Abstract
The effect of two types of pectins with different degrees of esterification on dough and bread characteristics was analysed. A high methoxyl pectin (HMP) and a low methoxyl pectin (LMP) were assayed in dough without or with salt (2%) at levels ranging from 0.25% to 2.0%. Farinographic water absorption increased when pectins were incorporated in dough with salt, whereas this effect was not observed in dough without salt. Pectin addition diminished the stability of dough in all cases. Texture profile analysis showed that pectins softened the dough, particularly when salt was added. Cohesiveness was also higher in doughs with salt at the maximum level of hydrocolloid addition. In dough with salt, HMP decreased the elastic and viscous moduli, while the values for tan (δ) were increased with respect to control. SEM micrographs showed that dough with pectin has a filamentous structure. In the breadmaking process, dough with HMP showed a better performance, leading to higher specific volumes and softer crumbs both in fresh and stored bread.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pectin is a structural carbohydrate present in the middle lamellae of primary cell walls of plants. From a chemical point of view, pectins are water-soluble heteropolysaccharides mostly consisting of a linear chain of d-galacturonic acid and d-galacturonic acid methyl ester residues, which are linked together by α-1, 4 glucosidic bonds. The number of residues per chain ranges from 200 to 1,000 (Christensen 1982). The degree of esterification (DE) is defined as the percentage of esterified carboxyl groups; high methoxyl pectins (HMP) or high ester pectins have a DE higher than 50%, while low methoxyl pectins (LMP) or low ester pectins have a DE below 50%. The deesterification of HMP in mildly acidic or alkaline conditions leads to LMP. If ammonia is used for the process, the LMP will contain galacturonamide units in the chain. For standardization purposes, commercial pectin is added with sugars, and it is also mixed with suitable food-grade buffer salts for pH control and desirable setting characteristics.
Pectins are classified as dietary fibre, since they escape hydrolysis, digestion and absorption in the small intestine (Prosky 1999). Though in the large intestine, pectins can be used by bacteria, they have an insignificant net calorie value. A reduction of plasma LDL cholesterol and also animal arteriosclerosis as a consequence of pectin ingestion has been reported. Their intake, also, can reduce the incidence of degenerative diseases, such as diabetes and coronary heart disease (Fernandez 2001).
Pectins are generally regarded as safe by the US legislation; acceptable daily intake level is “not specified” by both the Joint Food Experts Committee for the Codex Alimentarius purposes and the Scientific Committee for the Food in the European Union (May 2000).
In the food industry, pectins have a wide range of applications. Their main use is in fruit jams with 60–70% total soluble solids and pH of 3.0–3.3, in which case HMPs can be added, while low-sugar jams and jellies are usually prepared with amidated LMPs. In dairy products, pectins can have two different functions; HMP is able to stabilize protein dispersions at reduced pH as in yoghurt, whereas LMP behaves as a gelling agent in the presence of calcium in either milk or more acid products. Pectins can also be used as an alternative to gelatin in fruit desserts, trifles and bakery fillings. Lately, pectins have been assayed for formulations of edible films (Giancone et al. 2009; Karimi et al. 2009) In all cases, pectins’ effect depends on pH, ionic strength, medium composition and the proportion and type of sweeteners added to the system (May 2000).
In spite of the extensive use of pectins in food industry, they are not commonly applied in breadmaking. Ribotta et al. (2005) have studied the effect of commercial hydrocolloids (pectins, λ-carrageenans and alginates) on dough rheology and bread quality. Particularly, they found that HMP strengthened wheat dough and improved loaf volume, through the formation of hydrophilic complexes between anionic groups (carboxyl) of pectins and gluten proteins. In addition, HMPs are also capable to establish hydrophobic interactions through their methoxyl groups (Christensen 1982). Bárcenas et al. (2009) have reported that HMP mainly affected gluten hydration, modifying its quantity and quality, meanwhile Bollaín and Collar (2004) found adequate rheological trends to obtain high-quality bread by addition of a mixture of high ester pectin, transglutaminase and emulsifier (diacetyl tartaric acid ester of mono-diglycerides) into dough formula. Collar et al. (1999) studied the single or combined addition of certain hydrocolloids to improve wheat bread dough functionally. High ester pectin combined with α-amylase was found to have a strengthening effect on gluten network. Gómez et al. (2007) have employed HMP in yellow layer cakes obtaining a harder and less cohesive product with a higher volume than the formulation without gum. Even though HMPs have been evaluated as dough and bread improvers, LMPs have been much less explored. The objectives of the present work have been (a) to study the potentiality of commercial pectins (a high esterified HMP and an amidated low esterified LMP ones) as dough and breadmaking improvers and (b) to relate the changes observed on dough performance to microstructural modifications.
Materials and Methods
Materials
Wheat commercial flour of standard quality provided by Molino Campodónico S.A. (La Plata, Argentina) was used for dough preparation. Flour composition was: proteins, 11.4% (Kjeldahl factor = 5.7); lipids, 1.4%; moisture, 14.2%; and ash, 0.678%. Alveographic parameters of flour were: P = 96 mm H2O, L = 93 mm, W = 326 × 10–4 J, and the falling number was 486 s. Two types of food grade citrus pectins were used as additives: a low methoxyl pectin (LMP), Genu Pectin 8001 (CP Kelco, USA) and a high methoxyl pectin (HMP), Genu Pectin 105 (CP Kelco, USA).
Methods
Pectin Characterization
Pectin purity, degree of esterification (as percent of total carboxyl groups) of HMP and LMP and degree of amidation of LMP were determined as described by FAO JECFA (2001). Molecular weights were calculated by viscosity measurements on purified isolated pectins applying the Mark–Houwink equation (Anger and Berth 1986). For purification, pectins were washed with a concentrated hydrochloric acid–60% ethanol mixture, and dried for 2.5 h in an oven at 105 °C.
Water Imbibing Capacity
The water imbibing capacity (WIC) of flour and flour–pectin mixtures (1% and 2%) was determined using the Baumann method. The equipment consists of a perforated base covered by a filter paper that is connected to a graduated pipette through a flexible tube; the paper and pipette are horizontal and at the same level (Pilosof 2000). The system is filled with water, and a uniform layer of sample (50 mg) is spread over the filter paper. The water volume was measured as a function of time till a constant value was obtained. The WIC value was calculated as:
Farinographic Assays
Farinographic assays were performed following the American Association of Cereal Chemists (AACC) method 54–21 (AACC International 2000) in a 300-g Brabender instrument (Duisburg, Germany). Flour–pectin mixtures were prepared without and with salt (2 g/100 g flour). In both cases, dry ingredients were premixed. Pectins were tested at 0.25%, 0.5%, 1.0%, 1.5% and 2.0%. The following farinographic parameters were determined: water absorption (A), defined as the percentage of water necessary to yield a consistency of 500 BU; development time (t d), as the time to reach up to 500 BU; and dough stability, the time that dough remains at a consistency of 500 BU. The assay was made at least in duplicate.
Dough Preparation for Rheological Assays
Dough formulation employed for rheological assays was: flour, 100 g; NaCl, 2 g; water according to farinographic absorption and pectins: 0 (control), 1 and 2 g. Doughs were prepared without yeast in a small-scale planetary kneader (Kenwood, Italy). For each blend, the farinographic development time was used as the mixing time. To improve gluten development, dough was sheeted four times, turning 90° after each passage by the rods. Dough was then covered with a plastic film, to avoid dehydration, and left to rest for 15 min at room temperature.
Dough was laminated and cut in cylindrical pieces 3 cm in diameter and 1 cm in height for texture assays, whereas pieces 3 cm in diameter and 2 mm in height were employed for rheometric assays. For both assays, the doughs were prepared in duplicate.
Dough Characterization
Water Availability
Moisture Content. It was determined by air-drying in an oven at 135 °C for 2 h (AACC International 2000; method 44–19).The assay was made in duplicate.
Water Activity. Measurements were performed at 25 °C in an AquaLab (Decagon Devices, Inc, Pullman and EUA). The assay was made in duplicate.
Wet Gluten/Dry Gluten. The assays were performed according to a modification of AACC 38–12 (AACC International 2000; Correa et al. 2010). A Glutomatic System (Perten Instruments, Sweden) was used, and dough samples were prepared as for the rheological assays. Each sample was measured at least in duplicate.
Rheological Assays
Dynamic Rheometry. Dynamic oscillatory tests were performed in a Haake RS600 controlled stress oscillatory rheometer (Haake, Germany). The assay was executed at 25 ± 0.1 °C, using a serrated plate–plate sensor system with a 1.5-mm gap between plates. To prevent sample dehydration during the assay, semisolid Vaseline oil was applied. Before measurements, samples were allowed to rest 15 min between plates to relax. Deformation and frequency sweeps were carried out. Deformation sweeps were performed at a constant frequency (1 Hz) in order to determine the linear viscoelastic range of each sample (data not shown); frequency sweeps (from 0.005 to 100 Hz) were performed at a constant stress (5 Pa) within the linear viscoelastic range. Dynamic moduli G′ (elastic or storage modulus), G″ (viscous or loss modulus) and tan δ (G″/G′) (related with the overall viscoelastic response) were obtained as a function of frequency. Doughs were prepared in duplicate, and three measurements were performed on each dough.
Texture Profile Analysis. Cylindrical samples (ten pieces) with a diameter of 3 cm and height of 1 cm were obtained from dough. Dough texture parameters were evaluated using a TA.XT2i Texture Analyzer (Stable Micro Systems, Surrey, UK) with a software Texture Expert for Windows, version 1.2. Dough was submitted to two cycles of compression up to 40% of the original height with a cylindrical probe (diameter = 7.5 cm). Force time curves were obtained at a crosshead speed of 0.5 mm/s. Dough hardness, adhesiveness, consistency, cohesiveness, springiness and resilience were determined. Hardness is defined as the maximum force registered during the first compression cycle. Adhesiveness is the negative area obtained during the first cycle. Consistency was calculated as the area of the first peak plus the area of the second peak. Cohesiveness was determined as the ratio between the positive area of the second cycle and the positive area of the first cycle. Springiness was measured by the distance of the detected height of the product on the second compression divided by the original compression. Finally, resilience is calculated as the area during the withdrawal of the first compression divided by the area up to the maximum of the first cycle.
Puncture Test. Using a TA.XT2i Texture Analyzer (Stable Micro Systems, Surrey, UK), cylindrical samples (ten pieces) with a diameter of 3 cm and height of 1 cm were penetrated until 40% in depth of the original height with a cylindrical probe SMS P/3 (diameter = 3 mm). Dough firmness was determined as the maximum force registered during the penetration.
Scanning Electron Microscopy
Micrographs of control and samples with pectins at the highest level (2.0%) and salt (2.0%) were taken. Dough was prepared as described for rheological assays. Small portions of each sample were cut, fixed in 10% glutaraldehyde and sequentially embedded in acetone solutions of increasing concentration to ensure full dehydration. Samples were dried at the critical point and coated with gold particles. A scanning electron microscope (JEOL 35 CF, Japan), with a magnification of ×500 was used.
Breadmaking
Fermentation Curves
Dough formulation on a 100-g flour basis was: compressed yeast, 3.0% (Calza, Argentina); salt, 2.0%; water according to farinographic absorption and pectins (used at two levels, 1.0% and 2.0%). Dough without pectin was used as control. Mixing times were fixed according to development times obtained from farinograph assays. A planetary kneader (Kenwood, Italy) was used. Dry ingredients were premixed, and the yeast was dissolved in the added water. Dough was divided into pieces (50 g), placed in a graduated cylinder and left to leaven in a 30-°C chamber. The increase in volume (ΔV) was defined as the difference between the volume at a certain time and the initial volume, and it was registered as a function of time. The highest increase in volume (ΔVmax) was obtained, and the time required to reach up to 3/4 of ΔVmax was used as fermentation time.
Breadmaking Process
Formulation and dough preparation were the same as described in “Fermentation Curves” Section. Dough was left to rest for 10 min at room temperature, sheeted four times and left to rest again. Then, it was divided into 90-g portions. Each portion was rounded, left to rest for 15 min and then shaped into bread loaves. These pieces were proofed at 30 °C according to their fermentation time and baked in a convection oven for 26 min at 210 °C. Breads were packaged in plastic bags and stored for 0, 1 and 3 days at 20 °C.
Characterization of Bread
Specific Volume. After cooling, bread pieces were weighted, and the volume was determined by rapeseed displacement. The specific volume was determined as volume/piece weight.
Crumb Texture Profile Analyses. The texture profile analysis (TPA) of bread slices was performed on fresh bread and after 1 and 3 days of storage at 20 °C. From the middle part of each bread piece, two slices of 2 cm height were obtained. A texture analyzer TA.XT2i (Stable Micro Systems, Surrey, UK) equipped with a 25-kg load cell was used to perform the TPA of crumb. The slices were subjected to a double compression cycle up to 40% of the original height using a 2.5-cm-diameter cylindrical probe and 0.5 mm s−1 crosshead speed. The parameters determined were: hardness, cohesiveness, consistency, springiness, resilience and chewiness.
Crumb Moisture. It was determined on fresh bread and after 1 and 3 days of storage at 20 °C. The assay was performed according the AACC 44–19 (AACC International 2000) method.
Crust Colour. It was measured using a colorimeter (Chroma Meter CR-300C, Minolta, Osaka, Japan). The Hunter scale parameters were determined: L (lightness, ranging from 0 to 100, from black to white), a (positive values indicating redness and negative values, greenness) and b (positive values indicate yellowness and negative values, blueness)
Crumb Image Analysis. Crumb grain characteristics of bread were assessed using a digital image analysis system. Images were previously acquired at 350 dots per inch with a HP scanner 4070 model. The analysis was performed on the centre of the slice. Images were processed using ImageJ software. The crumb grain characteristics studied were: mean cell area (square centimetre), mode cell area (square centimetre), circularity and cell to total area ratio (or void fraction).
Statistical Analysis
To discriminate among means, Bonferroni’s multiple comparison procedure was applied at 95% confidence level. Statgraphics Plus software was used.
Results and Discussion
Pectin Characteristics
Purity of both pectins was the same: 57% (w/w). The degree of esterification was 67% for HMP and 43% for LMP. The degree of amidation for LMP was 16%. Molecular weight was determined as 5 × 104 Da for both pectins.
Water Absorption of Pectin–Flour Mixtures
In Table 1, the results for farinographic water absorption and WIC are shown. In general, a tendency to higher farinographic water absorption values was observed as the level of pectin increased, but only in samples with salt was a significant difference found. In agreement, higher WIC values were obtained for samples with pectins added at the maximum level.
Dough Characterization
As expected, moisture values were higher in doughs with pectins when compared to control. Moisture levels of samples without salt and with pectins ranged between 45.4 and 45.9 (control = 44.5%). With salt, the range was between 42.9 and 44.9 (control = 43.2%). At the highest level of additive, samples with salt showed a significant increase in moisture with respect to control. Water activity was significantly lower for samples with salt (from 0.9693 to 0.9726) than for samples without salt (from 0.9865 to 0.9876). No significant differences were found within each group.
The ratio between wet gluten (WG) to dry gluten (DG), related to the hydration capacity of gluten proteins, ranged from 2.8 to 3.1. In general, no significant differences were found in WG or in WG/DG ratio independently of the presence or absence of salt. The farinographic development time was not significantly modified by pectin addition; however, in the presence of salt, higher times were obtained.
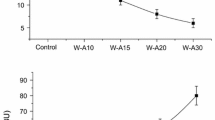
In Fig. 1, stability values of mixtures with and without salt are shown. As it can be seen from comparison between control doughs with and without salt (black bars), stability is markedly increased by the addition of salt. Wherle et al. (1997) reported that the addition of salt (2%) led to softer doughs with more stability. The increased stability is probably due to a reinforcement of the gluten network. Kinsella and Hale (1984) studied the effect on dough of anions of different degrees of chaotropic character. They stated that in the presence of F− and Cl−, hydrophobic interactions between gluten proteins were promoted, remaining more aggregated and resistant to hydration. In spite of requiring extensive kneading to be developed, the resulting network was much more stable in the presence of NaCl.
When pectins were added in both samples without or with salt, dough stability tended to diminish. This decrease was more pronounced in the case of HMP addition (up to 25%), suggesting that interactions between pectins and proteins negatively affected gluten network.
Results for TPA on doughs are shown in Fig. 2. From comparison between controls with and without salt, it is observed that softer doughs were obtained in the absence of salt. This point is controversial since different authors (Wherle et al. 1997; Larsson 2002; Salvador et al. 2006; Lynch et al. 2009) do not agree with respect to the effect of salt on textural quality; these divergences are probably due to other variables such as type of flour, dough formulation and breadmaking process. When pectins were added, hardness decreased both in doughs with or without salt, but the effect was more pronounced in the presence of salt. Similar results were observed in puncture test, where the penetration force diminished more markedly with respect to control in the presence of salt (Table 2). In all cases, springiness (related to sample recovery after deformation) and adhesiveness were decreased by the addition of pectins. Cohesiveness is related to the degree of sample integrity when deformed. In this case, the effect of pectins was different according to the presence or absence of salt: both pectins (LMP and HMP) at the highest level drastically decreased cohesiveness in samples without salt. When salt was present, cohesiveness was slightly increased at the highest level of hydrocolloid, indicating an improvement in the integration of dough components. In general, it can be said that pectins had a disruptive effect on gluten matrix leading to softer and less elastic doughs.
In Fig. 3 is shown the typical frequency sweep obtained for doughs by rheometric assays. It can be seen that G′ > G″ in all the tested range, and both moduli are frequency dependent, showing a solid viscoelastic behaviour. HMP dough with salt exhibited significantly lower values of all moduli (G′, G″, G*; Fig. 4) and a significantly higher value of tan δ (more viscous and lesser elastic behaviour). This effect was not observed in samples without salt nor with LMP dough. Though rheometric measurements reflect the behaviour of dough under small strains, analogous trends were obtained with larger deformation tests like TPA and puncture assay.
Rheometric parameters (G′, G″, G*, tan δ) of dough without and with salt at two levels of pectin addition. All parameters were measured at 1 Hz. Black bar 0%, low density dotted bar 1%, high density dotted bar 2%. Different letters within the same group of bars indicate significant differences (P < 0.05). Error bars correspond to standard error
From rheological tests, it can be assessed that doughs with HMP in the presence of salt are softer, less adhesive, more cohesive and less elastic (i.e. more viscous) than the control. Though both pectins can establish ionic interactions through carboxylic groups, HMP (DE = 67%) would be more able than LMP (DE = 43%) to establish, additionally, hydrophobic unions with proteins, a type of interaction particularly favoured by the presence of NaCl.
Figure 5 shows representative scanning electron microscopy (SEM) micrographs of control and pectin samples at 2% hydrocolloid level, in the presence of salt. The control sample exhibits a distinguishable gluten film (GF) structure, surrounding starch granules. Some gluten strands (GS) are also evident, whereas doughs containing pectins show a more filamentous structure. When HMP is used, a more porous (open) structure is observed. This is coincident with the diminished stability and hardness of this sample as well as its more fluid character.
Breadmaking
Fermentation curves were adjusted by the sigmoid function shown in Eq. 2. This model was empirically selected among other experimental equations on the basis of the best fit.
Where ΔV is the volume increment, t is the time (minutes) and a, b and c are constants. Parameter a corresponds to the maximum achieved volume increment.
In Table 3, values of parameters a, b and c are shown. The highest fermentation volumes were obtained for doughs added with HMP. When LMP was used, final volumes were lower than those obtained with the control dough. Increasing levels of HMP tended to increase the final volume, whereas a contrary effect was observed when LMP was used. To prevent dough collapse during the oven spring, the fermentation time was fixed as the time necessary to reach three quarters of the maximum volume. Thus, doughs with HMP needed higher fermentation times. Higher rates of volume increment (compared to control dough) were observed at the beginning of the curves.
Specific volume of breads with pectins was compared to that of control bread (Table 4). Loaves added with LMP 1% and 2% showed specific volumes of 3.8 and 3.3 cc g−1, respectively, and these values did not significantly differ from control (3.3 cc g−1). However, when HMP was added, the specific volume of loaves significantly increased up to 4.0 and 4.2 cc g−1 for 1% and 2% levels, respectively. This indicates a better performance of HMP in both concentrations. These results were in accord with those of fermentation curves since doughs with HMP reached higher volumes. The evaluation of crust colour showed that pectin addition did not lead to changes since there were not significant differences among samples, with “a” value ranging from 9.8 to 10.8, “b” from 34.2 to 35.7 and “L” value from 59.9 to 62.6. With respect to crumb quality, moisture levels of samples with pectins were not significantly different from control, ranging from 43.9% to 44.9%.
In Table 4, the structural properties of bread crumb are summarized. The void percentage, which measures the proportion of the cross-sectional area of the crumb containing gas cells (Zghal et al. 1999), was slightly lower for samples with LMP, while HMP and control samples had similar values. The mode cell area (more frequent area value) was in all cases lower than the control. So, there are more cells of larger diameter in bread crumb without pectins than in crumb of breads with pectins. According to Gan et al. (1995), alveolus stabilization depends primarily on gluten–starch matrix and in a second instance on the liquid lamella that is surrounding the cell. So, a possible explanation for the diminished size of alveolus in the presence of pectins could be related to an enhancement of the viscosity of these lamellae by the presence of hydrocolloids that hinders alveolus expansion. On the other hand, circularity, an indicator of alveolus symmetry, was improved by pectins.
In Fig. 6, textural attributes of samples with and without pectins are shown for fresh and stored bread. For fresh bread (0 day), it is observed that hardness values of samples containing pectins were lower than the values for the control. The softest crumbs were obtained with HMP at both levels, in accord with the higher specific volumes obtained. The consistency values (data not shown) followed the hardness trend. Samples with gums were significantly more cohesive than control without pectins, suggesting a more integrated matrix. Chewiness is calculated as the product of springiness, cohesiveness and hardness; although cohesiveness and springiness increase, the decrease in hardness was relatively higher, so chewiness was significantly lower for pectin-added samples, particularly in the case of HMP. Resilience is related to the instantaneous ability of crumb for recovering the original geometry (instantaneous elasticity) and was significantly higher for samples with gums, indicating a positive effect on the crumb matrix.
Bread staling was evaluated through the changes observed in the above described textural attributes along the 3-day storage period and also in moisture variation. In Table 4, moisture values of bread crumb at different days of storage are shown. Samples stored for 3 days at room temperature diminished their moisture up to 14.5% with respect to the initial value. The driest crumb was found for the control, while the moisture was better maintained for breads with HMP 2%.
In all cases, bread samples stored increased hardness, but HMP breads maintained a significantly softer crumb with respect to control even in the third day of storage, whereas resilience and cohesiveness decrease with time. On the other hand, chewiness increased with storage though HMP samples were less affected. Thus, chewiness values of HMP samples after the 3-day storage period were similar to those of fresh breads, indicating a protective effect of this hydrocolloid.
Conclusions
Commercial standardized pectins of different degrees of esterification were satisfactorily assayed in breadmaking, rendering different dough characteristics and bread quality. In spite of being intended for other purposes, they can be used under commercial presentation (standardized). HMP addition led to softer, more cohesive and more viscous doughs than control and LMP samples. SEM microstructure analysis revealed a different, more porous type of gluten matrix. These doughs were more stable and able to support a greater expansion during fermentation, reaching up to higher loaf volumes. Specific volume of breads was significantly improved with HMP, and a softer, more cohesive and more resilient crumb with a different alveolus structure was obtained. In general, pectins exerted a protective action with respect to staling, but HMP was more effective. The dissimilar effect of HMP or LMP on breadmaking steps can be attributed to the specific interactions of each pectin with gluten proteins, with HMP probably being able to establish more hydrophobic bonds due to the major proportion of methoxyl groups.
References
AACC International. (2000). Methods 54–21, 38–12, 44–19. Approved methods of the American Association of Cereal Chemists (10th ed.). St. Paul: The American Association of Cereal Chemist, Inc.
Anger, H., & Berth, G. (1986). Gel permeation chromatography and the Mark Houwink relation for pectins with different degrees of esterification. Carbohydrate Polymers, 6, 193–202.
Bárcenas, M. E., De la O-Keller, J., & Rosell, C. M. (2009). Influence of different hydrocolloids on major wheat dough components (gluten and starch). Journal of Food Engineering, 94, 241–247.
Bollaín, C., & Collar, C. (2004). Dough viscoelastic response of hydrocolloid/enzyme/surfactant blends assessed by uni- and bi-axial extension measurements. Food Hydrocolloids, 18(3), 499–507.
Christensen, S. H. (1982). Pectins. In M. Glicksman (Ed.), Food hydrocolloids (Vol. 3, pp. 205–230). Boca Raton: CRC Press.
Collar, C., Andreu, P., Martinez, J. C., & Armero, E. (1999). Optimization of hydrocolloid addition to improve wheat bread dough functionality: a response surface methodology study. Food Hydrocolloids, 13(6), 467–475.
Correa, M. J., Añón, M. C., Perez, G. T., & Ferrero, C. (2010). Effect of modified celluloses on dough rheology and microstructure. Food Research International, 43, 780–787.
FAO JECFA. (2001) FNP52 add 9. Pectins. http://www.fao.org/ag/agn/jecfa-additives/specs/Monograph1/Additive-306.pdf
Fernandez, M. L. (2001). Pectin. In S. Sungsoo Cho & M. L. Dreher (Eds.), Handbook of dietary fiber (Vol. Chapter 30). New York: Marcel Dekker, Inc.
Gan, Z., Ellis, P. R., & Schofield, J. D. (1995). Gas cell stabilisation and gas retention in wheat bread dough. Mini review. Journal of Cereal Science, 21, 215–230.
Giancone, T., Torrieri, E., Di Pierro, P., Cavella, S., Giosafatto, C.V. L., Masi, P (2009). Effect of surface density on the engineering properties of high methoxyl pectin-based edible films. Food and Bioprocess Technology, doi:10.1007/s11947-009-0208-9 (in press).
Gómez, M., Ronda, F., Caballero, P. A., Blanco, C. A., & Rosell, C. M. (2007). Functionality of different hydrocolloids on the quality and shelf-life of yellow layer cakes. Food Hydrocolloids, 21, 167–173.
Karimi, Y., Maftoonazad, N., Ramaswamy, H.S., Prasher S.O, Marcotte, M. (2009) Application of hyperspectral technique for color classification avocados subjected to different treatments. Food and Bioprocess Technology, doi:10.1007/s11947-009-0292-x (in press).
Kinsella, J. E., & Hale, M. L. (1984). Hydrophobic associations and gluten consistency: effects of specific anions. Journal of Agriculture and Food Chemistry, 32, 1054–1056.
Larsson, H. (2002). Effect of pH and sodium chloride on wheat flour dough properties: ultracentrifugation and rheological measurements. Cereal Chemistry, 79, 544–545.
Lynch, E. J., Dal Bello, F., Sheehan, E. M., Cashman, K. D., & Arendt, E. K. (2009). Fundamental studies on the reduction of salt on dough and bread characteristics. Food Research International, 42, 885–891.
May, C. D. (2000). Pectins (chapter 10). In G. O. Phillips & P. A. Williams (Eds.), Handbook of hydrocolloids. Cambridge: Woodhead Publishing Limited.
Pilosof, A.M.R. (2000) Propiedades de hidratación. In Caracterización funcional y estructural de proteínas (pp 17–29), Editorial Universitaria de Buenos Aires.
Prosky, L. (1999). What is fibre? Current controversies. Trends in Food Science and Technology, 10, 271–275.
Ribotta, P. D., Ausar, S. F., Beltramo, D. M., & Leon, A. E. (2005). Interactions of hydrocolloids and sonicated-gluten proteins. Food Hydrocolloids, 19, 93–99.
Salvador, A., Sanz, T., & Fiszman, S. M. (2006). Dynamic rheological characteristics of wheat flour-water doughs. Effect of adding NaCl, sucrose and yeast. Food Hydrocolloids, 20, 780–786.
Wherle, K., Grau, H., & Arendt, E. K. (1997). Effects of lactic acid, acetic acid, and table salt on fundamental rheological properties of wheat dough. Cereal Chemistry, 74, 739–744.
Zghal, M. C., Scanlon, M. G., & Sapirstein, H. D. (1999). Prediction of bread crumb density by digital image analysis. Cereal Chemistry, 76(5), 734–742.
Acknowledgements
The authors acknowledge the Agencia Nacional de Promocion Cientifica y Tecnologica–MINCYT, Consejo Nacional de Investigaciones Científicas y Tecnicas–CONICET, Universidad Nacional de La Plata and Universidad Nacional de Córdoba (Argentina) for the financial support. The authors want to specially thank to Dra. Laura Villata for the technical assistance in viscosity measurements needed to determine molecular weights.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Correa, M.J., Pérez, G.T. & Ferrero, C. Pectins as Breadmaking Additives: Effect on Dough Rheology and Bread Quality. Food Bioprocess Technol 5, 2889–2898 (2012). https://doi.org/10.1007/s11947-011-0631-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0631-6