater hygroscopicity (0.306 g H2O/g powder), a higher BET surface area (0.99 m2/g powder) and a lower BJH adsorption average pore diameter (31.48 nm) than the gold kiwifruit juice–milk powder (i.e. 0.248 g H2O/g powder, 0.89 m2/g powder and 35.12 nm, respectively). Although the particle size of the spray-dried powders was not determined directly, these results suggest that the green kiwifruit juice–milk powder possessed a smaller particle size. The relatively low hygroscopicity values suggest low risk of handling issues (unlike most fruit juice-containing powders which have high intrinsic hygroscopicity). The addition of milk to the spray-drying feed may confer some benefits in this regard. The time required for the green kiwifruit juice–milk powder to be fully reconstituted in water was shorter (i.e. 23 s) than the gold kiwifruit juice–milk powder (i.e. 28 s), again suggesting that the green kiwifruit juice–milk powder had a smaller particle size. The short reconstitution time (23–28 s) of present kiwifruit juice–milk powders suggests the suitability of both types of powder for powdered beverage applications. The type of fruit juice influences to some extent the physicochemical properties of spray-dried powders. The relatively low inlet temperature (i.e. 150 ± 1 °C) used in this study could lead to less denaturation of protein and aid powder solubility in water.
The aqueous fractions of green and gold kiwifruit purees had different compositions in terms of their total phenolic, vitamin C and pectin contents, although both aqueous fractions showed similarity in pH (~3.5-3.6) and the total soluble solid content (~14 °Brix). The aqueous fraction from green kiwifruit had lower total phenolic and vitamin C contents (i.e. 1.48 mg CtE/mL and 0.95 mg ascorbic acid/mL, respectively) but a slightly high pectin content (1.02 % w/w, as GalA), compared to the aqueous fraction from gold kiwifruit (1.70 mg CtE/mL and 1.06 mg ascorbic acid/mL, and 0.90 % w/w, respectively). The differences between the two types of kiwifruit aqueous fractions in their composition likely accounted for the differences in some of the physicochemical attributes of their derived spray dried powders such as hygroscopicity and surface characteristics. These physicochemical properties are associated with the interactions between the small molecules (such as phenolics, vitamin C, water and gases including O2) and macromolecules (such as milk proteins, pectins and maltodextrin) during spray drying. As a result, the solid network of the green and gold kiwifruit juice–milk powders differed, which may have influenced water permeability, water diffusion, water-holding capacity, and ultimately the solubility of the spray-dried powders and the release rate of encapsulated antioxidants on contact with the aqueous media (Lim et al. 2011).
Product yield during spray drying is well known to be affected by both the spray drying conditions and the characteristics of feed solution. In this study, the same processing conditions were applied to the green or gold kiwifruit juice–milk feed solutions. Increasing the concentration of encapsulants such as the pectin and other mucilaginous substances from kiwifruit, whilst keeping the maltodextrin and milk protein contents the same, can raise the glass transition temperature and increase the spray-drying yield. The encapsulant matrix would have encapsulated not only the phenolic and vitamin C antioxidants, but also the organic acids and simple sugars present in the feed solutions. A lower product yield is often associated with a larger particle size and hence reduced surface area, resulting from a poor drying efficiency through decreased interactions between the feed droplet and the drying medium (i.e. hot air).
Maltodextrins are known to alter the surface stickiness of fruit juice droplets and aid the production of free-flowing fruit juice powders with reduced wall deposition during spray drying (Goula and Adamopoulos 2004, 2008). Dextrose equivalency (DE) of maltodextrins is inversely associated with their average molecular weight and determines their functional properties as drying agents (Whistler and BeMiller 1997). Low DE maltodextrins facilitate a higher glass transition temperature and a low degree of caking, but also cause more viscous feed mixtures and subsequently a lower evaporation rate (Goula and Adamopoulos 2008; Werner et al. 2007). A maltodextrin with a higher DE leads to a slower development of stickiness and the production of denser and more oxygen-impermeable encapsulation matrices (which preserve antioxidants and pigments). This study used a maltodextrin with a moderate DE of 12, and led to the production of kiwifruit juice–milk powders that have low water activity, low hygroscopicity and high solubility, containing a significant amount of hydrophilic groups (to facilitate water adsorption and wetting) and a relatively low drying rate (to allow a low droplet temperature and subsequent reduction of antioxidant degradation). Furthermore, an increased maltodextrin concentration is often associated with a decrease in bulk density (Shrestha et al. 2007) and an increase in moisture content of spray dried powder, due to the difficulty for water to diffuse past large maltodextrin molecules (Adhikari et al. 2004). A higher concentration of maltodextrin also presents potential adverse health effects on humans and will dilute co-existing nutrients in a powder product. Thus, this study used a low concentration of maltodextrin (i.e. 3 % w/w) to minimise such adverse effects.
The Antioxidants in Spray-Dried Powders and Effects of Storage at 4 °C or 20 °C
It is well recognized that variations in genotype or cultivar, physiological maturity, harvest factors, storage conditions and analysis method, all contribute to the detected vitamin C content of a fruit product (Tavarini et al. 2008; Rasam and Laing 2005; Kalt 2005; Imeh and Khokhar 2002). Thus, it was not surprising that the vitamin C content in the aqueous fractions of green or gold kiwifruit puree differed significantly (P < 0.05), i.e. 0.95 and 1.16 mg ascorbic acid/mL, respectively (Table 1). Further, the vitamin C content in the derived green kiwifruit juice–milk powder was slightly lower (p < 0.05) than that in the gold kiwifruit juice–milk powder (Table 1). Spray drying process involves high heat and could cause a degree of vitamin C degradation. Clearly, spray drying caused a greater extent of vitamin C degradation during the production of the gold kiwifruit juice–milk powder, compared to the green kiwifruit juice–milk powder (Table 1). This indicates the matrix of the green kiwifruit juice–milk powder provided a better protection for vitamin C than the matrix of the gold kiwifruit juice–milk powder during spray drying. However, the fact that vitamin C derived from kiwifruit was considerably retained in the final spray dried powder is encouraging. An adequate quantity of vitamin C through dietary intake is very important for humans, as humans cannot synthesise vitamin C but demand a sufficient amount of this antioxidant to neutralize reactive oxygen species (Davey et al. 2000; Ferraroni et al. 1994).
Figure 1 shows that the vitamin C contents of the two types of kiwifruit juice–milk spray dried powder were stable at 4 °C. At 20 °C, 88–90 % and 78–81 % of the vitamin C content in the kiwifruit juice–milk powder were retained after 30 days or 60 days, respectively. The gold kiwifruit juice–milk powder, although had a higher initial vitamin C content (i.e. ~1.1 times as much), showed a slightly greater decrease in vitamin C over time than the green kiwifruit juice–milk powder at 20 °C. For the gold kiwifruit juice–milk powder, 88.7 % and 76.3 % of initial vitamin C were retained after 30 and 60 days, respectively, compared to the green kiwifruit juice–milk powder where 89.7 % and 80.7 % of initial vitamin C were retained after 30 and 60 days, respectively. Although the initial vitamin C content was higher in the freshly spray-dried gold kiwifruit juice–milk powder, the final vitamin C content in the gold kiwifruit juice–milk powder became almost the same as the green kiwifruit juice–milk powder after 60 days at 20 °C. These results suggest that the green kiwifruit juice–milk powder matrix may provide a greater protective effect on vitamin C than the gold kiwifruit juice–milk powder matrix. Therefore, the type of kiwifruit affected the stability of vitamin C in kiwifruit juice–milk powder matrices.
The gold kiwifruit juice–milk powder had 2.6 times the total carotenoids as the green kiwifruit juice–milk powder, but again the deterioration rate over the storages at 20 °C for either 30 days or 60 days was slightly higher in the case of the gold kiwifruit juice–milk powder (Fig. 1). Carotenoids are isoprenoids that have polyene chains containing conjugated double bonds; thus, they are susceptible to heat destruction and oxidation (Stefanovich and Karel 1982). The difference in the storage stability of carotenoids in the green and gold kiwifruit juice–milk powders may be due to the different interactions between the carotenoids and other co-existing components in the two types of kiwifruit juice–milk powders.
Figure 2 shows the carotenoid profile (λ = 450 nm) of the spray dried kiwifruit juice–milk powders. Lutein and beta-carotene were identified as the major carotenoids in the powders (0.78 and 1.28 μg/g powder for gold kiwifruit juice–milk powder and 0.40 and 0.33 μg/g powder for green kiwifruit juice–milk powder). The green kiwifruit juice–milk powder also contained chorophylls (i.e. mainly chlorophyll a 0.54 μg/g powder). Kiwifruits with different fruit flesh colours have significantly different pigment compositions i.e. varying in carotenoid, chlorophyll and anthocyanin contents. Carotenoids that occur most abundantly during fruit development are lutein and beta-carotene (Ampomah-Dwamena et al. 2009). Thus, it is expected that these two compounds would be the dominant carotenoids in the spray dried powders. The retention of lutein, beta-carotene and chlorophyll after spray drying indicates these compounds may be quite stable in the kiwifruit juice–milk spray-dried powder matrices.
The total phenolic content of the green or gold kiwifruit juice–milk powders after spray drying was 1.68 and 2.36 mg CtE/g powder. Figure 3 shows the HPLC chromatograms of the two types of kiwifruit juice–milk spray dried powders at a wavelength of 280 nm. The phenolic profiles exhibit differences as a function of both the type of kiwifruit and the storage conditions. Phenolic compounds identified in the spray-dried powders included caffeic acid derivatives, protocatechuic acid, chlorogenic acid, gallic acid, hydroxybenzoic acids, quercetin glycosides, epicatechin and/or procyanidin. The presence of these phenolics in the spray-dried powders confirmed that the phenolics intrinsically occurring in green or gold kiwifruit juice were significantly retained after spray drying in the presence of milk and maltodextran (Sun-Waterhouse et al. 2009a, 2013a; McGhie and Ainge 2002; Dawes and Keene 1999). Differences in the concentrations of these compounds before and after storage indicate the different extractabilities and stabilities of these antioxidants in the green or gold kiwifruit juice–milk powder matrices at 4 °C or 20 °C. The relatively short retention times of these compounds, detected by reverse-phase HPLC method, suggest the dominance of phenolic compounds with high polarity in the two types of kiwifruit juice–milk powders.
HPLC chromatograms (λ = 280 nm) of the kiwifruit juice–milk spray-dried powders on day 0, day 30 after a storage at 4 °C, day 30 after a storage at 20 °C and day 60 after a storage at 20 °C: a green kiwifruit and b gold kiwifruit. Peak 1 ascorbic/citric acid, peak 2 unknown, peak 3 caffeic acid disaccharide, peak 4 caffeic acid derivative, peak 5 protocatechuic acid, peak 6 chlorogenic acid, peak 7 caffeic acid hexoside, peak 8 caffeic acid pentoside, peak 9 dimethyl-caffeic acid hexoside, peak 10 internal standard (phlorizin), peak 11 quercetin-glucoside, peak 12 quercetin-rhamnoside, peak 13 gallic acid, peak 14 3,4-dihydroxybenzoic acid, peak 15 p-hydroxybenzoic acid, peak 16 caffeic acid hexoside, peak 17 epicatechin and/or procyanidin B1
For the green kiwifruit-milk powder, caffeic acid hexoside (peak 7, initial concentration 0.13 mg/g powder) exhibited a marginal increase with storage. Dimethyl-caffeic acid hexoside (peak 9, initial concentration 0.31 mg/g powder) displayed a 9.5 %, 12.9 % and 13.5 % loss after a storage for 30 days at 4 °C, 30 days at 20 °C and 60 days at 20 °C, respectively. The concentration of protocatechuic acid (peak 5) increased 2.4, 2.0 and 4.9 times, respectively, compared to Day 0 value (0.43 mg/g powder), after the three different storage regimes. Caffeic acid derivative (peak 4) increased by 2.8, 4.3 or 7.5 times, respectively, compared to the concentration on Day 0 (0.22 mg/g powder). Quercetin-glucoside (peak 11, Day 0 concentration 0.18 mg/g powder) exhibited a 15 %, 28 % and 37 % increase after the three different storage regimes. The concentration of quercetin-rhamnoside (peak 12, initial concentration 0.15 mg/g powder) displayed a 15 % and 5 % increase after a 30-day storage at 4 °C and 20 °C, respectively, but a 2 % reduction after 60 days at 20 °C .
For the gold kiwifruit juice–milk powder, caffeic acid derivative (peak 4) with an initial concentration of 0.03 mg/g powder increased to 7.3, 8.7 and 39.1 times after 30 days at 4 °C, 30 days at 20 °C and 60 days at 20 °C, respectively. For protocatechuic acid (peak 5), storage at 4 °C for 30 days, at 20 °C for 30 days, or at 20 °C for 60 days led to 1.9, 1.6 and 2.5 times increase compared to the initial concentration of 0.60 mg/g powder. Dimethyl-caffeic acid hexoside (peak 9) at an initial concentration of 0.38 mg/g powder exhibited 13 %, 14 % and 16 % loss, respectively, over the three different storage regimes. The concentration of gallic acid (peak 13) rose 1.3, 2.5 and 25 times as much as the Day 0 concentration (i.e. 0.02 mg/g powder) over the three different storage treatments. The concentration of coumaric acid derivative (peak 17, with Day 0 concentration of 0.29 mg/g powder) was increased by 24 % and 28 % after a 30 days storage at 4 °C or 20 °C, respectively, but displayed a 6 % reduction after 60 days at 20 °C. A slight reduction of 8 %, 10 % and 14 %, respectively, was observed for quercetin-glucoside (peak 11, with an initial concentration of 0.34 mg/g powder) following the three different storage regimes. The concentration of quercetin-rhamnoside (peak 12, with an initial concentration was 0.31 mg/g powder) showed a decrease by 5 %, 12 % and 16 %, respectively, following the three different storage treatments. The increased concentrations of some phenolics such as caffeic acid, coumaric acid and quercetin derivatives in the kiwifruit juice–milk powders after storage at 20 °C, could result from the decomposition of some complex phenolic compounds into simpler phenolics, or the changes in fruit juice–milk powder matrices over storage causing decreased affinity of the phenolics to the matrix network. Such elevated concentrations of these health beneficial bioactives may present a nutritional advantage because of their high extractabilities and potential bioaccessibility from their powder matrices.
The milk proteins, kiwifruit polysaccharides (like pectins), maltodextrin and sugars that co-exisited in the feed solution for spray drying reduces the moisture content of the product powders, and formed a matrix network to protect bioactive compounds via interactions such as hydrogen bonding between the –OH groups of kiwifruit polysaccharides or maltodextrin and the polar groups of milk proteins. The differences in the composition and physicochemical attributes between green and gold kiwifruit juices are largely responsible for the detected differences in their derived kiwifruit juice–milk powders. When the aqueous fraction of kiwifruit puree was mixed with milk and maltodextrin, interactions among all the constituents in kiwifruit juice and milk and maltodextrin could influence the structure and function of the active components initially present such as vitamin C, carotenoids and phenolics. Interactions between these active components have been previously found, for example, phenolic-induced complexation through intermolecular or intramolecular co-pigmentation effects (Rein and Heinonen 2004), preservative effect on vitamin C by pectin (Sun-Waterhouse et al. 2008a, b), and matrix effect of the kiwifruit juice–milk ice cream on the vitamin C, phenolic and carotenoid contents (Sun-Waterhouse et al. 2013a). The stress of heat and pressure encountered during the high shear homogenisation and spray drying steps could potentially damage the sensitive phenolic compounds. The retention of the kiwifruit phenolics in the kiwifruit juice–milk powder after spray drying and storages depends on the chemical structure of the phenolic compounds (which directly affected the susceptibility to oxidation), and the powder physicochemical attributes like surface area (which directly affected the interactions via chemical bonding or physical effect among other matrix components, heat and oxygen) (Soukoulis et al. 2014; Sun-Waterhouse et al. 2013a; Lin et al. 2007; Rice-Evans et al. 1996). In this study, a relatively low inlet temperature was employed thus enabling a good retention of phenolics as increasing inlet temperature would cause a greater loss of phenolic content during spray drying (Murugesan and Orsat 2011).
Viscosity of Aqueous Solutions of the Spray Dried Powders
Both types of kiwifruit juice–milk powder exhibited shear thinning characteristics when they were reconstituted in water (Fig. 4). It is not surprising that the kiwifruit juice–milk powders were pseudoplastic-like materials as they contained biopolymers such as polysaccharides and proteins. Thus, fluids obtained by powder reconstitution in water belong to structured fluids, in which particle size and particle orientation would be altered upon the application of a force/stress. The differences between the fluids produced from the green and gold kiwifruit juice–milk powders were associated with their structural and adhesive characteristics (which was governed by the powder composition). At higher shear rates, the decreasing viscosity indicated reduction of resistance to flow (Fig. 4). The decreasing rate of viscosity was smaller at mid-range shear rates (i.e. appeared like a relatively flat plateau region), indicating mid-range shear rates facilitated the occurrence of a shear-oriented microstructure in which almost all the macromolecules in the solution were in an aligned conformation and oriented to the shear direction completely. Further increase shear rate would have disturbed such a microstructure and some polymer particles would move around again (i.e. in random structure) causing frictional resistance.
The viscosity of aqueous solutions obtained upon reconstitution of spray-dried powders depended markedly on the fruit variety, although here similar behaviour was found for both types of kiwifruit powder (Fig. 4). The green kiwifruit juice–milk powder had a higher viscosity in water within the shear rate range including from 30 to 60 s−1. This suggests that the reconstituted aqueous solution of the green kiwifruit juice–milk had a more viscous texture and better sensory consistency (Wood and Goff 1973). Milk constituents, maltodextrin and the water soluble polysaccharides including pectins in the aqueous fraction of kiwifruit puree all likely contributed to the detected viscosities (Sun-Waterhouse et al. 2009b, 2013a; Yuliarti et al. 2008). For the same feed formulation, the aqueous fraction from green kiwifruit had a higher pectin content than that from gold kiwifruit (Table 1). The green kiwifruit was found to have a greater amount of dry matter, pectins and total oxalates, especially at acidic pHs and ~20 °C (Nguyễn and Savage 2013). Viscosity increases with increasing polysaccharide concentrations and increasing soluble solid content. Thus, it is understandable that the reconstituted green kiwifruit juice–milk aqueous solutions was slightly more viscous. Furthermore, differences in microstructure and surface area of the two kiwifruit juice–milk powder also led to variations in molecular bonding and polymer chain interactions, e.g. the hydrogen bonds between water and carbohydrates (including simple sugars, pectins and mucilages of kiwifruits, and food additive maltodextrin), milk proteins and kiwifruit phenolics, and the interaction between maltodextrin and kiwifruit polysaccharides (Goula and Adamopoulos 2008; Nishiyama 2007; Whistler and BeMiller 1997). These ultimately influence the connectivity of matrix network components and the behaviour of kiwifruit juice–milk powders in water, e.g. dissolution efficiency and viscosity. The data collected here suggest that the spray-dried kiwifruit juice–milk powders are suitable for functional beverage applications incorporating multiple nutrients and bioactives whilst also providing viscosity-modifying function.
Effect of Pretreatment on the Physicochemical Attributes of Kiwifruit Juice–Milk Powders
The green kiwifruit juice–milk powder had L*, a* and b* values of 82.3, −3.09 and 24.3, while the gold kiwifruit juice–milk powder had L*, a* and b* values of 83.9, −1.05 and 25.7 (Table 2). The colour of the kiwifruit juice–milk powders reflects the differences in the initial concentrations of pigments, phenolics and vitamin C between the green and gold kiwifruits, as well as the interactions of these compounds with the constituents of the feed solutions during spray drying (Sun-Waterhouse et al. 2013a; Tavarini et al. 2008; Maskan 2001; Wong and Stanton 1989).
Preheating the feed solutions at 50 °C for 1 min prior to spray drying led to changes in the physicochemical attributes and bioactive profiles of the kiwifruit juice–milk powders: The resultant L* and b* values indicate an increase of lightness and yellowness in both types of the kiwifruit juice–milk powders, and the resultant a* values suggest a slight rise in greenness in the green kiwifruit juice–milk powder but no change in the gold kiwifruit juice–milk powder (Table 2). The calculated ΔE* values (ΔE* values < 3) indicate that there was no visual difference in colour for the same type powder with and without the feed preheating (i.e. either green or gold kiwifruit juice–milk powder; Martinez-Cervera et al. 2011). Moreover, while the water activity remained almost the same, the preheating caused an increase in bulk density, dissolution efficiency and total phenolic content, and a small loss of vitamin C content for both types of kiwifruit juice–milk powders. Preheating increased the total phenolic content by 51 % and 28 %, whilst the vitamin C content reduced by 11 % and 22 %, respectively, for the green or gold kiwifruit juice–milk powders (Table 2).
Previous studies reported the presence of enzymes such as polyphenol oxidase (PPO), peroxidase (POD), protease actinidin, pectinmethylesterase (PME) and polygalacturonase (PG) in the aqueous fractions of kiwifruit puree (Nieuwenhuizen et al. 2007; Bublin et al. 2004; Lamikanra 2002; Richard-Forget and Gauillard 1997; Wegrzyn and MacRae 1992). PPO, POD and actinidin could breakdown the phenolics and proteins in the feed solution, and also cause browning of product via enzymatic reactions (Sun-Waterhouse et al. 2013a; Gastaldi et al. 1996, 1997; Holt 1995; Fox 1989). Further, it was previously found that mild preheating, such as 50 °C for 1 min, could eliminate or at least partially inactivate these enzymes without denaturing milk proteins, thus reducing browning effects and preserving the matrix network for encapsulating pigments and phenolics (Sun-Waterhouse and Waterhouse 2014; Sun-Waterhouse et al. 2013a; Katsaros et al. 2009; Nam et al. 2006; Vasbinder and de Kruif 2003; Corredig and Dalgleish 1996; Wilson and Burns 1983). However, such preheating of the feed solution could activate PME and PG, thereby potentially causing some pectin chain shortening, reduction in the degree of methylation and extent of polymerisation of pectins, increase of pectin solubilisation and decrease in the viscosity of feed solutions (Landi et al. 2014; Anthon et al. 2005; Redgwell et al. 1992). Thus, improved dissolution efficiency of spray-dried powder in water and elevated TPEC in spray-dried powders due to increased extractability of phenolics from powder matrices for the Folin–Ciocalteu assay are expected. Decreased feed viscosity, which resulted from higher feed temperature (i.e. 50 °C) and actions of PME and PG, could aid the spray drying process and reduce clogging of the nozzle, thus minimising degradation of antioxidants and interconversion of α- and β-anomers, resulting in a more stable and consistent powder composition and structure.
Differences in the enzymes present in green and gold kiwifruits could cause variations in the enzymatic reactions, e.g. PPO-catalyzed oxidation of the phenolics in the presence of oxygen during spray drying, which produced o-quinones that are further polymerized melanin; actinidin-catalyzed hydrolysis of milk proteins, which ultimately influenced the characteristics of encapsulant matrix and the stability of the encapsulated antioxidants. The green and gold kiwifruit differed in abundance and cysteine protease activity (Nieuwenhuizen et al. 2007). Gold kiwifruit has a smaller total cysteine protease activity and does not express an acidic isoform, i.e. KFAct1a, comparied to green kiwifruit (Nieuwenhuizen et al. 2007). Moreover, a higher pectin content was detected in the aqueous fraction of green kiwifruit puree compared to the gold kiwifruit puree (Table 1). Sun-Waterhouse et al. (2008a, b) reported a significant stablising effect of pectin on ascorbic acid in an aqueous system. Thus, it was possible that the greater amount of pectin present in the green kiwifruit juice–milk feed solution led to a greater protection of vitamin C.
Effect of Addition of Kiwifruit Pulp Residue on the Physicochemical Properties of Kiwifruit Juice–Milk Powders
After prelimirary trials, it was found that blending a portion of the pulp residue from kiwifruit puree with the feed solution (i.e. at a 15:85 mass ratio), along with adjusting the pH to higher values close to neutral, and adding a small amount of Zn2+, offered advantages in the sensory attibutes (i.e. retained significantly the desired green colours of green kiwifruit) and shelf life (i.e. decreased water activity) of the kiwifruit juice–milk powders, with minimal detrimental effects on the dissolution efficiency and the total phenolic and vitamin C contents (Table 2). However, further increase of the proportion of pulp residue in the feed solution could cause difficulties with spray drying system due to nozzle blocking. Adding a portion of pulp residue led to reduced water activity and increased bulk density for both the green and gold kiwifruit juice–milk powders. The viscosity of the feed solution was increased to some extent upon the addition of pulp residue. When the same spray drying conditions and feed formulation were applied (i.e. a relatively low inlet temperature and a low maltodextrin concentration, i.e. 3 % w/w), the addition of pulp residue might lead to the surface of particles not completely solidified or dried, thereby causing stickiness and a greater extent of particle agglomeration and air exclusion (Goula and Adamopoulos 2004).
Colour and flavour enhancement as a result of pulp residue addition are expected because kiwifruit puree almost resembles whole kiwifruit in terms of composition. The centrifugation step has removed the solid tissues of kiwifruit and associated pectins, the bound/entrapped pigments and flavour compounds and other organic constituents from the aqueous fraction (but has concentrated these substances in the pulp residues). Adding back a portion of the pulp residues was expected to cause the changes in colour, flavour and antioxidant contents of the final powder products, given the differences between the kiwifruit purees and their derived aqueous fractions (Table 1). The green or gold kiwifruit puree had a slightly higher pH, total soluble solids, vitamin C and pectin contents but a lower total phenolic content, compared to its corresponding aqueous fraction. Interestingly, the addition of pulp residue from kiwifruit puree did not cause unfavorable changes in powder’s phenolic and vitamin C contents, i.e. negligible differences were found in the antioxidant contents between the kiwifruit juice–milk powder and the kiwifruit juice–milk–pulp residue powders.
The loss of green color in food products is reported to be due to the substitution of Mg2+ in chlorophyll a and b by H+ during pheophytin formation (Cassano et al. 2006; Johnson and Steele 1995). The addition of alkaline agents such as zinc and copper salts during heating is an approach to preserve the green colour via the formation of Zn2+/Cu2+–chlorophyll complexes (Ngo and Zhao 2005; Guzmán et al. 2002; Maharaj and Sankat 1996). The pH range of 4.0–8.5 favors the zinc complex formation and the development of green colour (Ngo and Zhao 2005; LaBorde and von Elbe 1990). Further, the milk casein micelles are stable around the neutral pH (e.g. the pH of natural milk; Walstra 1990). When kiwifruit juice fractions are mixed with milk, the pH of the mixture is lowered. This would induce hydrophobic interactions and neutralisation of the negative charges on the casein micelles, causing destabilisation or aggregation of casein micelles. Thus, the pH adjustment above the PI of calcium caseinate (i.e. 4.6) in this study, along with the enrichment of soluble polysaccharides in the feed solutions, facilitated a stable and tighter protein-containing network with fine protein particles and good water-binding activity (Fagan et al. 2006; Langton and Hermansson 1992). The approach of zinc fortification at 15 ppm aided the retention of green colour via an increase of cross-linking and stabilisation of the pectin-containing encapsulation matrix (Pillay et al. 2005).
Conclusions
This study is the first reported attempt to produce stable and readily reconstitutable kiwifruit juice–milk powders via spray drying. The obtained products have desirable colours and physicochemical properties and high nutritional quality, and are based on novel formulations containing the aqueous fraction of green or gold kiwifruit puree, skimmed milk, fortifying zinc and minimal amounts of maltodextrin. The spray drying method is effective for preserving, via in-situ encapsulation, the pigments and antioxidants including vitamin C, phenolics and carotenoids of kiwifruit juice, yielding powders with desirable density for packaging (i.e. 0.40–0.57 g/cm3), ‘instant’ reconstitution properties in water (i.e. dissolution efficiency 21–28 s, viscosity 2–23 mPa · s) and good shelf life (i.e. low risk of microbial spoilage due to water activity 0.22–0.28). Both the green and gold kiwifruit juice–milk powders were pseudoplastic-like materials, and the viscosity of their reconstituted water solutions showed a shear rate dependence. The initial differences in the composition and physicochemical attributes between green and gold kiwifruit purees caused differences in the interactions among the components of the feed solutions for spray drying, which led to kiwifruit juice–milk powders with different matrix networks, colours and bioactives. Storage at 4 °C was the best for retaining the phenolic, vitamin C and caroteinoid contents of the as-prepared products, whilst storage at room temperature was also acceptable. Fortification with zinc not only stablises the green kiwifruit colour and the encapsulation matrix structure, but also provides additional nutritional value derived from zinc. Modification of the formulation (i.e. adding a portion of pulp residue from kiwifruit puree) or processing steps (i.e. preheating the feed solutions at 50 °C for 1 min prior to spray drying) allows the final powder characteristics to be tuned for specific applications. This study conclusively demonstrates approaches to successfully produce kiwifruit juice–milk powders that contain health-promoting bioactives and nutrients as well as desirable product attributes, which can be used for various purposes including beverages, foods and nutraceutical products.
Abbreviations
- FDA:
-
Food and Drug Administration
- BET:
-
Brunauer–Emmett–Teller
- BJH:
-
Barrett–Joyner–Halenda
- ASE:
-
accelerated solvent extraction
- TEPC:
-
total extracted phenolic content
- SPE:
-
solid phase extraction
- CtE:
-
catechin equivalent
- HPLC:
-
high performance liquid chromatography
- AOAC:
-
Association of Official Analytical Chemists
- UA:
-
uronic acid
- GalA:
-
galacturonic acid
- DE:
-
dextrose equivalency
- PPO:
-
polyphenol oxidase
- POD:
-
peroxidase
- PME:
-
pectinmethylesterase
- PG:
-
polygalacturonase
References
Adhikari, B., Howes, T., Bhandari, B. R., & Troung, V. (2004). Effect of addition of maltodextrin on drying kinetics and stickiness of sugar and acid-rich foods during convective drying: Experiments and modelling. Journal of Food Engineering, 62, 53–68.
Ampomah-Dwamena, C., McGhie, T., Wibisono, R., Montefiori, M., Hellens, R. P., & Allan, A. C. (2009). The kiwifruit lycopene beta-cyclase plays a significant role in carotenoid accumulation in fruit. Journal of Experimental Botany, 60(13), 3765–3779.
Anthon, G. E., Blot, L., & Barrett, D. M. (2005). Improved firmness in calcified diced tomatoes by temperature activation of pectin methylesterase. Journal of Food Science, 70, C342–C347.
AOAC. (1990). Official methods of analysis of the Association of Official Analytical Chemists, 15th edn. Arlington, VA. 1058–1059.
Bakar, J., Ee, S. C., Hashim, D. M., & Adzahan, N. (2013). Spray-drying optimization for red pitaya peel (Hylocereus polyrhizus). Food and Bioprocess Technology, 6, 1332–1342.
Bhandari, B. R., Senoussi, A., Dumoulin, E. D., & Lebert, A. (1993). Spray drying of concentrated fruit juices. Drying Technology, 11(5), 1081–1092.
Bhandari, B. R., Data, N., & Howes, T. (1997). Problems associated with spray drying of sugar-rich foods. Drying Technology, 15(2), 671–684.
Brand-Miller, J., Atkinson, F., & Rowan, A. (2013). Effect of added carbohydrates on glycemic and insulin responses to children’s milk products. Nutrients, 5, 23–31.
Bublin, M., Mari, A., Ebner, C., Knulst, A., Scheiner, O., Hoffmann-Sommergruber, K., Breiteneder, H., & Radauer, C. (2004). IgE sensitization profiles toward green and gold kiwifruits differ among patients allergic to kiwifruit from 3 European countries. Journal of Allergy and Clinical Immunology, 114, 1169–1175.
Cano-Chauca, M., Stringheta, P. C., Ramos, A. M., & Cal-Vidal, J. (2005). Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innovative Food Science & Emerging Technologies, 6(4), 420–428.
Cassano, A., Figoli, A., Tagarelli, A., Sindona, G., & Drioli, E. (2006). Integrated membrane process for the production of highly nutritional kiwifruit juice. Desalination, 189, 21–30.
Chan, A. O. O., Leung, G., Tong, T., & Wong, N. Y. H. (2007). Increasing dietary fiber intake in terms of kiwifruit improves constipation in Chinese patients. World Journal of Gastroenterology, 13, 4771–4775.
Cilliers, J. J. L., & Singleton, V. L. (1989). Nonenzymic autoxidative phenolic browning reactions in a caffeic acid model system. Journal of Agricultural and Food Chemistry, 37(4), 890–896.
Condon, J. B. (2006). Surface area and porosity determinations by physisorption: measurements and theory (1st ed.). Boston: Elsevier.
Corredig, M., & Dalgleish, D. G. (1996). Effect of temperature and pH on the interactions of whey proteins with casein micelles in skim milk. Food Research International, 29, 49–55.
Davey, M. W., Van Montagu, M., Inze, D., Sanmartin, M., Kanellis, A., Smirnoff, N., Benzie, I. F. F., Strain, J. J., Favell, D., & Fletcher, J. (2000). Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. Journal of the Science of Food and Agriculture, 89, 825–860.
Dawes, H. M., & Keene, J. B. (1999). Phenolic composition of kiwifruit juice. Journal of Agriculture and Food Chemistry, 47, 2398–2403.
Duttaroy, A. K., & Jorgensen, A. (2004). Effects of kiwi fruit consumption on platelet aggregation and plasma lipids in healthy human volunteers. Platelets, 15, 287–292.
Ersus, S., & Yurdagel, U. (2007). Microencapsulation of anthocyanin pigments of black carrot (Daucuscarota L.) by spray drier. Journal of Food Engineering, 80, 805–812.
Fagan, C. C., O’Donnell, C. P., Cullen, P. J., & Brennan, C. S. (2006). The effect of dietary fibre inclusion on milk coagulation kinetics. Journal of Food Engineering, 77, 261–268.
Ferraroni, M., Heinonen, O. P., Albanes, D., Heinonen, M., Rikkala, E., & Teppo, L. (1994). Selected micronutrients intake and the risk of colorectal cancer. British Journal of Cancer, 70, 1150–1155.
Filisetti-Cozzi, T. M. C. C., & Carpita, N. C. (1991). Measurement of uronic acids without interference from neutral sugars. Analytical Biochemistry, 197, 157–162.
Forastiere, F., Pistelli, R., Sestini, P., Fortes, C., Renzoni, E., Rusconi, F., Dell’Orco, V., Ciccone, G., & Bisanti, L. (2000). Consumption of fresh fruit rich in vitamin C and wheezing symptoms in children. Thorax, 55, 283–288.
Fox, P. F. (1989). Heat-induced changes in milk. In P. F. Fox (Ed.), Bulletin of the International Dairy Federation (No. 238). Brussels: International Dairy Federation.
Gastaldi, E., Lagaude, A., & Tarodo De La Fuente, B. (1996). Micellar transition state in casein between pH 5.5 and 5.0. Journal of Food Science, 61(Tarodo De La Fuente, B), 59–64. 68.
Gastaldi, E., Lagaude, A., Marchesseau, S., & Tarodo de la Fuente, B. (1997). Acid milk gel formation as affected by total solids content. Journal of Food Science, 62(4), 671–687.
Goula, A. M., & Adamopoulos, K. G. (2004). Influence of spray conditions on residue accumulation—simulation using CFD. Drying Technology, 22, 1107–1128.
Goula, A. M., & Adamopoulos, K. G. (2008). Effect of maltodextrin addition during spray drying of tomato pulp in dehumidified Air: II. Powder Properties. Drying Technology, 26(6), 726–737.
Guzmán, G. R., Dorantes, A. L., Hernández, U. H., Hernández, S. H., Ortiz, A., & Mora, E. R. (2002). Effect of zinc and copper chloride on the color of avocado puree heated with microwaves. Innovative Food Science and Emerging Technologies, 3, 47–53.
Holt, C. (1995). Effect of heating and cooling on the milk salts and their interaction with casein. In P. F. Fox (Ed.), Heat induced changes in milk (pp. 105–133). Brussels: International Dairy Federation.
Imeh, U., & Khokhar, S. (2002). Distribution of conjugated and free phenols in fruits: antioxidant activity and cultivar variations. Journal of Agricultural and Food Chemistry, 50, 6301–6306.
Jeffrey, S. W., Mantoura, R. F. C., & Wright, S. W. (1997). Phytoplankton pigments in oceanography: guidelines to modern methods. Paris: UNESCO Publications.
Johnson, R. L., & Steele, R. J. (1995). New strategies for kiwifruit processing. International Journal of Food Science and Technology, 30, 13–21.
Kalt, W. (2005). Effects of production and processing factors on major fruit and vegetable antioxidants. Journal of Food Science, 70, R11–R19.
Katsaros, G. I., Katapodis, P., & Taoukis, P. S. (2009). Modeling the effect of temperature and high hydrostatic pressure on the proteolytic activity of kiwi fruit juice. Journal of Food Engineering, 94, 40–45.
Kennedy, J. F., Rivera, Z. S., Warner, F. P., Lloyd, L. L., & Jumel, K. (1989). Analysis of carbohydrates and amino acids in aqueous solutions of L-ascorbic acid and correlation of their role in the nonenzymatic browning of vitamin C. Journal of Micronutrient Analysis, 6, 1–17.
Kha, T. C., Nguyen, M. H., & Roach, P. D. (2010). Effects of spray drying conditions on the physicochemical and antioxidant properties of the Gac (Momordica cochinchinensis) fruit aril powder. Journal of Food Engineering, 98, 385–392.
LaBorde, L. F., & von Elbe, J. H. (1990). Zinc complex formation in heated vegetable purees. Journal of Agricultural and Food Chemistry, 38, 484–487.
Lamikanra, O. (2002). Enzymatic effects on flavor and texture of fresh-cut fruits and vegetables. In Fresh cut fruits and vegetables: science, technology, and market (pp. 127–147). Boca Raton: CRC Press.
Landi, M., Tardelli, F., Remorini, D., Massai, R., & Guidi, L. (2014). Do sun- versus shade-grown kiwifruits perform differently upon storage? An overview of fruit maturity and nutraceutical properties of whole and fresh-cut produce. Journal of Agricultural and Food Chemistry, 62(19), 4377–4383.
Langton, M., & Hermansson, A. M. (1992). Fine-stranded and particulate gels of betalactoglobulin and whey protein at varying pH. Food Hydrocolloids, 5, 523–539.
Lim, L. H., Tan, A., Simovic, S., & Prestidge, C. A. (2011). Silica-lipid hybrid microcapsules: influence of lipid and emulsifier type on in vitro performance. International Journal of Pharmaceutics, 409, 297–306.
Lin, Q. L., Wang, J., Qin, D., & Bergenståhl, B. (2007). Influence of amphiphilic structures on the stability of polyphenols with different hydrophobicity. Science in China Series B: Chemistry, 50(1), 121–126.
Lodge, N., & Robertson, G. L. (1990). Processing of kiwifruit. In I. J. Warrington & G. C. Weston (Eds.), Kiwifruit: science and management (pp. 460–484). Auckland: New Zealand Society for Horticultural Science.
Maharaj, V., & Sankat, C. K. (1996). Quality changes in dehydrated dasheen leaves: effects of blanching pre-treatments and drying conditions. Food Research International, 29, 563–568.
Martinez-Cervera, S., Salvador, A., Muguerza, B., Moulay, L., & Fizman, S. M. (2011). Cocoa fibre and its application as a fat replacer in chocolate muffins. LWT-Food Science and Technology, 44, 729–736.
Maskan, M. (2001). Kinetics of colour change of kiwifruits during hot air and microwave drying. Journal of Food Engineering, 48, 169–175.
McGhie, T. K., & Ainge, G. D. (2002). Color in fruit of the genus Actinidia: carotenoid and chlorophyll compositions. Journal of Agriculture and Food Chemistry, 50, 117–121.
Montoya, C. A., Hindmarsh, J. P., Gonzalez, L., Boland, M. J., Moughan, P. J., & Rutherfurd, S. M. (2014). Dietary Actinidin from kiwifruit (Actinidia deliciosa cv. Hayward) increases gastric digestion and the gastric emptying rate of several dietary proteins in growing rats. Journal of Nutrition, 144(4), 440–446.
Murugesan, R., & Orsat, V. (2011). Spray drying of elderberry (Sambucus nigra L.) juice to maintain its phenolic content. Drying Technology, 29(14), 1729–1740.
Nam, S., Walsh, M. K., & Yang, K. (2006). The enzymatic properties of actinidine from kiwifruit. Food Science Biotechnology, 15(3), 453–457.
Ngo, T., & Zhao, Y. (2005). Retaining green pigments on thermally processed peels-on green pears. Journal of Food Science, 70(9), 568–574.
Nguyễn, H. V., & Savage, G. P. (2013). The effects of temperature and pH on the extraction of oxalate and pectin from green kiwifruit (Actinidia deliciosa L.), golden kiwifruit (Actinidia chinensis L.), kiwiberry (Actinidia arguta) and persimmon (Diospyros kaki). International Journal of Food Science & Technology, 48, 794–800.
Nieuwenhuizen, N. J., Beuning, L. L., Sutherland, P. W., Sharma, N. N., Cooney, J. M., Bieleski, L. R. F., Schröder, R., MacRae, E. A., & Atkinson, R. G. (2007). Identification and characterisation of acidic and novel basic forms of actinidin, the highly abundant cysteine protease from kiwifruit. Functional Plant Biology, 34, 946–961.
Nishiyama, I. (2007). Fruits of the Actinidia genus. Advances in food and nutrition research, 52, 293–324.
Obon, J. M., Castellar, M. R., Alacid, M., & Fernández-López, J. A. (2009). Production of a red-purple food colorant from Opuntia stricta fruits by spray drying and its application in food model systems. Journal of Food Engineering, 90, 471–479.
Parkar, S. G., Redgate, E. L., Wibisono, R., Luo, X., Koh, E. T. H., & Schröder, R. (2010). Gut health benefits of kiwifruit pectins: comparison with commercial functional polysaccharides. Journal of Functional Foods, 2210–2218.
Pearce, R. J. (1983). Thermal separation of beta-lactoglobulin and alpha-lactalbumin in bovine cheddar cheese whey. Australian Journal of Dairy Technology, 38, 144–149.
Perez-Jimenez, J., & Saura-Calixto, F. (2006). Effect of solvent and certain food constituents on different antioxidant capacity assays. Food Research International, 39, 791–800.
Philpott, M., Mackay, L., Ferguson, L. R., Forbes, D., & Skinner, M. (2007). Cell culture models in developing nutrigenomics foods for inflammatory bowel disease. Mutation Research, 622, 94–102.
Pillay, V., Danckwerts, M. P., Muhidinov, Z., & Fassihi, R. (2005). Novel modulation of drug delivery using binary zinc-alginate-pectinate polyspheres for zero-order kinetics over several days: experimental design strategy to elucidate the crosslinking mechanism. Drug Development and Industrial Pharmacy, 31, 191–207.
Rasam, M., & Laing, W. (2005). Variation in ascorbic acid and oxalate levels in the fruit of Actinidia chinensis tissues and genotypes. Journal of Agricultural and Food Chemistry, 53, 2322–2326.
Rashidinejad, A., Birch, E. J., Sun-Waterhouse, D., & Everett, D. W. (2013). Effects of catechin on the phenolic content and antioxidant properties of low-fat cheese. International Journal of Food Science and Technology, 48(12), 2448–2455.
Rawel, H., Kroll, J., & Hohl, U. (2001). Model studies on reactions of plant phenols with whey proteins. Nahrung, 45, 72–78.
Redgwell, R. J., Melton, L. D., & Brasch, D. J. (1992). Cell-wall dissolution in ripening kiwifruit-solubilization of the pectic polymers. Plant Physiology, 98(1), 71–81.
Rein, M. J., & Heinonen, M. (2004). Stability and enhancement of berry juice colour. Journal of Agriculture and Food Chemistry, 52, 3106–3114.
Reineccius, G. A. (2004). The spray drying of food flavors. Drying Technology, 22(6), 1289–1324.
Rice-Evans, C. A., Miller, N. J., & Paganga, G. (1996). Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine, 20, 933–956.
Richard-Forget, F. C., & Gauillard, F. A. (1997). Oxidation of chlorogenic acid, catechins, and 4-methylcatechol in model solutions by combinations of pear (Pyrus communis cv. Williams) polyphenols oxidase and peroxidase: a possible involvement of peroxidase in enzymatic browning. Journal of Agricultural and Food Chemistry, 45, 2472–2476.
Righetto, A. M., & Netto, F. M. (2005). Effect of encapsulating materials on water sorption, glass transition and stability of juice from immature acerola. International Journal of Food Properties, 8(2), 337–346.
Rodriquez-Amaya, D. B. (1999). A guide to carotenoid analysis in foods. Washington, DC: ILSI Press.
Rush, E., Ferguson, L., Cumin, M., Thakur, V., Karunasinghe, N., & Plank, L. (2006). Kiwifruit consumption reduces DNA fragility: a randomised controlled pilot study in volunteers. Nutrition Research, 26, 197–201.
Saénz, C., Tapia, S., Chávez, J., & Robert, P. (2009). Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chemistry, 114, 616–622.
Shinde, T., Sun-Waterhouse, D., & Brooks, J. (2014). Co-extrusion encapsulation of probiotic Lactobacillus acidophilus alone or together with apple skin polyphenols: an aqueous and value-added delivery system using alginate. Food and Bioprocess Technology, 7(6), 1581–1596.
Shrestha, A. K., Ua-arak, T., Adhikari, B. R., Howes, T., & Bhandari, B. R. (2007). Glass transition behavior of spray dried orange juice powder measures by differential scanning calorimetry (DSC) and thermal mechanical compression test (TMCT). International Journal of Food Properties, 10, 661–673.
Singleton, V. L., Orthofer, R., & Lamuela-Ravento, M. (1997). Analysis of total phenols and other oxidation substrates and antioxidants by means of the Folin-Ciocalteu reagent. Methods in Enzymology, 229A, 152–178.
Soukoulis, C., Behboudi-Jobbehdar, S., Yonekura, L., Parmenter, C., & Fisk, I. (2014). Impact of milk protein type on the viability and storage stability of microencapsulated Lactobacillus acidophilus NCIMB 701748 using spray drying. Food and Bioprocess Technology, 7, 1255–1268.
Stefanovich, A. F., & Karel, M. (1982). Kinetics of β-carotene degradation at temperatures typical of air drying of foods. Journal of Food Processing and Preservation, 6, 227–242.
Stevenson, D., Wibisono, R., Jensen, D., Stanley, R., & Cooney, J. (2006). Direct acylation of flavonoid glycosides with phenolic acids catalysed by candida antarctica lipase B (Novozym 435®). Enzyme and Microbial Technology, 39, 1236–1241.
Sun-Waterhouse, D., & Waterhouse, G. I. N. (2014). Novel fruit juice beverages with health-promoting bioactives for “everyday consumption” (Chapter 10). In K. E. Elder (Ed.), Fruit Juices: Types, Nutritional Composition & Health Benefits. Nova Science Publishers, Inc. ISBN: 978-1-63321-134-6.
Sun-Waterhouse, D., Melton, L. D., O’Connor, C. J., Kilmartin, P. A., & Smith, B. G. (2008a). Effect of apple cell walls and their extracts on the activity of dietary antioxidants. Journal of Agricultural and Food Chemistry, 56(1), 289–295.
Sun-Waterhouse, D., Smith, B. G., O’Connor, C. J., & Melton, L. D. (2008b). Effect of raw and cooked onion dietary fibre on the antioxidant activity of ascorbic acid and quercetin. Food Chemistry, 111, 580–585.
Sun-Waterhouse, D., Wen, I., Wibisono, R., Melton, L. D., & Wadhwa, S. (2009a). Evaluation of the extraction efficiency for polyphenol extracts from by-products of green kiwifruit juicing. International Journal of Food Science and Technology, 44, 2644–2652.
Sun-Waterhouse, D., Chen, J., Chuah, C., Wibisono, R., Melton, L. D., Laing, W., Ferguson, L. R., & Skinner, M. A. (2009b). Kiwifruit-based polyphenols and related antioxidants for functional foods: kiwifruit extract-enhanced gluten-free bread. International Journal of Food Science and Nutrition, 60(S7), 251–264.
Sun-Waterhouse, D., Teoh, A., Massarotto, C., Wibisono, R., & Wadhwa, S. (2010). Comparative analysis of fruit-based functional snack bars. Food Chemistry, 119, 1369–1379.
Sun-Waterhouse, D., Edmonds, L., Wadhwa, S. S., & Wibisono, R. (2013a). Producing ice cream using a substantial amount of juice from kiwifruits with green, gold or red flesh. Food Research International, 50(2), 647–656.
Sun-Waterhouse, D., Wadhwa, S. S., & Waterhouse, G. I. N. (2013b). Spray-drying microencapsulation of polyphenol bioactives: a comparative study using different natural fibre polymers as encapsulants. Food and Bioprocess Technology, 6, 2376–2388.
Tan, J. S., Wang, J. J., Flood, V., Rochtchina, E., Smith, W., & Mitchell, P. (2008). Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye study. Ophthalmology, 115(2), 334–341.
Tavarini, S., Degl’Innocenti, E., Remorini, D., Massai, R., & Guidi, L. (2008). Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chemistry, 107, 282–288.
Vasbinder, A. J., & de Kruif, C. G. (2003). Casein-whey protein interactions in heated milk: the influence of pH. International Dairy Journal, 13, 669–677.
Villacrez, J. L., Carriazo, J. G., & Osorio, C. (2014). Microencapsulation of Andes berry (Rubus glaucus Benth.) aqueous extract by spray drying. Food and Bioprocess Technology, 7(5), 1445–1456.
Walstra, P. (1990). On the stability of casein micelles. Journal of Dairy Science, 73, 1965–1979.
Wegrzyn, T. F., & MacRae, E. A. (1992). Pectinesterase, polygalacturonase and beta-galactosidase during softening of ethylene-treated kiwifruit. Hortscience, 27(8), 900–902.
Werner, S. R. L., Jones, J. R., & Paterson, A. H. J. (2007). Stickiness of maltodextrins using probe tack test during in-situ drying. Journal of Food Engineering, 80, 859–868.
Whelan, W. J. (2004). New York times horror—the wars of the carbohydrates: Part 3: maltose. IUBMB Life, 56(10), 641.
Whistler, R. L., & BeMiller, J. N. (1997). Carbohydrate chemistry for food scientists (pp. 203–210). St. Paul: The American Association of Cereal Chemists.
Wilson, E. L., & Burns, D. J. W. (1983). Kiwifruit juice processing using heat treatment techniques and ultrafiltration. Journal of Food Science, 48, 1101–1105.
Wong, M., & Stanton, D. W. (1989). Nonenzymaic browning in kiwifruit juice concentrate systems during storage. Journal of Food Science, 54, 669–673.
Wood, F. W., & Goff, T. C. (1973). The determination of the effective shear rate in the Brabender Viscograph and in other systems of complex geometry. Starch, 25(3), 89–91.
Yuliarti, O., Goh, K., Matia-Merino, L., Mawson, J., Drummond, L., & Brennan, C. S. (2008). Effect of extraction techniques and conditions on the physicochemical properties of the water soluble polysaccharides from gold kiwifruit (Actinidia chinensis). International Journal of Food Science and Technology, 43, 2268–2277.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun-Waterhouse, D., Waterhouse, G.I.N. Spray-Drying of Green or Gold Kiwifruit Juice–Milk Mixtures; Novel Formulations and Processes to Retain Natural Fruit Colour and Antioxidants. Food Bioprocess Technol 8, 191–207 (2015). https://doi.org/10.1007/s11947-014-1397-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-014-1397-4